Summary
Carbapenem resistance due to acquired metallo-beta-lactamases (MBLs) is considered to be more serious than other resistance mechanisms. The aim of this study was to evaluate the antibacterial activity of Zataria multiflora Boiss and Carum copticum plants on IMP-producing P.aeruginosa strains. This experimental study was carried out on hospitalized burn patients during 2011 and 2012. Antibiotics and extracts susceptibility tests were performed by disc diffusion and broth microdilution methods. MBL detection was performed by Combination Disk Diffusion Test (CDDT). The bla(VIM) and bla(IMP) genes were detected by PCR and sequencing methods. Using Combination Disk Diffusion test method, it was found that among 83 imipenem resistant P.aeruginosa strains, 48 (57.9%) were MBL producers. PCR and sequencing methods proved that these isolates were positive for blaIMP-1 genes, whereas none were positive for bla(VIM) genes. The mortality rate of hospitalized patients with MBL-producing Pseudomonas infection was 4/48 (8.3%). It was shown that Zataria multiflora and Carum copticum extracts had a high antibacterial effect on regular and IMP-producing P. aeruginosa strains in 6.25 mg/ml concentration. The incidence of MBL-producing P. aeruginosa in burn patients is very high. In our study, all MBL-producing isolates carry the blaIMP-1 gene. Therefore, detection of MBL-producing isolates is of great importance in identifying drug resistance patterns in P. aeruginosa, and in prevention and control of infections. In this study, it was shown that extracts of Z. multiflora and C. copticum have high antibacterial effects on ß-lactamase producing P. aeruginosa strains.
Keywords: P. aeruginosa, Metallo-ß-lactamases, Zataria multiflora Boiss, Carum copticum
Abstract
La résistance aux carbapénèmes en raison de l’acquisition des métallo-bêta-lactamases (MBL) est considéré comme plus grave que d’autres mécanismes de résistance. Le but de cette étude était d’évaluer l’activité antibactérienne de Zataria multiflora Boiss et Carum copticum sur des souches de Pseudomonas aeruginosa. Cette étude expérimentale a été réalisée sur les patients brûlés hospitalisés au cours de 2011 et 2012. Les antibiotiques et des extraits des tests de sensibilité ont été réalisés par diffusion du disque et les méthodes de microdilution de bouillon. La détection des MBL a été réalisée par des tests basés sur l’utilisation de disques de combinaison. Les gènes bla(IMP) et bla(VIM) ont été détectés par des méthodes de PCR et séquençage. En utilisant la méthode de test de diffusion sur disque de combinaison, il a été constaté que, parmi les 83 souches de Pseudomonas aeruginosa résistant à l’imipénème, 48 (57,9%) étaient des producteurs de MBL. Méthodes de PCR et séquençage ont montré que ces isolats étaient positifs pour les gènes blaIMP-1, alors aucun n’était positif pour les gènes bla (VIM). Le taux de mortalité des patients hospitalisés avec une infection de Pseudomonas producteurs des MBL était de 4/48 (8,3%). Il a été montré que les extraits de Zataria multiflora et Carum copticum ont eu un effet antibactérien élevé sur des souches de P. aeruginosa réguliers et producteurs d’IMP dans 6,25 mg/ml de concentration. L’incidence du P. aeruginosa producteur des MBL chez les patients brûlés est très élevée. Dans notre étude, tous les isolats producteurs des MBL sont porteurs du gène blaIMP-1. Par conséquent, la détection des isolats qui produisent des MBL sont d’une grande importance dans l’identification des profils de résistance aux médicaments chez P. aeruginosa et dans la prévention et le contrôle des infections. Dans cette étude, il a été montré que des extraits de Z. multiflora et C. copticum ont des effets antibactériens élevés sur les souches de P. aeruginosa producteurs de ß-lactamase.
