Abstract
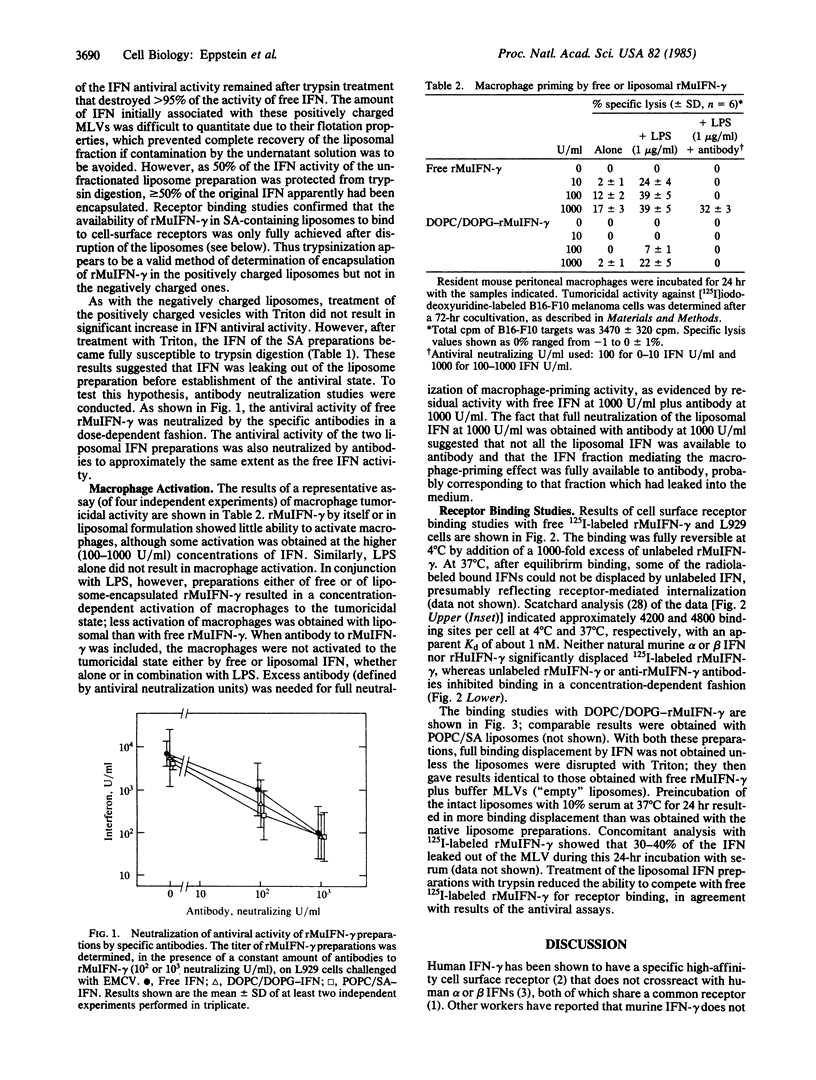
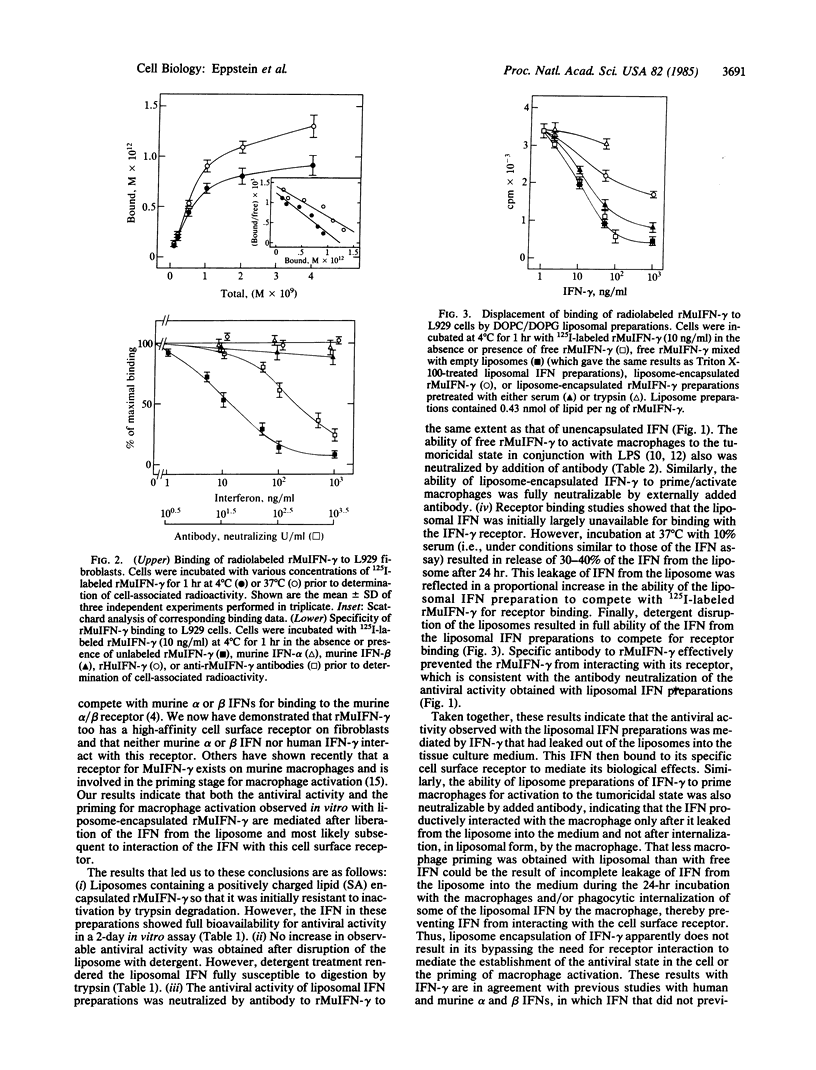
Recombinant murine gamma interferon (rMuIFN-gamma) was found to bind reversibly to a specific high-affinity surface receptor on L929 cells; neither murine alpha or beta nor human gamma IFN competed for receptor binding. Encapsulation of the rMuIFN-gamma in either negatively or positively charged liposomes reduced its immediate ability to bind to this surface receptor. Disruption of liposome integrity with detergent resulted in full ability of the rMuIFN-gamma to bind to the membrane receptor. Incubation of the liposomal IFN in serum-containing medium resulted in significant leakage so that the IFN was able to bind to its surface receptor. Assessment of the biological activity of the rMuIFN-gamma preparations revealed that full antiviral activity was observed in vitro with the liposomal IFN preparations without their prior disruption by detergent. The antiviral activity observed with either free or liposomal IFN was neutralized completely by antibodies against rMuIFN-gamma. Both free and liposomal rMuIFN-gamma, in conjunction with bacterial lipopolysaccharide, were also able to activate murine peritoneal macrophages to the tumoricidal state. Again, this activity of both free and liposomal IFN could be neutralized completely by antibody. These results indicate that although rMuIFN-gamma can be effectively incorporated into liposomes, it must ultimately leak out of the liposome in order to mediate its biological effects; these effects are triggered after the IFN binds to its cell surface receptors.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguet M., Belardelli F., Blanchard B., Marcucci F., Gresser I. High-affinity binding of 125I-labeled mouse interferon to a specific cell surface receptor. IV. Mouse gamma interferon and cholera toxin do not compete for the common receptor site of alpha / beta interferon. Virology. 1982 Mar;117(2):541–544. doi: 10.1016/0042-6822(82)90497-4. [DOI] [PubMed] [Google Scholar]
- Anderson P., Yip Y. K., Vilcek J. Specific binding of 125I-human interferon-gamma to high affinity receptors on human fibroblasts. J Biol Chem. 1982 Oct 10;257(19):11301–11304. [PubMed] [Google Scholar]
- Branca A. A., Baglioni C. Evidence that types I and II interferons have different receptors. Nature. 1981 Dec 24;294(5843):768–770. doi: 10.1038/294768a0. [DOI] [PubMed] [Google Scholar]
- Celada A., Gray P. W., Rinderknecht E., Schreiber R. D. Evidence for a gamma-interferon receptor that regulates macrophage tumoricidal activity. J Exp Med. 1984 Jul 1;160(1):55–74. doi: 10.1084/jem.160.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppstein D. A. Altered pharmacologic properties of liposome-associated human interferon-alpha. II. J Interferon Res. 1982;2(1):117–125. doi: 10.1089/jir.1982.2.117. [DOI] [PubMed] [Google Scholar]
