Summary
Multidrug-resistant Acinetobacter baumanii is a major pathogen encountered in pyogenic infections, especially from burns patients in hospital settings. Often there is also coexistence of multiple beta-lactamase enzymes responsible for beta-lactam resistance in a single isolate, which further complicates treatment options. We conducted a study on burn wound pus samples obtained from the burns unit of our hospital. Phenotypic tests were used to determine the Extended Spectrum Beta-Lactamase, AmpC Beta-Lactamase and Metallo-Beta-Lactamase producing status of the isolates. Almost half of the samples from the burn wounds yielded Acinetobacter baumanii as the predominant pathogen (54.05%). Coexistence of the three resistance mechanisms was seen in 25 of the 100 (25%) isolates of Acinetobacter baumanii. This study emphasizes the need for the detection of isolates that produce these enzymes to avoid therapeutic failures and nosocomial outbreaks.
Keywords: Acinetobacter baumanii, Extended Spectrum Beta-Lactamase, AmpC Beta-Lactamase, Metallo-Beta-Lactamase
Abstract
Acinetobacter baumannii multirésistante est un pathogène majeur rencontré dans les infections pyogènes, en particulier parmi les patients brûlés en milieu hospitalier. Il n’est également pas rare de trouver coexistence de plusieurs enzymes bêta-lactamases responsables de la résistance bêta-lactame dans un seul isolat, ce qui complique encore les options de traitement. Nous avons mené une étude sur les échantillons de pus des plaies de brûlures obtenus à partir de l’unité de soins aux brûlures de notre hôpital. Les isolats qui produisent des Bêta-Lactamases à Spectre Étendu, à l’AmpC et des métallo-bêta-lactamase ont été déterminés sur la base des tests phénotypiques. Près de la moitié des échantillons des plaies de brûlures a donné l’Acinetobacter baumannii comme l’agent pathogène prédominant (54,05%). La coexistence de ces trois mécanismes de résistance a été observée dans 25 des 100 (25%) des isolats d’Acinetobacter baumannii. Cette étude met l’accent sur la nécessité pour la détection des isolats qui produisent ces enzymes pour éviter les échecs thérapeutiques et des épidémies nosocomiales.
Introduction
Acinetobacter baumanii has emerged as an important opportunistic pathogen within the hospital environment, being able to colonize and produce infections in ventilator associated pneumonia, secondary meningitis, urinary tract, septicaemia and other conditions in intensive care unit (ICU) patients.1 It is also causing infections in other immunocompromised patients, including burns patients.2 Antimicrobial treatment of such severe infections is complicated by a wide spread multidrug resistance pattern. One of the most striking features of A.baumanii is its extraordinary ability to develop resistance against major antibiotic classes by various mechanisms.3 A. baumanii resistance is mediated by the presence of mechanisms such as various enzymes, penicillin binding protein alterations and reduced penetration across the outer membrane.4 In isolates of A. baumanii, sometimes there can be co-expression of multiple mechanisms in the same isolate, resulting in total resistance with the exception of colistin and tigecycline. 5 Beta-lactam resistance appears to be primarily caused by ß-lactamase production, including the extended spectrum ß-lactamases, AmpC beta-lactamases, metallo-ß-lactamases and oxacillinases. Other modalities of beta-lactam resistance are antibiotic target site alterations, efflux pumps and finally porin channel deletion, which also appears to contribute to ß-lactam resistance.4 Because of the multiple antibiotic resistance exhibited by A.baumanii, serious infections caused by this organism are difficult to treat.3 In A.baumanii, phenotypic tests can be used to detect resistance to various ß-lactam group drugs via ESBLs, AmpC, and MBL, but for others it is necessary to resort to molecular mechanisms.6 Phenotypic testing may also be misleading, especially when both ESBL and Amp C ß-lactamases co-exist and mask each other, which results in misreporting and failure in clinical treatment of patients.6 For this reason, different inhibitors have been used to improvise the phenotypic tests for B-lactamase detection.6 In patients suffering from burns infected with these drug resistant pathogens, their detection and that of their resistance patterns becomes a priority in order to avoid further complications, as well as to commence appropriate antibiotics. In our study, we considered the detection of all three of these ß-lactamases in A. baumanii, which were found in patients admitted to the burns unit of our hospital based on phenotypic tests involving various beta-lactam antibiotics and inhibitors.
