Abstract
Background
Low total lymphocyte count (TLC) and lymphocyte-to-neutrophil ratio have been found to be poor prognostic indicators in several different tumor types at various stages. Although immune-based therapies are under rapid development, it is not known whether baseline complete blood counts, particularly lymphocytes, are associated with the clinical outcomes of patients receiving immunotherapies.
Methods
We performed a retrospective analysis of complete blood count for 59 patients enrolled onto a phase II trial evaluating the integration of an adjuvant immunotherapy—irradiated granulocyte-macrophage colony-stimulating factor (GM-CSF) secreting allogeneic pancreatic tumor vaccine (GVAX)—with standard chemoradiation.
Results
After adjusting for nodal status, individuals with a TLC of <1,500 cells/mm3 (10 patients) had significantly higher risk, both in terms of overall survival (OS) [adjusted hazard ratio 2.63, 95 % confidence interval (CI) 1.22–5.67, p = 0.013] and progression-free survival (adjusted hazard ratio 3.07, 95 % CI 1.03–6.93, p = 0.003), compared to those with a TLC of ≤1,500 cells/mm3 (49 patients). Adjuvant chemoradiation significantly reduced lymphocyte counts from baseline values. Patients with suppression of their lymphocytes to <500 cells/mm3 after chemoradiation also had shorter disease-free and OS.
Conclusions
Immunosuppressive conditions associated with surgical procedures and chemoradiation may affect the efficacy of immunotherapy.
Immunotherapies for the treatment of cancers hold great promise to be among the most important medical achievements of the recent era. Some have recently proven their success in treating certain malignancies, while many others are anticipated to have successful mid- or late-stage trial data in the near future.1–4 Yet treating gastrointestinal malignancies, particularly pancreatic adenocarcinoma (PDA), remains a challenge. Pancreaticoduodenectomy is the only curative treatment option for the 15–20 % of patients who present with resectable PDA.5 Even within this subset of PDA patients with potentially curable cancer, median survival is 13–20 months. The addition of adjuvant radiation and/or chemotherapy has demonstrated only modest improvement in survival.6 Additional multimodal therapeutic approaches such as immunotherapy are needed for all stages of PDA.
We have developed and reported the safety and efficacy of an irradiated granulocyte–macrophage colony-stimulating factor (GM-CSF)-secreting allogeneic pancreatic tumor vaccine (pancreatic GVAX) in the adjuvant setting when integrated with chemoradiation and in metastatic patients.7–9 Induction or enhancement of CD8+T cells specific for mesothelin, a putative PDA-associated tumor antigen, is associated with a survival benefit in all three published studies.8–10
A number of studies have reported an association between baseline components of the complete blood count—with total lymphocyte count (TLC) and neutrophil-to-lymphocyte ratio being the most common—and overall survival (OS) in pancreatic as well as other types of cancers.11–14 However, to our knowledge, no study has reported data evaluating changes in blood count components with response to an immunotherapy. However, this is a critical issue because often immunotherapies are provided either concurrently with other therapies or after treatment with other therapies that have the potential to suppress one or more components of the complete blood count. Clinical trials testing immunotherapies provide an opportunity to explore how peripheral blood counts react and affect the response to immune-based therapies. On the one hand, chemotherapy and radiotherapy are associated with neutropenia, anemia, and lymphopenia and can increase a patient’s risk for serious infections. Comprehensive surgeries including pancreatectomies have also been reported to be associated with postoperative immune dysregulation.15,16 On the other hand, the lymphodepletion that results from anticancer treatments may be in some cases advantageous in eliciting clinically relevant response to cancer immunotherapy, particularly if the anticancer agent suppresses subsets of regulatory immune cells. In fact, certain chemotherapy agents are known to have such immunomodulatory effects.17,18
We conducted a retrospective analysis of data collected from a reported phase II pancreatic GVAX study to determine whether components of the complete blood count are associated with survival. We focused our analysis on TLC in particular because GVAX has been demonstrated in preclinical and clinical studies to produce its antitumor response by inducing, activating, and expanding tumor targeted lymphocytes.