Introduction
Bacteria have the genetic ability to transfer and acquire drug resistance factors. In recent years, inappropriate use of antibiotics has led to drug resistance among bacteria, which is the cause of untreatable ‘superbugs’ and high death rates in clinics throughout the world, particularly among patients with suppressed immunity. It is reported that about 25,000 people in the E.U. and 63,000 in the U.S. die each year due to infectious diseases caused by multi-drug resistant (MDR) bacteria.1 P. aeruginosa is the common cause of nosocomial infections2 and, as an opportunistic pathogen, causes other infections such as pneumonia, septicemia, urinary tract infection, endocarditis, skin, ear and eye infections, as well as being a leading cause of morbidity and mortality among hospitalized burn patients.3 Carbapenems are the last generation of drugs for treatment of infections caused by MDR P. aeruginosa.4 Carbapenems, including imipenem, meropenem and doripenem are the most potent antibacterial agents for the treatment of P. aeruginosa infections.5 P. aeruginosa resistance to carbapenems is due to loss of OprD porin expression, high-level expression of AmpC enzymes, and increased expression of several efflux systems such as MexA–MexB–OprM. Among clinical isolates of Pseudomonas spp., resistance to carbapenems has been found to be mainly due to the production of carbapenemhydrolyzing enzymes such as metallo-ß-lactamases (MBLs).6 Carbapenem resistance due to acquired MBLs is considered to be more serious than other resistance mechanisms because MBLs can hydrolyze almost all beta-lactam antibiotics except monobactams. Furthermore, the MBL-encoding genes located on integrons can be disseminated easily from one bacterium to another.7 Many MBLs have been found in P. aeruginosa, including Imipenemase (IMP), São Paolo metallo (SPM), (Verona integron-encoded metallo-ß-lactamases (VIM)), Seoul imipenemase (SIM), Japan, Kyorin University Hospital Imipenemase (KHM), German imipenemase (GIM), New-Delhi metallo- beta-lactamase-1 (NDM-1) and Australian Imipenemase (AIM). The genes of both IMP and VIM-type in clinical isolates of P. aeruginosa are often encoded on mobile elements inserted into class 1 integrons.8 The integrons are located on transposons or plasmids, the distribution of which contributes to the wide spread of this resistance mechanism.9
Zataria multiflora Boiss, a member of the Labiatae family, grows in countries such as Pakistan, Afghanistan and Iran. Traditionally, it has been used as a diuretic, an antiseptic, a flavoring, a carminative, and as an antispasmodic agent, as well as for premenstrual pain, edema, sore throat, jaundice, chronic catharsis and asthma treatment. Z.multiflora has been reported to have applicable medical properties including pain-relieving, immunostimulant, antibacterial , anticandidal, antifungal , antioxidant, antinociceptive, and anti-inflammatory effects.10-13 Carum copticum grows in Egypt, East India and Iran, with bright flowers and brownish seeds which have a thymol-like odour. Its essential oil contains a-pinene, paracymene, terpinene, ß-pinene and other components such as thymol and carvacrol. The seeds can be used as a diuretic, analgesic, anti-asthma, anti-vomiting and anti-dyspnea agent. They also have a wonderful effect on skin and are good for neural and urinary tract disorders.14-16
Our study was conducted on hospitalized burn patients in Shahid Motahari Hospital, Tehran, Iran, from 2011 to 2012. The aims of this study were threefold: firstly, to determine the antibiotic resistance patterns of P. aeruginosa, secondly to detect blaVIM and blaIMP metallo-beta-lactamase genes, and thirdly to evaluate the effects of Z. multiflora and Carum copticum methanolic, acetonic and chloroformic extracts on P. aeruginosa strains producing metallo-ß-lactamase (blaIMP) isolated from the studied patients.
Materials and methods
Isolation and Clinical Identification
From September to January 2011, 100 isolates of P. aeruginosa were collected by sterile swabs from 448 burn patients referred to Shahid Motahari Hospital (level I burn care center) in Tehran, Iran. Prior to sampling, the wounds were washed by Physiological Serum. Samples were transferred in Stuart media, cultured on Cetrimide and Mac- Conkey agar and incubated at 37ºC for 24 hours. Pigmented and odoripherous colonies were analyzed using biochemical tests including an oxidase test, a catalase sugar fermentation test and growth ability at 42ºC. The isolates were stored at -200C in brain heart broth containing 20% glycerol. P. aeruginosa ATCC27853 was used as a control strain.
