Summary
Bacterial translocation after major burns plays an important role in burn sepsis and can be reduced with SDD. 30 patients with burns of 25-50% TBSA were divided into 2 groups. Group I received SDD regimen in the form of amikacin, miconazole, and colistin sulphate. Group II served as a control group. SDD treatment resulted in significant control of infectious episodes and multi-organ dysfunction syndrome (MODS). It also resulted in a reduction of mortality, although this was not statistically significant. Despite the statistical insignificance of the improved mortality rate, SDD treatment seems to be a useful tool in treating this group of highly critical patients.
Keywords: bacterial translocation, digestive decontamination, gut derived burn sepsis, burn sepsis
Abstract
La translocation bactérienne après des brûlures ha un rôle important dans le sepsis des brûlures et peut être réduite avec la décontamination sélective du tractus digestif (DSTD). 30 patients souffrant de brûlures de 25% à 50% de la surface corporelle totale (SCT) ont été divisés en 2 groupes. Groupe I a reçu DSDT sous la forme de l’amikacine, le miconazole et le sulfate de colistine. Groupe II a servi de groupe témoin. Le traitement DSTD a donné lieu à un contrôle important des épisodes infectieux et le syndrome de dysfonction d’organes multiples. Il a également entraîné une réduction de la mortalité, même si ce n’était pas statistiquement significative. En dépit la manque de la signification statistique du taux de mortalité amélioré, le traitement DSDT semble être utile pour ce groupe de patients très critiques.
Introduction
Major Burns result in alteration of the permeability of the gastrointestinal tract. This enables the microorganisms and/or their toxins to translocate across the gut barrier and invade the blood stream, leading to colonization of the burn wound and systemic organs as secondary sites.1 Yao et al.,2 and Peng et al.,3 correlated a strong association between multiple organ failure (MOF) and high plasma levels of endotoxin in absence of wound infection. Their findings strongly suggest the role of gut-derived endotoxin in triggering a postburn systemic inflammatory response leading to MOF.
Experimentally, the control of the pathogenic organisms in the bowel lumen by ligation of mesenteric lymph nodes prevented postburn spread of translocated bacteria.4 This suggests that elimination or reduction of pathogenic organisms in the gut can have a role in prevention of gut derived endotoxaemia. This can be achieved by means of selective digestive decontamination (SDD).5 SDD is an infection prophylaxis regimen that employs oral non absorbable antibiotics to eradicate pathogenic organisms from the gastrointestinal tract (GIT).6 The standard SDD regimen consists of two components, topical non-absorbable antimicrobials and antifungal.7
The aim of this work is to evaluate the role of SDD in the prevention and/or treatment of gut-derived burn sepsis, sepsis related internal organ derangement and mortality.
Methods and materials
This is a prospective comparative study that was conducted in the Burn Unit of Ain Shams University Hospitals, Egypt. The study included 30 (16 males and 14 females) acutely burned patients. Patients were admitted within 16 hours of injury. Patients were excluded if they had any previous history of GIT disease (such as inflammatory bowel disease or gastric ulcers), or were allergic to the drugs used. Any patients with inhalation injury were also excluded to avoid multiple variants that could affect patient outcome. The total body surface area (TBSA) burned ranged from 30-50 %. Full thickness injury was at least 15% of the TBSA.
Frequent bacteriological sampling in our burn unit showed a very high prevalence of gram –ve bacteria. The infection control unit advised using parenteral antibiotics for a period until the patient was stabilized and the burned skin was excised. This antibiotic was changed every 3 months. In this study, all patients received parenteral antibiotics (ciprofloxacin) in the first week.