PATIENTS AND METHODS
Study Design and Data Collection
The current study is a retrospective analysis of the 59 of 60 subjects with a histologic diagnosis of PDA after an R0 or R1 pancreaticoduodenectomy who were enrolled onto a recently published phase II clinical trial (NTC00084383) at the Johns Hopkins Hospital testing the safety and efficacy of a GM-CSF-secreting PDA vaccine (GVAX) provided in the adjuvant setting.9 One participant was excluded as a result of lack of laboratory data. The first vaccination was provided 8 weeks after surgery, followed by adjuvant 5-fluorouracil plus radiotherapy according to the standard arm of RTOG 9704.19 Those subjects without evidence of recurrence by computed tomographic criteria received repeated injections at weeks 40 (vaccine 2), 44 (vaccine 3), 48 (vaccine 4), and 52 (vaccine 5). Complete blood counts were obtained on day 0 just before each vaccination (Supplementary Fig. S1). The normal range for TLC in the reference laboratory is 1,100–4,500 cells/mm3. Cutoffs of 1,500 cells/mm3 before first vaccination and 500 cells/mm3 before second vaccination were selected on the basis of prior work by others that demonstrated that these values predicted cancer survival in the pre- and postchemotherapy setting, respectively.20,21 Patient characteristics, OS, and disease-free survival (DFS) data were obtained from the database used in the previously published primary results article.9
Statistical Methods
Comparisons between individuals with TLC of <1,500 cells/mm3 and those with TLC of ≥1,500 cells/mm3 before adjuvant chemoradiation and between those with TLC of <500 cells/mm3 and TLC of ≥500 cells/mm3 were performed by Fisher’s exact tests and Wilcoxon rank-sum tests for categorical and continuous variables, respectively. The change in TLC over time was analyzed using generalized estimating equations with an unstructured covariance structure to account for longitudinal within-patient measurements. Time-to-event outcomes (OS and DFS) were summarized by Kaplan–Meier estimates of the survival function. Comparisons between subgroups of interest were made by Cox proportional hazard models.
RESULTS
Fifty-nine of 60 study participants in this clinical trial had pre-adjuvant therapy blood counts available for analysis. The median OS for these 59 patients was 25.4 months [95 % confidence interval (CI) 21.2–31.6]. Thirty-five patients remained in the study and provided complete blood counts for analysis after the completion of adjuvant chemotherapy and radiotherapy (Supplementary Fig. S2). As expected for this disease, patients began to experience recurrence and drop out of the study over time. As such, additional data were available from 32, 27, and 13 individuals for vaccines 3, 4, and 5, respectively, who remained on the study protocol.
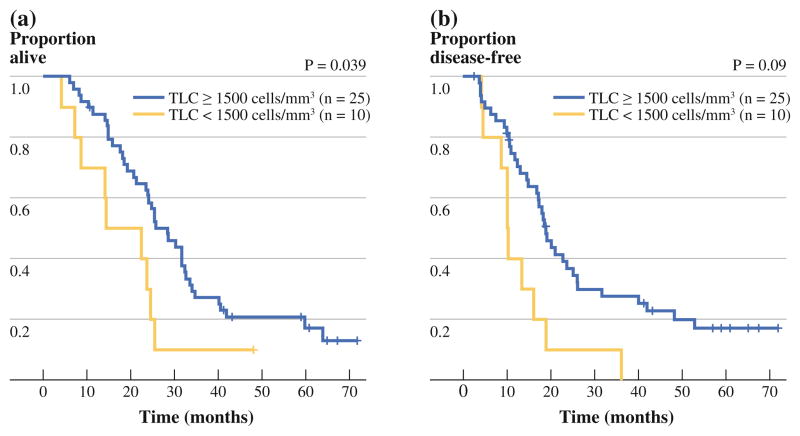