Antimicrobial susceptibility testing
Antimicrobial susceptibility to imipenem (IPM, 10 µg), meropenem (MEM, 10 µg), ceftazidime (CAZ, 30 µg), cefotaxime (CTX, 30 µg), amikacin (AK, 30 µg), tobramycin (TOB, 10 µg), piperacillin/Tazobactam(PTZ, 100/10 µg),ciprofloxacin (CIP, 5 µg), cefepime (FEP, 30 µg), ceftriaxone (CRO, 30 µg), aztreonam(ATM, 30 µg), gentamicin (GEN,10 µg) and carbenicillin (Car,100 µg) (Mast Group, Merseyside, UK) was tested on the isolated P. aeruginosa samples, as well as the control ATCC27853 strain by the Kirby-Bauer disk diffusion method on Mueller Hinton agar (Merck, Germany) based on Clinical Laboratory Standards Institute (CLSI) guidelines.
Minimum Inhibitory Concentration of Antibiotics
Minimum inhibitory concentration (MIC) of different antibiotics were determined according to the guidelines of the CLSI by broth microdilution method. Distinct doses of imipenem, meropenem, cefepime, ampicillin, piperacillin/tazobactam, cefotaxime, ceftriaxone and ceftazidime (GLAXO England Co. and Himedia Co.) were added to the culture medium (Muller-Hinton broth (Merck, Germany).
Phenotypic detection of MBL
In this study, Meropenem and Meropenem + EDTA, Imipenem and Imipenem + EDTA discs were used to detect MBL producing P. aeruginosa. MBL detection was performed by Combination Disk Diffusion Test (CDDT).17
Detection of blaIMP and blaVIM Genes by PCR Method
DNA templates were prepared by boiling method. The polymerase chain reaction (PCR) amplification for blaIMP and blaVIM were performed with primers VIM-F (5'-GTTTGGTCGCATATCGCAAC-3') and VIM-R (5'-AATGCGCAGCACCAGGATAG-3') for blaVIM gene and primers IMP-F (5'- GAAGGCGTTTATGTTCATAC-3') and IMP-R (5'-GTATGTTTCAAGAGTGATGC-3') for blaIMP gene under PCR conditions as reported previously.18
Sequencing
The PCR purification kit (Bioneer Co., Korea) was used to purify PCR products and sequencing of forward strand was performed by the Bioneer company (Korea). The nucleotide sequences were analyzed with Chromas 1.45 and MEGA- 4 softwares and BLAST in PubMed NCBI.
Nucleotide sequence accession number
The nucleotide sequence data reported in this paper have been submitted to the GenBank sequence database and assigned accession no. JX648311.
Plant material
Source, Collection and Identification
During 2012, leaves of Zataria multiflora Boiss and seeds of Carum copticum were collected from Fars province in Iran, during 2012 (Fig. 1). A voucher specimen was prepared and deposited at Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Fig. 1. Antibacterial potency of Carum copticum and Zataria multiflora extracts against IMP-producing P.aeruginosa strains.
Extraction Method
Leaves of the Zataria multiflora Boiss and seeds of Carum copticum plants (400gr) were dried at 25oC and then powdered using a mechanical grinder. 10gr of dried plant was soaked in 100ml methanol (96%, v/v), acetone (99%) and chloroform (99.4% purity) (Merck, Germany) for a period of 48 hours without heating. Each extract was first filtered through Whatman No. 1 filter paper to clarify and then through a 0.45 µm membrane filter. The filtrate was evaporated under reduced pressure in a vacuum evaporator and stored at 4oC. After drying, extracts were preserved in -20ºC.
Determination of MIC and MBC values by broth Microdilution assay
The minimum inhibitory concentration (MIC) of the extracts was determined according to the method described by CLSI, 2012. Zataria multiflora Boiss and Carum copticum extracts were diluted with 10% DMSO to yield concentrations ranging from 25 to 0.19 mg/ml. Cation-adjusted Muller Hinton broth was used as broth medium. After shaking, 0.1ml of each extract was added to one well of 96-well microtiter plates. Microbial suspensions were adjusted to 0.5 MacFarland and diluted 1:10 to yield 107CFU/ml and to each well, 0.005 ml of the bacterial inoculum were seeded. Control line with no bacterial inoculation and P. aeruginosa ATCC27853 was simultaneously maintained. Microplates were incubated aerobically at 37°C for 18-24 hours. The lowest concentration of the extracts that produced no visible bacterial growth was reported as the MIC. MBC values were determined using the first well showing no growth on media agar.