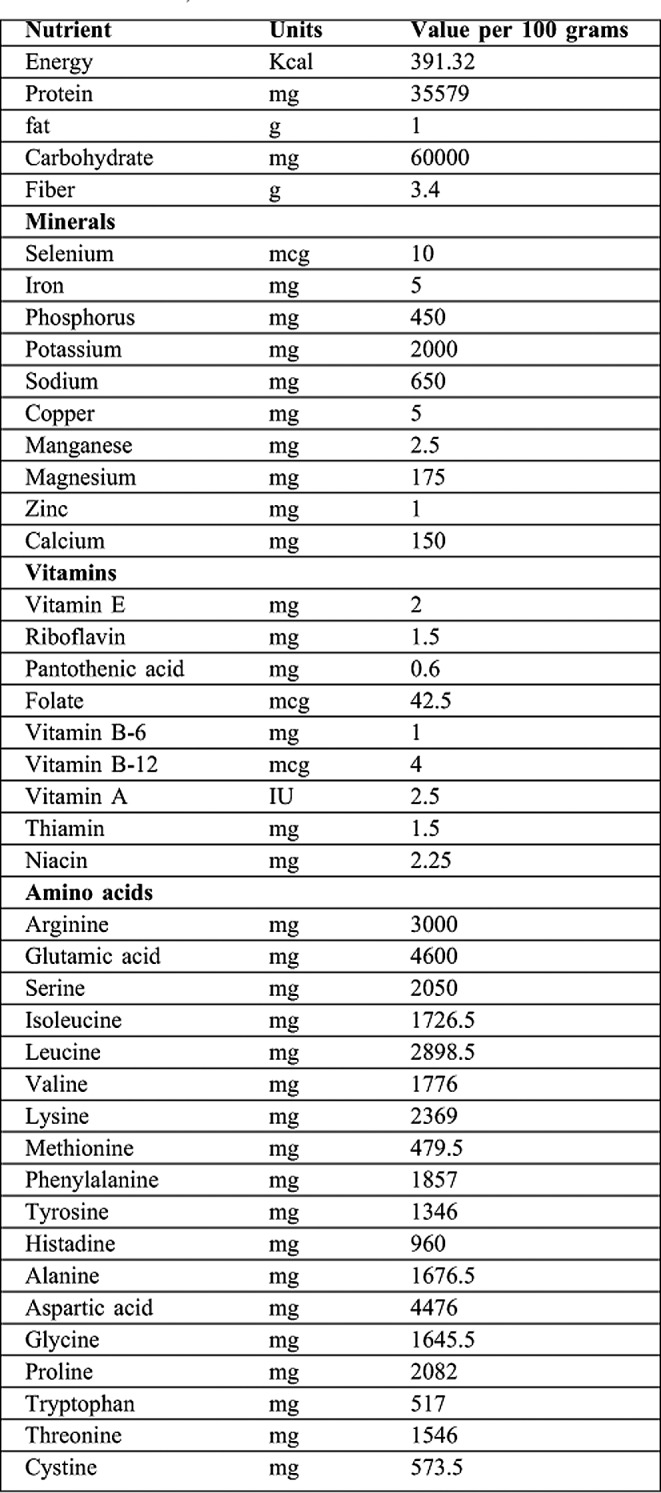
Resuscitation was performed using the Parkland formula. The caloric and protein requirements were calculated according to the Curreri formula.8 Feeding was started 8 hours after the injury occurred. The requirements were given either orally or by tube. Feeding was performed gradually to avoid any associated issues such as vomiting and ileus. Feeding was started using Bio gainers formula (BIO PHARMA EGYPT) (Table I) then replaced gradually with blended ordinary food.
Table I. showing constituents of feeding formula Bio Gainers (BIO PHARMA EGYPT).
Patients were divided into two groups of 15: Group I (treatment group) and Group II (plain control group). The patients in Groups I and II were treated consecutively to facilitate our work by enabling us to finish one group before starting the other and thus avoid any confusion or misunderstanding on the part of nursing staff and junior assistants. Group I patients underwent early excision of full thickness eschar on 2-3 stages within the first week and coverage was done either by homograft or amniotic membrane. The homograft were obtained from clean surgical procedures then processed in the burn unit skin bank laboratory. The amniotic membrane was prepared and sterilized by gamma irradiation (it was provided by the National Nuclear Energy Institute). Excision and auto-grafting was done in selected patients with a maximum of 35% burned surface area and at least one burn-free area (usually thighs or back) from which to harvest autografts. Otherwise, auto-grafting was performed 1-2 weeks after completion of total excision of burned skin, in accordance with our burn unit treatment protocol.
Group I received SDD regimen in the form of:
Amikacin: 15 mg/kg/day for adults and 5 mg/kg/day for children.
Miconazole: 1/2 tablespoon /6 hours.
Colistin sulphate: 0.15 million unit/kg/day.
These drugs were administered orally or by nasogastric tube 4 times daily for 4 weeks.
Group II patients underwent early excision and coverage exactly as Group I (staged excision with temporary coverage in the first week) without decontamination measures or placebo treatment.
Both groups received the same local burn wound treatment in the form of daily dressing using Betadine antiseptic solution and topical antibiotic treatment. In the case of infection, dressings were changed twice daily while in more superficial burns, dressings were changed every 2-3 days. Both groups had clinical, bacteriological, and laboratory follow-up. The clinical data gathered included length of hospital stay, wound healing, GIT complications, and other infections (respiratory tract infections, urinary tract infections, burn wound infection and bacteraemia). Laboratory tests were assessed at admission, third day post burn, and then once a week. They included routine laboratory investigation (CBC, coagulation profile, blood sugar, liver and kidney functions, total serum proteins, serum albumin and C-reactive protein), specific laboratory investigation for detection of organ dysfunction, and assessment of levels of serum cytokine interleukin 6 (IL 6). This was used as a marker to monitor the immune system inflammatory response. The samples of IL 6 were stored at -80º c. The samples were processed using (BIOSOURCE IL-6 EASIA KIT.CATALOGUE NUMBER: KAC1261; 96 DETERMINTION, BIOSOURCE EUROPE S.A). Routine bacteriological examination of urine, stools, blood and wound cultures were carried out. Cultures were obtained on admission and then once a week.