Patient demographics are compared for individuals with ≥1,500 cells/mm3 in the average TLC and those with <1,500 cells/mm3 in the average TLC before the first vaccination. Average TLC before the first vaccination was 1,977 cells/mm3 (range 700–3,890 cells/mm3), and 10 patients (17 %) had TLC of <1,500 cells/mm3. Individuals (10 patients) with counts of <1,500 cells/mm3 were slightly older (median age at surgery 67 vs. 62 years, p = 0.14), more likely to be male (80 vs. 57 %, p = 0.29) and margin positive (40 vs. 24 %, p = 0.13), and less likely to have histologic grade > 2 (10 vs. 43 %, p = 0.074) compared to those (25 patients) with counts of ≥1,500 cells/mm3, although the differences were not statistically significant (Table 1). The OS was significantly lower for those patients with TLC of <1,500 cells/mm3 (median 18.4 months, 95 % CI 4.1–24.6) compared to those with TLC of ≥1,500 [median 28.4 months, 95 % CI 21.1–32.5, unadjusted hazard ratio (HR) 2.14, 95 % CI 1.02–4.47, p = 0.039] (Fig. 1a). TLC of <1,500 cells/mm3 before the first vaccination was also associated with shorter DFS (HR 2.62, 95 % CI 1.27–5.40, p = 0.009) (Fig. 1b). Of the remaining risk factors, only node positivity was significantly associated with OS (HR 3.39, p = 0.021) or close to significantly associated with progression-free survival (HR 2.28, p = 0.08). After adjusting for nodal status, TLC of <1,500 cells/mm3 remained significantly associated with increased risk both in terms of OS [adjusted HR (aHR) 2.63, 95 % CI 1.22–5.67, p = 0.013] and progression-free survival (aHR 3.07, 95 % CI 1.03–6.93, p = 0.003).
TABLE 1.
Patient demographic and pathologic characteristics at vaccine 1, day 0
| Characteristic | TLC <1,500 cells/mm3 (n = 10) | TLC ≥ 1,500 cells/mm3 (n = 49) | pa |
|---|---|---|---|
| Age at surgery (year), median (range) | 67 (52–83) | 62 (40–82) | 0.137 |
| Male, n (%) | 8 (80 %) | 28 (57 %) | 0.288 |
| Lymph node positive, n (%) | 9 (90 %) | 43 (88 %) | > 0.99 |
| Margin positive, n (%) | 5 (50 %) | 12 (24 %) | 0.133 |
| Tumor size, cm, median (range) | 2.90 (1.50–4.50) | 3.00 (1.30–6.00) | 0.800 |
| Histologic grade > 2, n (%) | 1 (10 %) | 21(43 %) | 0.074 |
| Vaccine 2, day 0, TLC of < 500 cells/mm3, n (%)b | 2 (40 %) | 5 (17 %) | 0.256 |
TLC total lymphocyte count
p values are based on Wilcoxon rank sum and Fisher’s exact test for continuous and categorical variables, respectively
Percentage calculated from the 35 participants who proceeded to vaccine 2
FIG. 1.
Kaplan-Meier curves of overall (a) and disease-free (b) survival for individuals with total lymphocyte counts (TLC) <1,500 cells/mm3 versus those with TLC of ≥1,500 cells/mm3 immediately before the first vaccine
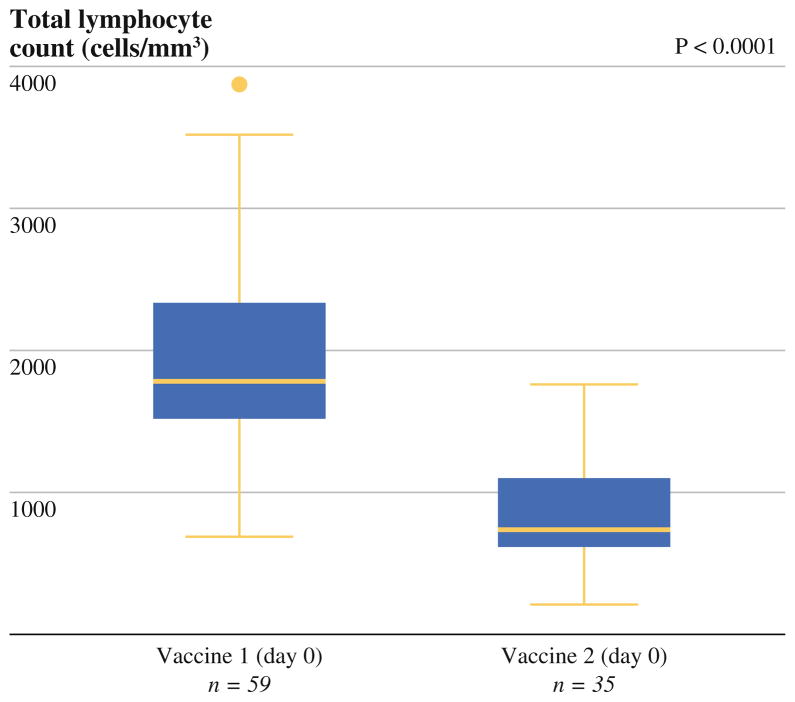