- Eppstein D. A., Marsh Y. V. Partial dissociation of antiviral and antimitogenic activities of murine interferon after its incorporation into liposomes. J Interferon Res. 1983;3(2):161–168. doi: 10.1089/jir.1983.3.161. [DOI] [PubMed] [Google Scholar]
- Eppstein D. A., Stewart W. E., 2nd Altered pharmacological properties of liposome-associated human interferon-alpha. J Virol. 1982 Feb;41(2):575–582. doi: 10.1128/jvi.41.2.575-582.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppstein D. A., Stewart W. E., 2nd Binding and capture of human interferon-alpha by reverse evaporation vesicles, multilamellar vesicles, and small unilamellar vesicles. J Interferon Res. 1981;1(4):495–504. doi: 10.1089/jir.1981.1.495. [DOI] [PubMed] [Google Scholar]
- Fidler I. J., Darnell J. H., Budmen M. B. In vitro activation of mouse macrophages by rat lymphocyte mediators. J Immunol. 1976 Aug;117(2):666–673. [PubMed] [Google Scholar]
- Fogler W. E., Raz A., Fidler I. J. In situ activation of murine macrophages by liposomes containing lymphokines. Cell Immunol. 1980 Jul 15;53(1):214–219. doi: 10.1016/0008-8749(80)90440-2. [DOI] [PubMed] [Google Scholar]
- Gray P. W., Goeddel D. V. Cloning and expression of murine immune interferon cDNA. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5842–5846. doi: 10.1073/pnas.80.19.5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray P. W., Goeddel D. V. Structure of the human immune interferon gene. Nature. 1982 Aug 26;298(5877):859–863. doi: 10.1038/298859a0. [DOI] [PubMed] [Google Scholar]
- Higashi Y., Sokawa Y. Microinjection of interferon and 2',5'-oligoadenylate into mouse L cells and their effects on virus growth. J Biochem. 1982 Jun;91(6):2021–2028. doi: 10.1093/oxfordjournals.jbchem.a133895. [DOI] [PubMed] [Google Scholar]
- Koff W. C., Fidler I. J., Showalter S. D., Chakrabarty M. K., Hampar B., Ceccorulli L. M., Kleinerman E. S. Human monocytes activated by immunomodulators in liposomes lyse herpesvirus-infected but not normal cells. Science. 1984 Jun 1;224(4652):1007–1009. doi: 10.1126/science.6426057. [DOI] [PubMed] [Google Scholar]
- Laurent G., Laduron C., Ruysschaert J. M., Deleers M. Inhibition of cationic liposomes of [3H]thymidine incorporation into DNA of L1210 cells. Res Commun Chem Pathol Pharmacol. 1981 Mar;31(3):515–527. [PubMed] [Google Scholar]
- Le J., Prensky W., Yip Y. K., Chang Z., Hoffman T., Stevenson H. C., Balazs I., Sadlik J. R., Vilcek J. Activation of human monocyte cytotoxicity by natural and recombinant immune interferon. J Immunol. 1983 Dec;131(6):2821–2826. [PubMed] [Google Scholar]