Material and methods
This study was conducted in the Microbiology Department of the Government Medical College Hospital, Chandigarh, India, on the burn wound pus specimens received from the burns unit of our hospital over a period of one year. We used Levine’s technique for preparation of wound beds before specimen collection, by which the immediate surface exudates from the burn wound were cleansed off with moistened sterile gauze and sterile normal saline solution.7 Dressed wounds were cleansed with sterile normal saline upon removal of the dressing. This way, surface contaminating bacteria were removed and the swab sample represented the bacterial flora in the deep wound compartment. For this, aseptically the end of a sterile cotton-tipped applicator was rotated over a 1 cm2 area for 5 seconds with sufficient pressure to express pus and bacteria to the surface from within the wound tissue. We took two wound pus swabs from each area at a given point of time. If the two swab samples differ in types of organisms during presumptive testing, this is also indicative of contamination.8 Wound specimens were then transported to the microbiology laboratory within half an hour by placing the swabs into sterile test tubes containing 1.0 ml of sterile normal saline solution. Bacteriological culture and further identification of the isolates was done by standard microbiological methods.9 The antibiotic susceptibility testing was done by Kirby-Bauer disk diffusion method according to Clinical Laboratory Standards Institute (CLSI) guidelines.10 ESBL production was detected by CLSI method (using ceftazidime and ceftazidime-clavulanic acid combination disks). ESBL non-producers were further confirmed by modifying phenotypic confirmatory tests, using boronic acid (BA) as the inhibitor.11 AmpC screening was done using cefoxitin disk testing and was confirmed by the combination disk test using BA (cefoxitin and cefoxitin/ boronic acid disk).12 MBL production was detected by the Imipenem-EDTA disk test.13 Combination disk test (CDT) was done to detect MBLs with the use of meropenem supplemented with 1000 µg of dipicolinic acid (DPA).14 All strains were susceptible to colistin. The antibiotic discs were obtained from Hi Media, India Ltd., while the powders were from Sigma Aldrich.
Results
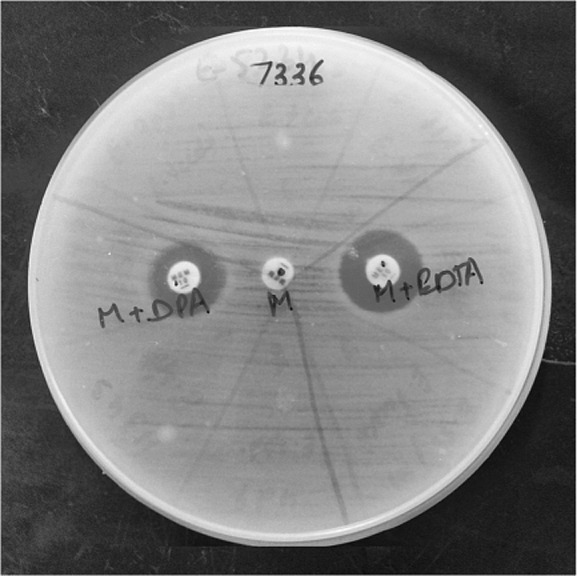
Out of a total of 185 wound samples received from burn patients, A. baumanii was isolated in 100 (54.05%) samples. Presence of all three enzymes (ESBL, AmpC and MBL) was seen in 25/100 (25%) isolates of A. baumanii. Co-production of AmpC and MBL was seen in 16/100 (16%) of the isolates. Coexistence of ESBL and MBL was seen in 9/100 (9%) of the isolates. Coexistence of AmpC and ESBL was not found in any of the isolates, and nor did we find ESBL and AmpC individually. However, 25/100 (25%) isolates showed only MBL production. Coexistence of ESBL with AmpC and MBL was detected only in the presence of boronic acid (Fig. 1). MBL detection was seen with EDTA and dipicolinic acid (Fig. 2). None of the enzymes were detected in 25 (25%) of the isolates, indicating some other method of resistance.