Statistical Analysis
The different effects of Zataria multiflora Boiss and Carum copticum extracts on P. aeruginosa were analyzed using one-way ANOVA via MINITAB Version 13 software. A p value <0.05 was considered significant.
Results
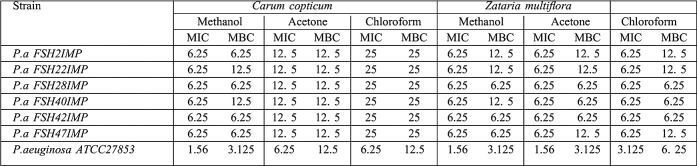
Out of the 100 P.aeruginosa isolates, 83 were resistant to Imipenem and Ceftazidime. The CDDT showed that among 83 Imipenem non-susceptible P.aeruginosa strains, 48 (57.9%) were metallo-beta-lactamase producers. All MBL-producing P.aeruginosa were resistant to Meropenem, Imipenem, Ceftazidime, Amikacin, Tobramycin, Ciprofloxacin, Aztreonam, Piperacillin/Tazobactam, Ceftriaxone, Cefepime and Carbenicillin; while 49% of isolates were resistant to Gentamicin, indicating that 100% of isolates were multi-drug resistant (MDR) (resistance to more than three antibiotics from different classes was defined as MDR). Minimum inhibitory concentration (MIC) of different antibiotics for IMP-producing P.aeruginosa strains is shown in Table I. Using PCR method, 6 isolates were positive for bla(IMP) gene, while bla(VIM) gene was not detected. Sequencing of PCR products showed conserved region for the restriction sequence blaIMP-1 gene which was confirmed by BLAST. 48 (57.9%) patients were infected with MBL-producing Pseudomonas strains of whom 4 (8.3%) died. Antibacterial potency of Carum copticum and Zataria multiflora extracts against six IMP-producing P.aeruginosa strains (Fig. 1) were evaluated by microdilution method as described by CLSI. MICs and MBCs (mg/ml) results of Carum copticum and Zataria multiflora against IMP-producing P.aeruginosa strains were presented in Table II.
Table I. Distribution of antibiotics MICs for IMP-producing P.aeruginosa strains.
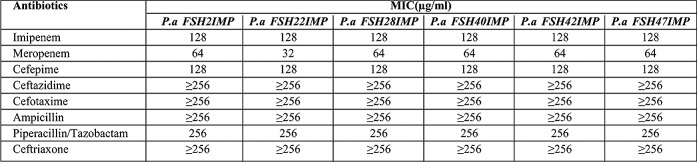
Table II. Frequency of minimum inhibitory concentration (MIC mg/ml) of Carum copticum and Zataria multiflora extracts for IMP-producing P.aeruginosa strains.
Discussion
The threat caused by P.aeruginosa in hospital-borne infections and the need for an effective antibiotic incited us to search for a potential anti-infective agent against this pathogen. It is assumed that herbal products could constitute a natural antibiotic for controlling and treating infection without risking the appearance of resistance strain.19
P.aeruginosa MBL producers are a major problem in medical centers because P.aeruginosa beta-lactamasesproducers are capable of hydrolyzing almost all ß-lactam antibiotics and are associated with high morbidity and mortality.4 In the past twenty years, P.aeruginosa was the most common bacteria in burn wards in Tehran, Iran.20 P.aeruginosa isolates in our study showed resistance to almost all antibacterial agents. All MBL-producing P.aeruginosa were resistant to Meropenem, Imipenem, Ceftazidime , Amikacin, Tobramycin, Ciprofloxacin, Aztreonam, Piperacillin/Tazobactam, Ceftriaxone, Cefepime and Carbenicillin; while 49% of isolates were resistant to Gentamicin. In addition to enzymes mechanism, another mechanism such as Efflux pump or KPC enzymes with loss of the OprD (outer membrane porin) can also cause drug resistance.6 Prevalence of drug resistance in burn wards has been reported from other parts of the world.4,9 Compared to reports from the USA,21 Spain,22 Korea, 23 and India,24 a higher prevalence of MBLs was found in Tehran probably due to different length of hospitalization, care