Among the 35 (60 %) individuals with total lymphocyte measurements available after completing adjuvant chemoradiation and just before receiving the second vaccine, treatment with adjuvant chemoradiation consistently decreased TLC when compared with the pre–adjuvant therapy counts, with a median reduction of 1,180 cells/mm3 (range 450–2,740, p < 0.0001, Fig. 2). Total lymphocyte measurements for this subgroup before adjuvant therapy was slightly higher than those for the entire cohort (median 1,960 cells/mm3, range 840–3,890 cells/mm3), although not significantly (p = 0.19). After adjuvant chemoradiation and just before the second vaccine, patients with a TLC of <500 cells/mm3, which represented 20 % (n = 7) of the 35 patients, had a significantly reduced OS (median OS 23.9 vs. 33.9 months, HR 6.34, 95 % CI 2.37–16.91, p < 0.0001) (Fig. 3). Median DFS was also significantly shorter among those with depressed lymphocyte counts <500 cells/mm3 (median DFS 19.0 vs. 26.0 months, HR 3.09, 95 % CI 1.14–8.33, p = 0.026). Only two of these seven patients had low postoperative lymphocyte counts. In fact, eight out of 10 patients who initially had low postoperative lymphocyte counts were able to maintain a higher level after adjuvant chemotherapy.
FIG. 2.
Comparison of total lymphocyte count at vaccine 1 (day 0) versus vaccine 2 (day 0) among the 35 patients who received adjuvant chemoradiation and the second vaccine
FIG. 3.
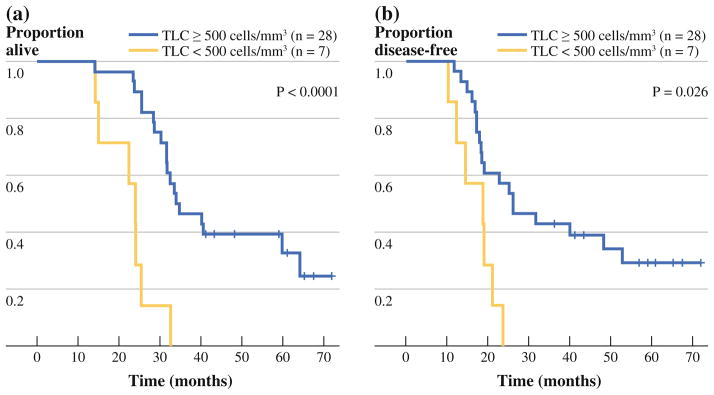
Comparison of the overall (a) and disease-free (b) survival for individuals with total lymphocyte counts (TLC) <500 cells/mm3 versus those with TLC of ≥ 500 cells/mm3 immediately before the second vaccine (i.e., after adjuvant chemoradiation)
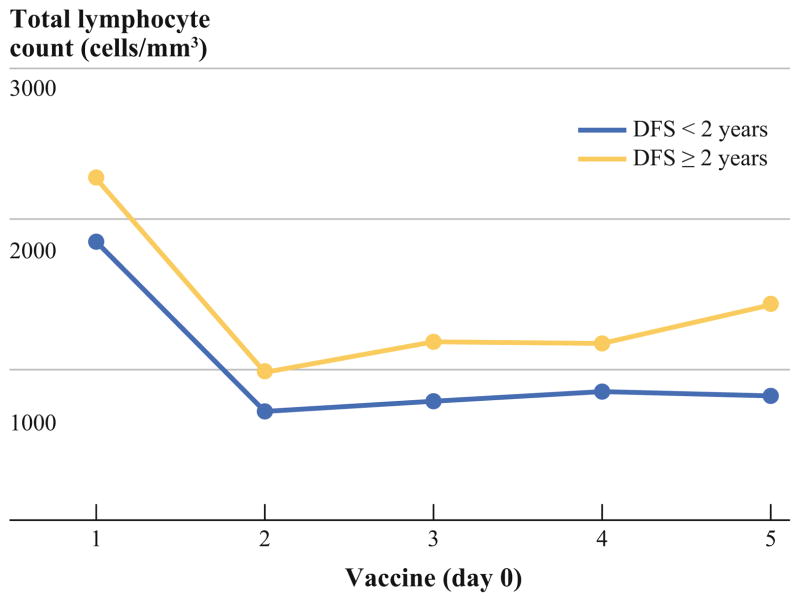
Subjects whose DFS was 2 years or more had significantly higher TLC before the first vaccination compared with patients who experienced disease recurrence in less than 2 years; these subjects continued to maintain higher TLC throughout the vaccine treatment course (mean difference 399, 95 % CI 117–629, p = 0.004; Fig. 4).
FIG. 4.
Comparison of total lymphocyte counts (TLC) at day 0 for vaccines 1 through 5 for individuals with disease-free survival (DFS) less than 2 years versus those with DFS of 2 years or more. The TLC for those with DFS less than 2 years was consistently lower than for those with DFS 2 years or more both before and after chemotherapy
In addition to TLC, other complete blood count measurements just before the first vaccination were evaluated. The total neutrophil counts fell within the normal range for almost all individuals (median 4,180 cells/mm3, range 1,790–9,940 cells/mm3), with one individual having a count of >7,500 cells/mm3. Neutrophil-to-lymphocyte ratio was >5 in only five individuals at baseline. The white blood counts were also within the normal range in general (median 7,180 cells/μL, range 4,020–12,500 cells/μL) with only two patients below 4,500 cells/μL. Only one individual had eosinophil counts of >300 cells/mm3 (median 170 cells/mm3; range 30–550 cells/mm3). Thus, a decrease in TLC does not appear to be due to an increase in myelopoiesis.