Fig. 1. ESBL and AmpC in the presence of boronic acid.
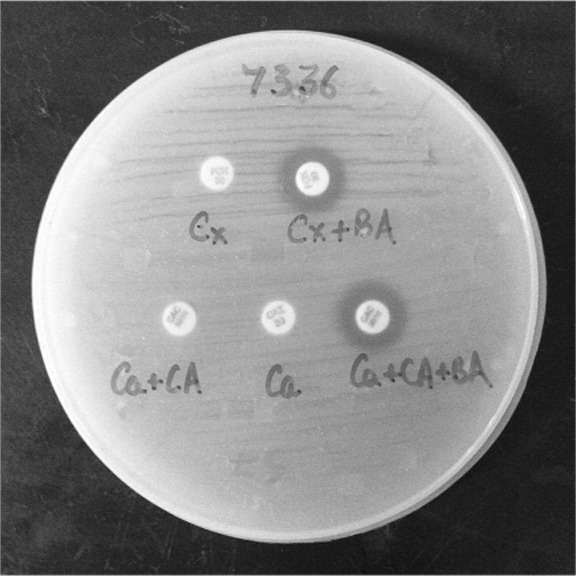
Fig. 2. MBL in the presence of EDTA and DPA.
Discussion
The most common pathogen isolated from burn wounds in our study was Acinetobacter baumanii. The high prevalence of Acinetobacter baumanii in our centre differs markedly from most other studies from Europe, the USA and South America. However, Acinetobacter spp. is highly prevalent in countries like Singapore and Turkey, a fact that may be explained by the tropical climatic conditions. Other studies have supported the hypothesis that Acinetobacter spp. might be more prevalent in warm climates, with corresponding increase in colonization and nosocomial infection. 15 In India, other common isolates from burn wounds are Pseudomonas aeruginosa and Acinetobacter sps.16 A. baumanii is a hardy pathogen associated with high mortality rates, especially in hospitalised patients. The pattern of bacterial resistance is important for epidemiological and clinical purposes. The results of the antimicrobial resistance pattern give serious cause for concern because the Acinetobacter spp. isolated as predominant bacterial isolates in burn wounds were highly resistant. In the recent past, due to the high resistance of Acinetobacter species to beta-lactams, carbapenems had been the drugs of choice for serious infections with A.baumanii. However, carbapenem resistant strains are rapidly emerging. There are several factors leading to carbapenem resistance in A.baumanii, most important being the acquisition of carbapenem hydrolysing ß-lactamases with porin mutations and other contributing strategies.17 Carbapenemases cause the most concern due to the high chance of rapid dissemination.18 These multidrug-resistant A. baumanii in burn patients are difficult to treat as there are very few alternative antibiotics left. The optimal finding of different resistance mechanisms is therefore essential to curtail the spread of these pathogens.
Based on phenotypic tests, we observed that almost 75% of the Acinetobacter isolates showed some mechanism of beta-lactamase resistance. In our study, none of the isolates showed ESBL production alone, while in another study, ESBL production was reported to be 28%.19 We could not find any isolated AmpC production, whereas, in another study, AmpC beta-lactamases were produced by 43% of isolates as the main method of carbapenem resistance. 20 We observed that 25% of the isolates showed MBL production, which is consistent with a prevalence of 26.5% identified by Yong et al.21 MBL and AmpC coexistence was seen in 16% of our isolates, and other studies have also shown the association of AmpC with MBL in Acinetobacters.22,23 We observed that 25% of our isolates showed co-expression of ESBL, AmpC and MBL. Thus, in our study, the main mechanism of resistance was found to be MBL enzyme production apart from coexistence of three enzymes, as was found in P. aeruginosa in another study.24 Recently, a strain of Acinetobacter has been described as carrying genes for multiple beta-lactamases from CVC infection in a burns patient.25
For phenotypic detection of various beta-lactamases, beta-lactams with various inhibitors were tried but, as regards tests with a high level of specificity, molecular methodologies must be used. In routine clinical microbiology laboratories we can use these basic phenotypic tests and then proceed with specific testing based on requirement and availability.