conditions and antibiotics utilization protocols. Globally, as well as in Iran, the VIM beta-lactamase is the most dominant MBL type.25-27 Previous research in Iran showed that 11.43% of P.aeruginosa isolates from Tehran and 19.51% from Ahwaz (in Iran) were VIM-type positive. However, in our study, IMP-1 producer P.aeruginosa was the dominant strain among the studied isolates, which is in concordance with other studies.28-30 In this study, 42 isolates were negative for bla(VIM) and bla(IMP) genes by PCR method. However, resistance may be caused by other genes such as AIM, SIM, KHM, SPM, GIM and NDM.6
In our study mortality rate due to infection with MBLproducing Pseudomonas strains was 4 (8.3%) and in Brazil the mortality rate of infection with MBL-producing Pseudomonas strains was 82.6%.31 The genes encoding these enzymes spread easily on plasmids, causing nosocomial infections and outbreaks with mortality rates from 25% to 75%.32 Only a few antibacterial drugs were effective on the P.aeruginosa MBL-producers that were isolated from Motahari hospital. Therefore there is a need to revise treatment protocols to prevent the spread of resistant genes. The antimicrobial activity of Carum copticum and Zataria multiflora have been demonstrated in many other studies but antibacterial effects of these plants against IMPproducing P.aeruginosa strains has not yet been reported. 10-13 The present study supports the idea that Carum copticum and Zataria multiflora extracts might be useful as antibacterial agents against IMP-producing P.aeruginosa strains. The acetonic, methanolic and chloroformic extracts of Zataria multiflora and Carum copticum methanolic extract have promising MIC values against all IMP-producing P.aeruginosa in 6.25 mg/ml concentration. In this study, the antibacterial activity of these plants against IMP-producing P.aeruginosa strains was evaluated for the first betime. In 2010, Saei-Dehkordi and et al. reported that P.aeruginosa growth can be inhibited by the use of 2-8 mg/ml of Zataria multiflora Boiss essential oil.10 Sharififar et al. have shown that essential oil and methanol extract of endemic Zataria multiflora Boiss has an inhibitory effect on S. aureus, E. coli, K. pneumoniae, S.epidermidis, E. faecalis, B. subtilis, S.typhi, S.marcescens and S.flexneri.11 Furthermore, Mahboubi et al. have shown that Staphylococcus growth can be inhibited by 0.5-1 µl/ml of Zataria multiflora Boiss essential oils.12
Conclusions
The incidence of MBL-producing P.aeruginosa in burn patients is very high. Therefore, detection of MBL-producing isolates is of great importance in identifying drug resistance patterns in P.aeruginosa and in preventing and controlling infections. The choloroformic, acetonic and methanolic extract of Zataria multiflora Boiss and Carum copticum methanolic extract had a better effect on regular and clinical MBL-producing P.aeruginosa strains. Other evaluations considering the different effects of various herbal extracts as antibacterial agents, as well as in vivo examination of these extracts, are required to provide a natural, cost effective and strong alternative for the traditionally less effective antibiotics which are normally used.
Acknowledgments
The authors would like to thank Dr. Mardaneh and Dr. Pourakbari from the Microbiology Department of Tehran University of Medical Sciences for providing IMP and VIM type positive isolates.