Eosinophil counts rose after each vaccination, likely as a result of the GM-CSF secreted from the vaccine cells and returned to the prevaccination baseline (Supplementary Fig. S3). This GM-CSF-associated change of eosinophil counts occurred in every patient and therefore did not account for the effect of prevaccination lymphocyte counts on patient survival. These data suggest that other peripheral blood count parameters are unlikely to have prognostic value for patients’ clinical outcomes in response to immunotherapy.
DISCUSSION
This study demonstrates the association between TLC and clinical outcomes in individuals receiving vaccine-based cancer immunotherapy. Immunotherapy has recently become a new modality for metastatic prostate cancer and melanoma. Sipuleucel-T, a dendritic cell vaccine, was approved by the U.S. Food and Drug Administration (FDA) for treating metastatic prostate cancer.1 Ipilimumab, a monoclonal antibody targeting an immune checkpoint, was approved by the FDA for treating metastatic melanoma.2 To our knowledge, whether patients’ general immune condition before therapy, as reflected by their lymphocyte count, is associated with their clinical outcome after receiving immunotherapy has not yet been studied. For pancreatic cancer, low preoperative lymphocyte counts has been identified to be a poor prognostic factor in resected patients.14,22,23
To our knowledge, our study is the first supporting the prognostic value of postoperative peripheral TLC in patients receiving vaccine-based immunotherapy. The results in our study suggest that patients who have postoperative baseline lymphopenia before initiating immunotherapy may not be as responsive to immunotherapy or receive the full benefit of the therapy. In contrast, postoperative lymphocyte counts did not appear to be associated with survival for patients who did not receive any immunotherapy after surgical resection of pancreatic cancer in a retrospective review in Japan.24 More recently, an analysis of patients who had surgical resection of PDAs at the Johns Hopkins Hospital who did not receive pancreatic cancer vaccines revealed that postoperative lymphocyte counts were not strongly associated with OS, being nonsignificant in the unadjusted model and only borderline significant after adjusting for other risk factors.25 The later cohort is more directly comparable to our study because it includes individuals used in the direct comparison of vaccine and nonvaccine patients discussed in the original clinical trial article, although a slightly different cutoff was used (1,000 vs. 1,500 cells/mm3). Nonetheless, a formal comparison between vaccinated and unvaccinated patients remains needed to substantiate the notion that the postoperative effects differ for the two types of therapy.