- Männel D. N., Falk W. Interferon-gamma is required in activation of macrophages for tumor cytotoxicity. Cell Immunol. 1983 Jul 15;79(2):396–402. doi: 10.1016/0008-8749(83)90082-5. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orchansky P., Novick D., Fischer D. G., Rubinstein M. Type I and Type II interferon receptors. J Interferon Res. 1984 Spring;4(2):275–282. doi: 10.1089/jir.1984.4.275. [DOI] [PubMed] [Google Scholar]
- Panzner E. A., Jansons V. K. Control of in vitro cytotoxicity of positively charged liposomes. J Cancer Res Clin Oncol. 1979 Sep;95(1):29–37. doi: 10.1007/BF00411106. [DOI] [PubMed] [Google Scholar]
- Poste G., Kirsh R., Fogler W. E., Fidler I. J. Activation of tumoricidal properties in mouse macrophages by lymphokines encapsulated in liposomes. Cancer Res. 1979 Mar;39(3):881–892. [PubMed] [Google Scholar]
- Roberts W. K., Vasil A. Evidence for the identity of murine gamma interferon and macrophage activating factor. J Interferon Res. 1982;2(4):519–532. doi: 10.1089/jir.1982.2.519. [DOI] [PubMed] [Google Scholar]
- Rothermel C. D., Rubin B. Y., Murray H. W. Gamma-interferon is the factor in lymphokine that activates human macrophages to inhibit intracellular Chlamydia psittaci replication. J Immunol. 1983 Nov;131(5):2542–2544. [PubMed] [Google Scholar]
- Schreiber R. D., Pace J. L., Russell S. W., Altman A., Katz D. H. Macrophage-activating factor produced by a T cell hybridoma: physiochemical and biosynthetic resemblance to gamma-interferon. J Immunol. 1983 Aug;131(2):826–832. [PubMed] [Google Scholar]
- Schultz R. M., Kleinschmidt W. J. Functional identity between murine gamma interferon and macrophage activating factor. Nature. 1983 Sep 15;305(5931):239–240. doi: 10.1038/305239a0. [DOI] [PubMed] [Google Scholar]
- Sone S., Poste G., Fidler I. J. Rat alveolar macrophages are susceptible to activation by free and liposome-encapsulated lymphokines. J Immunol. 1980 May;124(5):2197–2202. [PubMed] [Google Scholar]
- Svedersky L. P., Benton C. V., Berger W. H., Rinderknecht E., Harkins R. N., Palladino M. A. Biological and antigenic similarities of murine interferon-gamma and macrophage-activating factor. J Exp Med. 1984 Mar 1;159(3):812–827. doi: 10.1084/jem.159.3.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vengris V. E., Stollar B. D., Pitha P. M. Interferon externalization by producing cell before induction of antiviral state. Virology. 1975 Jun;65(2):410–417. doi: 10.1016/0042-6822(75)90046-x. [DOI] [PubMed] [Google Scholar]
- Zoon K. C., Arnheiter H. Studies of the interferon receptors. Pharmacol Ther. 1984;24(2):259–278. doi: 10.1016/0163-7258(84)90037-8. [DOI] [PubMed] [Google Scholar]