References
- 1.Dey D, Debnath S, Hazra S, Ghosh S, Ray R, Hazra B. Pomegranate pericarp extract enhances the antibacterial activity of ciprofloxacin against extended-spectrum beta-lactamase (ESBL) and metallo-beta-lactamase (MBL) producing Gram-negative bacilli. Food Chem Toxicol. 2012;50:4302–9. doi: 10.1016/j.fct.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Dharmalingam K, Tan BK, Mahmud MZ, et al. Swietenia macrophylla extract promotes the ability of Caenorhabditis elegans to survive Pseudomonas aeruginosa infection. J Ethnopharmacol. 2012;139:657–63. doi: 10.1016/j.jep.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 3.Picao RC, Poirel L, Gales AC, Nordmann P. Diversity of betalactamases produced by ceftazidime-resistant Pseudomonas aeruginosa isolates causing bloodstream infections in Brazil. Antimicrob Agents Chemother. 2009;53:3908–13. doi: 10.1128/AAC.00453-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yousefi S, Farajnia S, Nahaei MR, et al. Detection of metallo-beta-lactamase-encoding genes among clinical isolates of Pseudomonas aeruginosa in the northwest of Iran. Diagn Microbiol Infect Dis. 2009;68:322–5. doi: 10.1016/j.diagmicrobio.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 5.Poole K. Pseudomonas aeruginosa: Resistance to the max. Front Microbiol. 2011;2:65. doi: 10.3389/fmicb.2011.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cornaglia G, Giamarellou H, Rossolini GM. Metallo-beta-lactamases: A last frontier for beta-lactams? Lancet Infect Dis. 2011;11:381–93. doi: 10.1016/S1473-3099(11)70056-1. [DOI] [PubMed] [Google Scholar]
- 7.Miyajima Y, Hiramatsu K, Mizukami E, et al. In vitro and in vivo potency of polymyxin B against IMP-type metallo-beta-lactamase-producing Pseudomonas aeruginosa. Int J Antimicrob Agents. 2008;32:437–40. doi: 10.1016/j.ijantimicag.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Khosravi Y, Tay ST, Vadivelu J. Analysis of integrons and associated gene cassettes of metallo-beta-lactamase-positive Pseudomonas aeruginosa in Malaysia. J Med Microbiol. 2011;60:988–94. doi: 10.1099/jmm.0.029868-0. [DOI] [PubMed] [Google Scholar]
- 9.Viedma E, Juan C, Villa J, et al. VIM-2-producing multidrug-resistant Pseudomonas aeruginosa ST175 clone, Spain. Emerg Infect Dis. 2012;18:1235–41. doi: 10.3201/eid1808.111234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saei-Dehkordi SS, Tajik H, Moradi M, Khalighi-Sigaroodi F. Chemical composition of essential oils in Zataria multiflora Boiss from different parts of Iran and their radical scavenging and antimicrobial activity. Food Chem Toxicol. 2010;48:1562–7. doi: 10.1016/j.fct.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 11.Sharififar F, Moshafi MH, Mansouri SH, Khodashenas M, Khoshnoodi M. In vitro evaluation of antibacterial and antioxidant activities of the essential oil and methanol extract of endemic Zataria multiflora Boiss. Food control. 2007;18:800–5. [Google Scholar]
- 12.Mahboubi M, Bidgoli FG. Antistaphylococcal activity of Zataria multiflora essential oil and its synergy with vancomycin. Phytomedicine: International journal of phytotherapy and phytopharmacology. 2010;17:548–50. doi: 10.1016/j.phymed.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Misaghi A, Basti A.A. Effects of Zataria multiflora Boiss. essential oil and nisin on Bacillus cereus ATCC 11778. Food Control. 2007;18:1043–9. [Google Scholar]
- 14.Boskabady MH, Jandaghi P, Kiani S, Hasanzadeh L. Antitussive effect of Carum copticum in guinea pigs. J Ethnopharmacol. 2005;97:79–82. doi: 10.1016/j.jep.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 15.Mohagheghzadeh A, Faridi P, Ghasemi Y. Carum copticum Benth essential oil chemotypes. Food Chem Toxicol. 2007;100:1217–9. [Google Scholar]
- 16.Rezvani ME, Roohbakhsh A, Mosaddegh MH, Esmailidehaj M, Khaloobagheri F, Esmaeili H. Anticonvulsant and depressant effects of aqueous extracts of Carum copticum seeds in male rats. Epilepsy Behav. 2011;22:220–5. doi: 10.1016/j.yebeh.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 17.Galani I, Rekatsina PD, Hatzaki D, Plachouras D, Souli M, Giamarellou H. Evaluation of different laboratory tests for the detection of metallo-beta-lactamase production in Enterobacteriaceae. J Antimicrob Chemother. 2008;61:548–53. doi: 10.1093/jac/dkm535. [DOI] [PubMed] [Google Scholar]