Thus, the finding of multiple beta-lactamases emphasizes the need for the detection of these resistance patterns in order to formulate newer policies of treatment. These drug resistant pathogens are usually susceptible to colistin, as was found in our study. The need of the hour is, therefore, to understand and carry out active screening for these enzymatic coexistences.
Acknowledgments
Conflict of interest. The authors of this paper hereby declare that they have no conflict of interest.
Funding & support. None
References
- 1.Dijkshoorn L, Nemec A, Seifert H. An increasing threat in hospitals: Multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol. 2007;5:939–51. doi: 10.1038/nrmicro1789. [DOI] [PubMed] [Google Scholar]
- 2.Bayram Y, Parlak M, Aypak C, et al. Three-year review of bacteriological profile and antibiogram of burn wound isolates in Van, Turkey. Int J Med Sci. 2013;10:19–23. doi: 10.7150/ijms.4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perez F, Hujer AM, Hujer KM, et al. Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother. 2007;51:3471–84. doi: 10.1128/AAC.01464-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rahal JJ. Antimicrobial resistance among and therapeutic options against gram-negative pathogens. Clin Infect Dis. 2009;1:4–10. doi: 10.1086/599810. [DOI] [PubMed] [Google Scholar]
- 5.Castanheira M, Sader HS, Deshpande LM, et al. Antimicrobial activities of tigecycline and other broad-spectrum antimicrobials tested against serine carbapenemase- and metallo-beta-lactamase-producing Enterobacteriaceae: Report from the SENTRY Antimicrobial Surveillance Program. Antimicrob Agents Chemother. 2008;52:570–3. doi: 10.1128/AAC.01114-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomson KS. Extended-spectrum-beta-lactamase, AmpC, and carbapenemase issues. J Clin Microbiol. 2010;48:1019–25. doi: 10.1128/JCM.00219-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levine NS, Lindberg RB, Mason AD, et al. The quantitative swab culture and smear: A quick, simple method for determining the number of viable aerobic bacteria on open wounds. J Trauma. 1976;16:89–94. [PubMed] [Google Scholar]
- 8.Gardner SE, Frantz R, Hillis SL, et al. Diagnostic validity of semiquantitative swab cultures. Wounds. 2007;19:31–8. [PubMed] [Google Scholar]
- 9. Colle JG Fraser AG Marimon BP Simmon A , editor. Mackie & MacCartney practical medical microbiology. 14th ed. Edinburgh: Churchill Livingstone; 1996. Tests for identification of bacteria. pp. 151–79. [Google Scholar]
- 10.Performance standards for antimicrobial susceptibility testing: 18th Informational Supplement, M100-S18. 1. Vol. 28. Wayne, PA. Clinical and Laboratory Standards Institute; 2008. [Google Scholar]
- 11.Song W, Bae IK, Lee YN, et al. Detection of extended-spectrum beta-lactamases by using boronic acid as an AmpC beta-lactamase inhibitor in clinical isolates of Klebsiella spp. and Escherichia coli. J Clin Microbiol. 2007;45:1180–4. doi: 10.1128/JCM.02322-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee W, Jung B, Hong SG, et al. Comparison of 3 phenotypic-detection methods for identifying plasmid-mediated AmpC beta-lactamase-producing Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis strains. Korean J Lab Med. 2009;29:448–54. doi: 10.3343/kjlm.2009.29.5.448. [DOI] [PubMed] [Google Scholar]