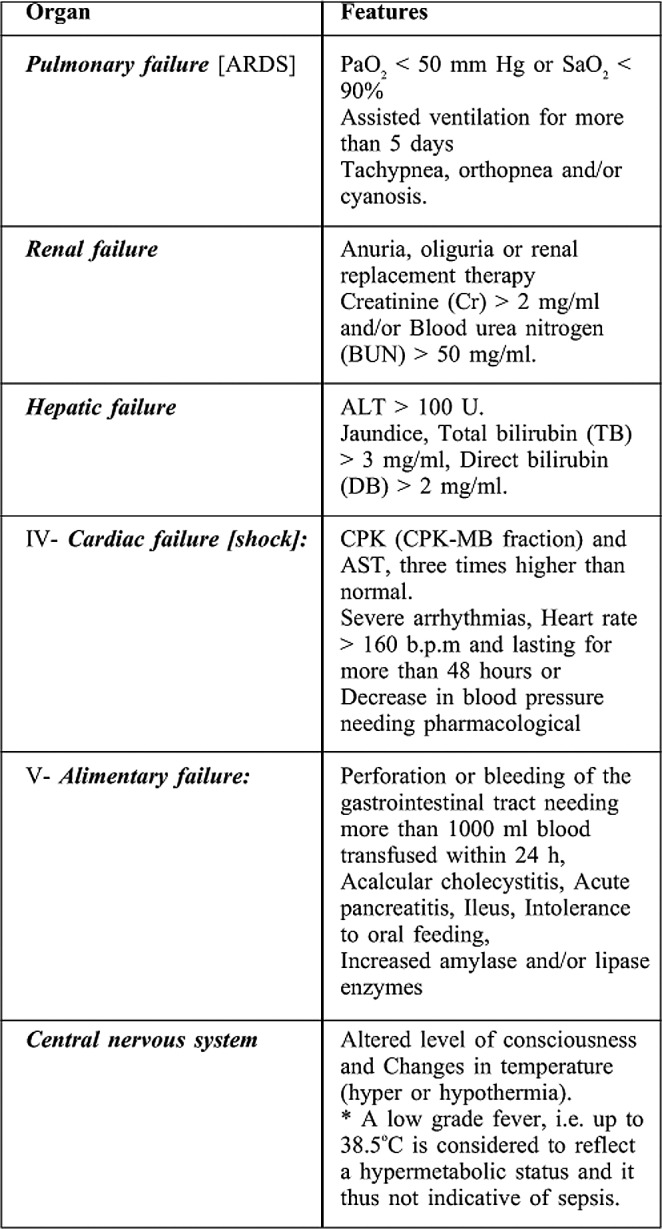
Organ dysfunction or failure was diagnosed based on clinical and/or laboratory criteria according to Yang et al.,9 (Table II).
Table II. shows criteria of diagnosing organ dysfunction syndrome.
Statistical analysis of ages was conducted using the Independent Samples Test. Comparison between BSA, hospital stay, wound closure time, and IL 6 was carried out using the Mann- Whitney Test. Comparison between mortality, infectious complications, organ dysfunction and routine laboratory tests was done via Chi- Square Tests.
Results
This study included 30 patients: 18 had flame burns (60 %), and 12 had scalds (40%). This work was approved by our scientific committee and informed consent was obtained from all included patients.
Patient Population
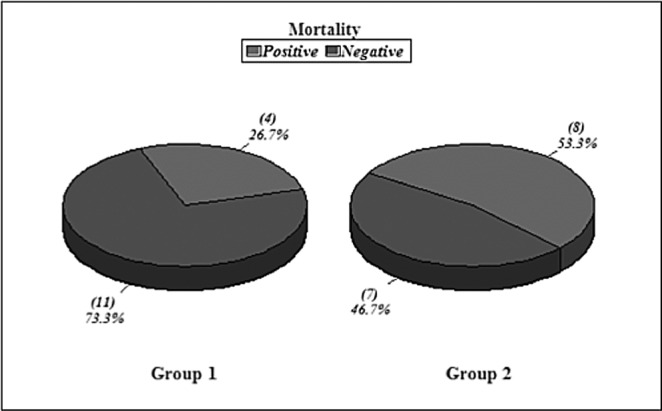
Group I included 11 males and 4 females with ages ranging from 22-49 years, giving a mean age of 31.8 years (standard deviation {SD} +9.05). The total body surface area burned (TBSA) ranged from 30-50%, with a mean of 35.8% (SD+8.7). The mean for deep burns was 23.3% TBSA (SD+12.8). The total mortality was 4 out of 15 (26.7%) (Fig. 1). Group II included 5 males and 10 females, with ages ranging from 3-50 years, giving a mean age of 22.5 years (with SD+13.3) {statistically significant in relation to Group I, p value<0.05. There were no children in Group I while Group II had 2 young children (3 and 5 years), both of whom survived. [The TBSA burned ranged from 30-50%, with a mean of 36.6% (SD+6.7)]. Deep burns ranged from 15-35% TBSA with a mean average of 22.8% (SD+8.09). The mortality rate was 8 out of 15 (53.3%). All epidemiologic data is statistically insignificant in relation to Group I, p value>0.05 (Fig. 1).