- 18.Khosravi AD, Mihani F. Detection of metallo-beta-lactamase-producing Pseudomonas aeruginosa strains isolated from burn patients in Ahwaz, Iran. Diagn Microbiol Infect Dis. 2008;60:125–8. doi: 10.1016/j.diagmicrobio.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Bag A, Bhattacharyya SK, Pal NK, Chattopadhyay RR. In vitro antibacterial potential of Eugenia jambolana seed extracts against multidrug-resistant human bacterial pathogens. Microbiol Res. 2012;167:352–7. doi: 10.1016/j.micres.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Estahbanati HK, Kashani PP, Ghanaatpisheh F. Frequency of Pseudomonas aeruginosa serotypes in burn wound infections and their resistance to antibiotics. Burns. 2002;28:340–8. doi: 10.1016/s0305-4179(02)00024-4. [DOI] [PubMed] [Google Scholar]
- 21.Aboufaycal H, Sader HS, Rolston K, et al. blaVIM-2 and blaVIM-7 carbapenemase-producing Pseudomonas aeruginosa isolates detected in a tertiary care medical center in the United States: Report from the MYSTIC program. J Clin Microbiol. 2007;45:614–5. doi: 10.1128/JCM.01351-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riera E, Cabot G, Mulet X, et al. Pseudomonas aeruginosa carbapenem resistance mechanisms in Spain: Impact on the activity of imipenem, meropenem and doripenem. J Antimicrob Chemother. 2011;66:2022–7. doi: 10.1093/jac/dkr232. [DOI] [PubMed] [Google Scholar]
- 23.Chin BS, Han SH, Choi SH, et al. The characteristics of metallo-beta-lactamase-producing gram-negative bacilli isolated from sputum and urine: a single center experience in Korea. Yonsei Med J. 2011;52:351–7. doi: 10.3349/ymj.2011.52.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De AS, Kumar SH, Baveja SM, et al. Prevalence of metallo-beta-lactamase producing Pseudomonas aeruginosa and Acinetobacter species in intensive care areas in a tertiary care hospital. Indian J Crit Care Med. 2010;14:217–9. doi: 10.4103/0972-5229.76089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Correa A, Montealegre MC, Mojica MF, et al. First report of a Pseudomonas aeruginosa isolate coharboring KPC and VIM carbapenemases. Antimicrob Agents Chemother. 2012;56:5422–3. doi: 10.1128/AAC.00695-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobson RK, Minenza N, Nicol M, Bamford C. VIM-2 metallo-beta-lactamase-producing Pseudomonas aeruginosa causing an outbreak in South Africa. J Antimicrob Chemother. 2012;67:1797–8. doi: 10.1093/jac/dks100. [DOI] [PubMed] [Google Scholar]
- 27.Sardelic S, Bedenic B, Colinon-Dupuich C, et al. Infrequent finding of metallo-beta-lactamase VIM-2 in carbapenem-resistant Pseudomonas aeruginosa strains from Croatia. Antimicrob Agents Chemother. 2012;56:2746–9. doi: 10.1128/AAC.05212-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoo JS, Yang JW, Kim HM, et al. Dissemination of genetically related IMP-6-producing multidrug-resistant Pseudomonas aeruginosa ST235 in South Korea. Int J Antimicrob Agents. 2012;39:300–4. doi: 10.1016/j.ijantimicag.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 29.Kitao T, Tada T, Tanaka M, et al. Emergence of a novel multidrug-resistant Pseudomonas aeruginosa strain producing IMPtype metallo-beta-lactamases and AAC(6’)-Iae in Japan. Int J Antimicrob Agents. 2012;39:518–21. doi: 10.1016/j.ijantimicag.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 30.Jeannot K, Poirel L, Robert-Nicoud M, Cholley P, Nordmann P, Plesiat P. IMP-29, a novel IMP-type metallo-beta-lactamase in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2012;56:2187–90. doi: 10.1128/AAC.05838-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marra AR, Pereira CA, Gales AC, Menezes LC, Cal RG, de Souza JM, et al. Bloodstream infections with metallo-beta-lactamase-producing Pseudomonas aeruginosa: epidemiology, microbiology, and clinical outcomes. Antimicrobial agents and chemotherapy. 2006;50:388–90. doi: 10.1128/AAC.50.1.388-390.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maltezou HC. Metallo-beta-lactamases in Gram-negative bacteria: introducing the era of pan-resistance? International journal of antimicrobial agents. 2009;33:405. doi: 10.1016/j.ijantimicag.2008.09.003. [DOI] [PubMed] [Google Scholar]