- 13.Lee K, Lim YS, Yong D, et al. Evaluation of the Hodge test and the imipenem-EDTA double-disk synergy test for differentiating metallo-beta-lactamase-producing isolates of Pseudomonas spp. and Acinetobacter spp. J Clin Microbiol. 2003;41:4623–9. doi: 10.1128/JCM.41.10.4623-4629.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pasteran F, Veliz O, Faccone D, et al. A simple test for the detection of KPC and metallo-ß-lactamase carbapenemase-producing Pseudomonas aeruginosa isolates with the use of meropenem disks supplemented with aminophenylboronic acid, dipicolinic acid and cloxacillin. Clin Microbiol Infect. 2011;17:1438–41. doi: 10.1111/j.1469-0691.2011.03585.x. [DOI] [PubMed] [Google Scholar]
- 15.Bayram Y, Parlak M, Aypak C, et al. Three-year review of bacteriological profile and antibiogram of burn wound isolates in Van, Turkey. Int J Med Sci. 2013;10:19–23. doi: 10.7150/ijms.4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta AK, Uppal S, Garg R, et al. A clinico-epidemiologic study of 892 patients with burn injuries at a tertiary care hospital in Punjab, India. J Emerg Trauma Shock. 2011;4:7–11. doi: 10.4103/0974-2700.76820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mugnier P, Poirel L, Pitout M, et al. Carbapenem-resistant and OXA-23-producing Acinetobacter baumannii isolates in the United Arab Emirates. Clin Microbiol Infect. 2008;14:879–82. doi: 10.1111/j.1469-0691.2008.02056.x. [DOI] [PubMed] [Google Scholar]
- 18.Poirel L, Nordmann P. Carbapenem resistance in Acinetobacter baumannii: Mechanisms and epidemiology. Clin Microbiol Infect. 2006;12:826–36. doi: 10.1111/j.1469-0691.2006.01456.x. [DOI] [PubMed] [Google Scholar]
- 19.Sinha M, Srinivasa H, Macaden R. Antibiotic resistance profile & extended spectrum beta-lactamase (ESBL) production in Acinetobacter species. Indian J Med Res. 2007;126:63–7. [PubMed] [Google Scholar]
- 20.Sinha M, Srinivasa H. Mechanisms of resistance to carbapenems in meropenem-resistant Acinetobacter isolates from clinical samples. Indian J Med Microbiol. 2007;25:121–5. doi: 10.4103/0255-0857.32717. [DOI] [PubMed] [Google Scholar]
- 21.Yong D, Choi YS, Roh KH, et al. Increasing prevalence and diversity of metallo-ß-lactamases in Pseudomonas spp., Acinetobacter spp. and Enterobacteriaceae from Korea. Antimicrob Agents Chemother. 2006;50:1884–6. doi: 10.1128/AAC.50.5.1884-1886.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sinha N, Agarwal J, Srivastava S, et al. Analysis of carbapenemresistant Acinetobacter from a tertiary care setting in North India. Indian J Med Microbiol. 2013;31:60–3. doi: 10.4103/0255-0857.108724. [DOI] [PubMed] [Google Scholar]
- 23.Upadhyay S, Sen MR, Prakash P, et al. Detection and characterisation of AmpC ß-lactamase-producing isolates of Acinetobacter spp. in North India. Int J Antimicrob Agents. 2010;36:472–3. doi: 10.1016/j.ijantimicag.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 24.Upadhyay S, Sen MR,Bhattacharjee A. Presence of different beta-lactamase classes among clinical isolates of Pseudomonas aeruginosa expressing AmpC beta-lactamase enzyme. J Infect Dev Ctries. 2011;4:239–42. doi: 10.3855/jidc.497. [DOI] [PubMed] [Google Scholar]
- 25.Pan YH, Su QH, Cheng HL, et al. A strain of Acinetobacter baumannii colonising a central venous catheter and carrying four beta-lactamase genes. J Hosp Infect. 2009;72:87–8. doi: 10.1016/j.jhin.2009.01.009. [DOI] [PubMed] [Google Scholar]