Fig. 1 . Mortality Rates in Groups I & II.
Clinical Results
Group I
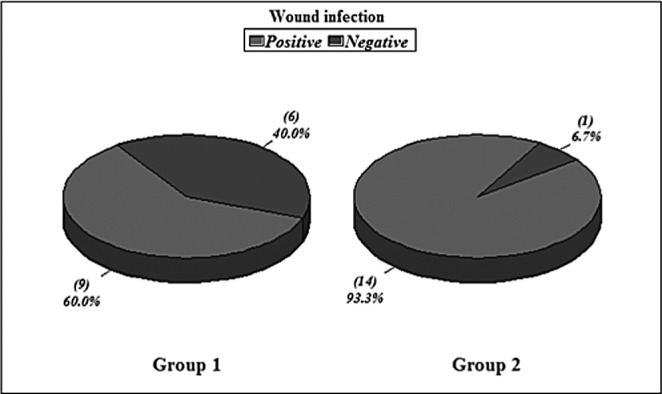
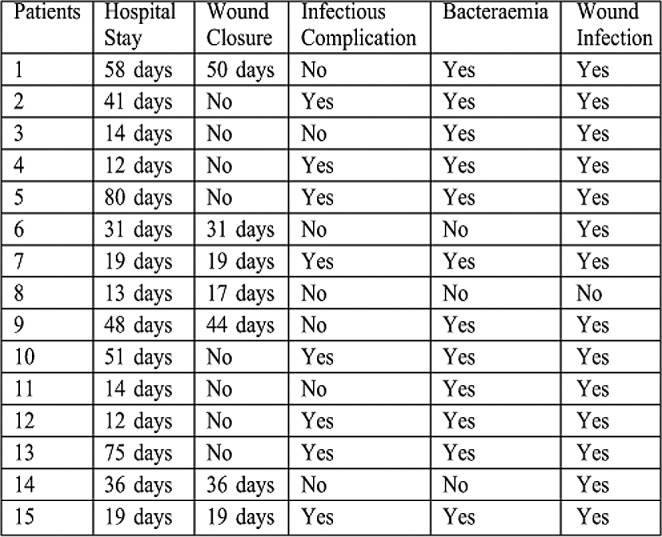
Hospital stay ranged from 6-39 days in survivors with a mean average stay of 22.1 days. The wound closure time (in survivors) ranged from 12-45 days with a mean of 22.4 days (SD+ 11.1). Non survivors died with open wounds (4 patients). The infectious complications occurred in 2 patients (pulmonary infection). Wound infection occurred in 9 patients (two in the week 1 and three in week 2) and was diagnosed clinically and proven in the laboratory by both wound culture swabs and wound surgical biopsy. Bacteraemia occurred in 4 patients (2 patients had no other positive cultures and the other 2 patients had positive wound cultures simultaneously). All organisms isolated from blood were gram negative organisms (Figs.2-4).
Fig. 2 . Bacteraemia in Groups I & II.
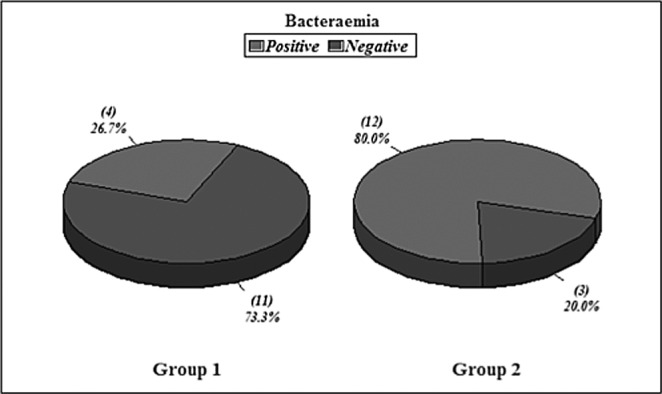
Fig. 4 . Infectious complications in Groups I & II.
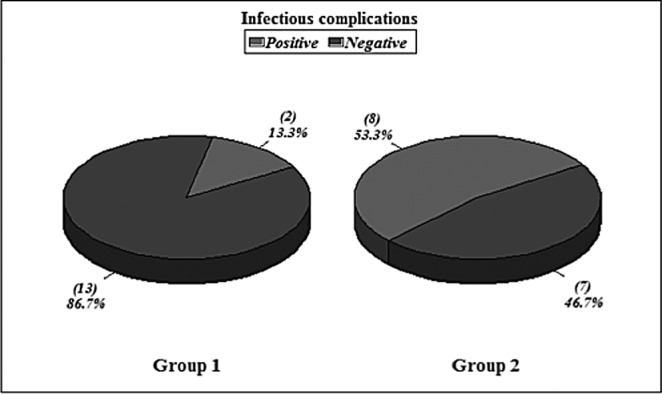
Fig. 3 . Wound infection in Groups I & II.
Group II
Hospital stay ranged from 12-58 days with a mean average of 33.5 days. Wound closure time (in the 7 surviving patients) ranged from 17-50 days with a mean of 30.8 days (SD+13.1) {statistically insignificant in relation to group I, p value>0.05}. Infectious complications occurred in 8 patients (2 patients had pulmonary infection, 2 patients had GIT infection, and 4 patients had urinary tract infection). Wound infection occurred in 14 patients. Bacteraemia occurred in 12 patients. All organisms isolated from blood were gram negative organisms (Figs.2-4). All these results are statistically significant in relation to group I, p value<0.05.
Organ Dysfunction
In Group I, 6 patients (40%) had MODS, occurring simultaneously not sequentially. Two of these patients had 6 occurrences of organ dysfunction and died (CVS, pulmonary, renal, hepatic, CNS, and GIT dysfunctions). The other 4 patients had 3 organs dysfunction, 2 of whom survived while the other 2 did not. The 2 patients that survived had tachypnea in week 1, elevated liver enzymes in weeks 3 and 4, and elevated cardiac enzymes in week 3. The other 2 patients that died had failed weaning, cyanosis, oliguria and cardiac support by the end of week 1. 5 patients (33.3%) had single organ dysfunction at a time and all of them survived (Fig. 5).
Fig. 5 . Organ dysfunction in Groups I & II.
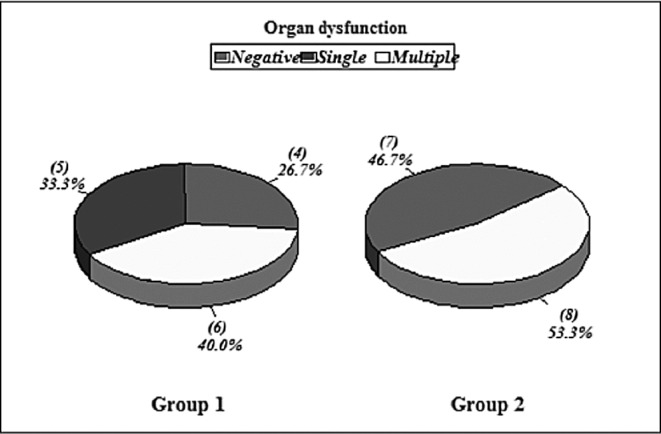
In Group II, 8 patients (50%) had MODS that occurred simultaneously. 6 patients had 4 organs dysfunction and 2 patients had 2 organs dysfunction. The 6 patients that had 4 organs dysfunction breakdown as follows: 2 of them had CNS (fever and disorientation), CVS (inotropic support), renal (anuria) and pulmonary dysfunction (tachypnea and artificial ventilation); another 2 patients had CNS (fever and disorientation), CVS (resistant hypotension), GIT (vomiting and hematemesis) and pulmonary issues (tachypnea); and 2 patients had CNS (fever), CVS (inotropic support), and renal (oliguria) and hepatic dysfunction (high enzymes). The 2 patients with dysfunction of 2 organs had CNS (fever and disorientation) and pulmonary dysfunction (artificial ventilation). All 8 patients died {statistically significant in relation to Group I, p value<0.05}.
Laboratory Results
All laboratory results of Group II were not significantly different from those of Group I {p value>0.05} (Table III and IV). All isolated organisms were gram –ve organisms (mainly Pseudonomas aeruginosa) in both groups. Antibiotics were prescribed according to culture and sensitivity results but the most frequently used antibiotic was Tazocin (piperacillin and tazobactam).
Table III. Clinical Results of Group I.
Table IV. Clinical Results of Group II.
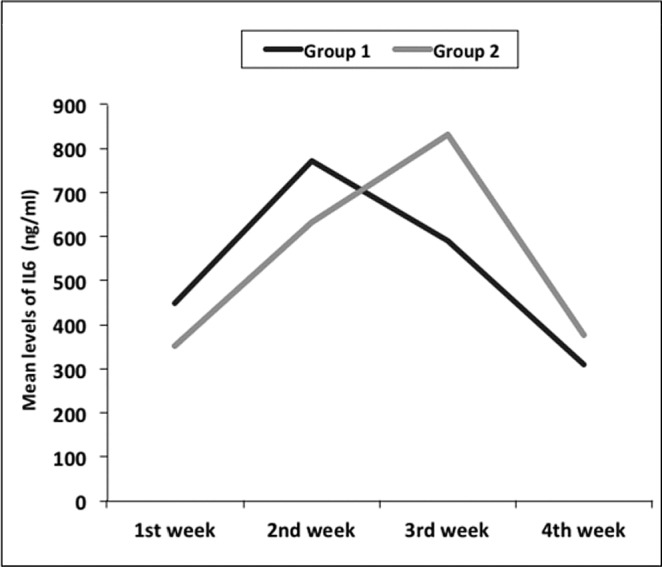
The mean of IL 6 level in serum was 450 ng/ml at admission and reached its peak in week 2 in Group I (771 ng/ ml), then declined in weeks 3 and 4 to 590 and 310 ng/ ml respectively. In Group II, the mean of IL 6 level in serum was 351ng/ml at admission and reached high levels in week 2 (632ng/ml). It continued rising in week 3, reaching its peak (830 ng/ ml) and declining to 377 ng/ml in week 4 (Fig. 6). Differences between the two groups were not statistically significant (p value> 0.05).
Fig. 6 . Serum IL 6 (ng/ ml) levels in Groups I & II.
Discussion
The combined effects of protein coagulation and the avascularity of the burn eschar result in a high risk of infection. 10 Infection further impacts the immune system through the liberation of bacterial toxins (whether the endotoxin of gram-negative rods, or the exotoxin of grampositive species). Thus, as a result of immunosuppression, the persistent inflammation in systemic organs (e.g. heart, liver, kidney, and GIT) results in dysfunction, followed by complete failure.11 The GIT lumen is the largest source of microorganisms and endotoxins in the body. Major Burns alter the GIT permeability leading to bacterial translocation to the blood stream. This leads to colonization of the burn wound and systemic organs as secondary sites.1 Aerobic gram-positive or gram-negative bacteria and fungi can translocate across the barrier after thermal injury.12 There has been controversy regarding the issue of whether anaerobes cross the intestinal barrier, however, anaerobic bacteria are rarely isolated from infectious foci in patients with burns.13 There are various approaches to prevent the intestinal permeability alteration. Early enteral feeding improves intestinal blood flow,14,15 decreases intestinal permeability, preserves the intestinal mucosal barrier, reduces gut derived endotoxaemia 3 and decreases the incidence of sepsis syndrome in patients with severe burns.16 Administration of growth factors and/or intravenous nutrients (e.g. epidermal growth factor, glutamine) may enhance mucosal growth and gut barrier function.17
In our study, the patients included had burn injuries ranging from 30% to 50% TBSA. We chose a minimum of 30% TBSA burns as these are regarded as major burns which are associated with significant bacterial translocation. The upper limit (50% TBSA) was chosen to meet our burn unit protocol for patients that will undergo early burn wound excision. The higher percentages were not included due to limited supply of homografts. Our results showed improved survival rate in the SDD treatment group. However, this improvement did not reach a statistical significance (p value >0.05), which may be due to small sample size. Even so, this difference is certainly of clinical importance. The mean hospital stay in survivors of Group I was shorter than those of Group II (19.3 days and 29.7 days respectively). These findings were similar to those of Munster et al.18 and Jones,19 who saw dramatic reduction in gut-derived endotoxaemia, although this was not accompanied by a significant reduction in mortality. Other studies revealed significant improvement in survival rates either experimentally2 or clinically. 5,20 On the contrary, Jones et al.21 failed to show any improvement in survival rate experimentally.
In our study, the SDD group had a statistically significant reduction in wound infection, septic episodes and bacteraemia. Furthermore, the mean time of wound closure was shorter in this group compared to Group II (22.4 days and 30.8 days respectively), although this difference was statistically insignificant. These results were comparable to many other studies. 5,22,23,24,25,26,27 Lorente et al.28 had better control of MRSA septic episodes after enteral vancomycin. Our findings were in contrast to the findings of Barret et al.,29 who found similar wound colonization rates in both the SDD treatment and placebo groups alike.
The status of organ function and the effects of SDD treatment on organ dysfunction in burn patients have not been clearly discussed in detail in previous studies. SDD treatment resulted in a reduction of organ dysfunction occurrence that is statistically significant (p value<0.05). We observed that the 4 patients who died from MODS in Group I and the 8 patients in Group II had cardiovascular system (CVS) dysfunction that needed inotropic support and pulmonary dysfunction that needed artificial ventilation. Both CVS and pulmonary failure indicate worse prognosis. None of the patients in either group who developed simultaneous CVS and respiratory failure survived. In contrast, single organ dysfunction had less influence on patient outcome.
The mean levels of IL6 assay showed that initial measurements were higher in Group I than in Group II and, furthermore, started to rise dramatically in both groups by week 2. The difference between the 2 groups was apparent in week 3 because levels began to decline in Group I while they continued rising dramatically in Group II. In week 4, IL6 levels declined in both groups and Group I showed lower levels than Group II. These findings suggest that SDD treatment could result in a better immune response despite initial higher levels of IL6. However, these differences are statistically insignificant (p value> 0.05), which could be due to small sample size. These results are similar to those of Barret et al.27 and in contrast to those of Horton et al.,30 who found that SDD attenuated cytokine secretion.
Conclusion
Our findings showed that SDD treatment resulted in better control of infectious episodes which could lead to fewer incidences of MODS. SDD also resulted in a lower mortality rate. In this case, the reduction in mortality did not prove statistically significant, but this statistical insignificance could be due to small sample size. Overall, the use of SDD treatment seems to be of benefit in patients with extensive burns as these patients are considered highly critical and have a high potential of infection related morbidity and mortality.
References
- 1.Ryan CM, Yarmusch ML, Burke JF, et al. Increased gut permeability early after burns correlates with the extent of burn injury. Cri Care Med. 1992;20:1508. doi: 10.1097/00003246-199211000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Yao YM, Sheng ZY, Tian HM, Yu Y, et al. The association of circulating endotoxaemia with the development of multiple organ failure in burned patients. Burns. 1995;21:255–258. doi: 10.1016/0305-4179(95)93867-j. [DOI] [PubMed] [Google Scholar]
- 3.Peng YZ, Yuan ZQ, Xiao GX. Effects of early enteral feeding on the prevention of enterogenic infection in severely burned patients. Burns. 2001;27:145–9. doi: 10.1016/s0305-4179(00)00078-4. [DOI] [PubMed] [Google Scholar]
- 4.Tokyay R, Zeigler ST, Loick HM, Heggers JP, De la Garza P, Traber DL, Herndon DN. Mesenteric lymphadenectomy prevents postburn systemic spread of translocated bacteria. Arch Surg. 1992;127:384–8. doi: 10.1001/archsurg.1992.01420040026003. [DOI] [PubMed] [Google Scholar]
- 5.Manson WL, Sauer EW. Selective intestinal decontamination of the digestive tract: A tool for infection prophylaxis in burns. Ann Medit Burns Club. 1992;7:2. [Google Scholar]
- 6.Nathens AB, Marshall JC. Selective decontamination of the digestive tract in surgical patient: A systematic review of the evidence. Arch Surg. 1999;134:170–6. doi: 10.1001/archsurg.134.2.170. [DOI] [PubMed] [Google Scholar]
- 7.Van Saene HK, Stoutenbeek CP, Hart CA. Selective decontamination of the digestive tract (SDD) in intensive care patients: A critical evaluation of the clinical, bacteriological and epidemiological benefits. J Hosp Intect. 1991;18:261–77. doi: 10.1016/0195-6701(91)90184-a. [DOI] [PubMed] [Google Scholar]
- 8.Curreri PW, Richmond D, Masrvin J, et al. Dietary requirements of patients with major burns. Journal of American Diet Association. 1974;65:415. [PubMed] [Google Scholar]
- 9.Yang ZC, Zhao ZD, Huang YS. Clinical study of the pathogenesis of multiple organ failure after burns. Chin J Plast Surg Burns. 1992;8:8–16. [Google Scholar]
- 10.Pruitt BA, Lindberg RB, McManus WF, Mason AD. Current approach to prevention and treatment of pseudomonas aeruginosa infections in burned patients. Rev of Inf Dis. 1983;5:889–97. doi: 10.1093/clinids/5.supplement_5.s889. [DOI] [PubMed] [Google Scholar]
- 11.Arturson G. Pathophysiology of the burn wound and pharmacological treatment: The Rudi Hermans lecture. Burns. 1995;22:255–274. doi: 10.1016/0305-4179(95)00153-0. [DOI] [PubMed] [Google Scholar]
- 12.Alexander JW, Gianotti L, Pyleo T, Carey MA, Babcock GF. Distribution and survival of Escherichia coli translocating from the intestine after thermal injury. Ann Surg. 1991;213:558–67. doi: 10.1097/00000658-199106000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lawrence JC. The bacteriology of burns. J Hosp Infect. 1985;6:3–17. doi: 10.1016/s0195-6701(85)80081-5. [DOI] [PubMed] [Google Scholar]
- 14.Inoue S, Luke S, Alexander JW, et al. Increased gut blood flow with early enteral feeding in burned guinea pigs. J Burn Care Rehabil. 1989;10:300–8. doi: 10.1097/00004630-198907000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Redan JA, Rush BF, Lysz TW, et al. Organ distribution of gutderived bacteria caused by bowel manipulation or ischemia. Am J Surg. 1990;159:85–90. doi: 10.1016/s0002-9610(05)80611-7. [DOI] [PubMed] [Google Scholar]
- 16.Pereira JL, Gomez-Cia T, Garrido M, et al. Decrease of the incidence of sepsis syndrome after early enteral nutrition of patients with severe burns. Nutr Hosp. 1996;11:274–8. [PubMed] [Google Scholar]
- 17.Saito H, Trocki O, Alexander JW, et al. The effect of route of nutrient administration on the nutritional state, catabolic hormone secretion and gut mucosal integrity after burn injury. J Parenter Enter Nutr. 1987;11:1–7. doi: 10.1177/014860718701100101. [DOI] [PubMed] [Google Scholar]
- 18.Munster AM, Xiao G-X, Guo Y, Wong LA, Winchurch RA. Control of endotoxaemia in burn patients by use of polymyxin B. J Burn Care Rehabil. 1989;10:327. doi: 10.1097/00004630-198907000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Jones EB. Prophylactic anti-lipopolysaccharide freeze-dried plasma in major burns: A double blind controlled trial. Burns. 1995;21:267–71. doi: 10.1016/0305-4179(94)00004-h. [DOI] [PubMed] [Google Scholar]
- 20.De La Cal MA, Cerda E, Garcia-Hierro P, et al. Survival benefit in critically ill burned patients receiving selective decontamination of the digestive tract: A randomized, placebo-controlled, doubleblind trial. Ann Surg. 2005;241:424–30. doi: 10.1097/01.sla.0000154148.58154.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones WG, Barber AE, Minei JP, Fahey TJ, Shires OTI, Shires GT. Antibiotic prophylaxis diminishes bacterial translocation but not mortality in experimental burn sepsis. J Trauma. 1990;30:737. doi: 10.1097/00005373-199006000-00015. [DOI] [PubMed] [Google Scholar]
- 22.Jarrett F, Balish E, Moylan JA, Ellerbe S. Clinical experience with prophylactic antibiotic bowel suppression in burn patients. Surgery. 1978;83:523–7. [PubMed] [Google Scholar]
- 23.Manson WL, Dijkema H, Klasen HJ. Alteration of wound colonization by selective intestinal decontamination in thermally injured mice. Burns. 1990;16:166–8. doi: 10.1016/0305-4179(90)90031-q. [DOI] [PubMed] [Google Scholar]
- 24.Manson WL, Klasen HJ, Sauer EW, Olieman A. Selective intestinal decontamination for prevention of wound colonization in severely burned patients: A retrospective analysis. Burns. 1992;18:98–102. doi: 10.1016/0305-4179(92)90002-c. [DOI] [PubMed] [Google Scholar]
- 25.Mackie DP, Van Herturn WAJ, Schumburg T. Prevention of infection in bums: Preliminary experience with selective intestinal decontamination of the digestive tract in patients with extensive injuries. J Trauma. 1992;32:570–3. [PubMed] [Google Scholar]
- 26.Shalaby HA, Higazi M, El Far N. Selective gastrointestinal decontamination and burn wound sepsis. Annals of Burns and Fire Disasters. 1998;9:1. [Google Scholar]
- X.Abecasis F, Sarginson R, Kerr S, Taylor N, van Saene H. Is selective digestive decontamination useful in controlling aerobic gram-negative bacilli producing extended spectrum beta-lactamases? Microbial Drug Resistance. 2011;17:17–23. doi: 10.1089/mdr.2010.0060. [DOI] [PubMed] [Google Scholar]
- 28.Cerda E, Abella A, De la Cal M, et al. Enteral ancomycin controls methicillin-resistant staphylococcus aureus endemicity in an intensive care burn unit: A 9-year prospective study. Ann Surg. 2007;245:397–407. doi: 10.1097/01.sla.0000250418.14359.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barret JP, Jeschkea MG, Herndon DN. Selective decontamination of the digestive tract in severely burned pediatric patients. Burns. 2001;27:439–45. doi: 10.1016/s0305-4179(00)00147-9. [DOI] [PubMed] [Google Scholar]
- 30.Horton JW, Tan J, White DJ, Maass DL, Thomas JA. Selective decontamination of the digestive tract attenuated the myocardial inflammation and dysfunction that occur with burn injury. Am J Physiol Heart Circ Physiol. 2004;287:2241–51. doi: 10.1152/ajpheart.00390.2004. [DOI] [PubMed] [Google Scholar]