Low TLC has been broadly associated with survival in both solid and hematologic malignancies (including pancreatic cancer, as described above) and has been retrospectively studied in the prechemotherapy, postchemotherapy, postsurgical, and adjuvant settings. A detailed review on the subject has been performed.26 In our study, there was an association between postoperative lymphocyte count and survival, suggesting that in patients receiving adjuvant immunotherapy, postoperative TLC potentially can be used to predict which patients may benefit more from immunotherapy than others. However, it remains to be determined whether low postoperative lymphocyte counts in the 10 patients were predetermined preoperatively at their baseline or were acquired postoperatively. The fact that lymphocyte counts in the majority of these patients (8 of 10) were not depressed after adjuvant therapy suggests that their prognosis was not changed with recovery of lymphocyte counts. Nevertheless, low post-chemoradiation lymphocyte count is also associated with poor survival.
In this study, we found that lymphocytes in patients whose DFS was longer than 2 years gradually recovered to normal levels, whereas most patients whose DFS was less than 2 years had persistent lymphopenia after adjuvant chemoradiation. It is possible that disease recurrence leads to a lymphopenic state. However, in this study, the onset of lymphopenia precedes disease recurrence, especially after adjuvant therapy. An alternative explanation is that persistent depression of TLC contributes to insufficient immune surveillance and ineffectiveness of immunotherapy treatment, thereby setting the stage for early recurrence. Our results have thus implicated the potential challenge of combining immunotherapy with conventional anticancer treatments. Both the surgical resection and adjuvant chemoradiation are considered to be essential treatments for resectable cancer. However, they are also known to cause immune dysregulation.27 It was reported that the degree of lymphocyte suppression correlates with the magnitude of surgery.28 Operative stress and poor postoperative nutrition status are thought to be among the potential causes of immunosuppression.28 Laparoscopic or minimally invasive surgery has the potential to offer beneficial immunomodulatory effects compared to conventional approaches.29,30 It will be interesting to test in the future whether combining less invasive laparoscopic surgical approaches and chemotherapy regimens that have less immunosuppression may lead to improved survival outcomes with and without the addition of immunotherapy. It will also be important to assess both preoperative and postoperative lymphocyte counts in the future studies of immunotherapy.
Immunotherapies are approved for the treatment of some cancers and are under rapid development for most others. This study is the first to suggest a prognostic value from determining baseline peripheral TLC in patients receiving vaccine-based immunotherapy. However, this study is limited to its retrospective nature, lack of control arm, and small sample size. Whether lymphocyte count is a prognostic factor for pancreatic cancer patients receiving immunotherapy requires further investigation through prospective clinical studies.
Supplementary Material
Acknowledgments
This study was supported by the NCI SPORE in Gsastrointestinal Cancers P50 CA062924-14 (E. M. J.), Viragh Foundation (D. L., E. M. J., E. A. S), NIH K23 CA93566 (D. L.), the Sol Goldman Pancreatic Cancer Center (L. Z.), NIH K23 CA148964 (L. Z.) and a Johns Hopkins University School of Medicine Clinician Scientist Award (L. Z.). Dr. Jaffee is the first recipient of the Dana and Albert “Cubby” Broccoli Endowed Professorship.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1245/s10434-013-3262-5) contains supplementary material, which is available to authorized users.
DISCLOSURE Under a licensing agreement between Aduro Bio-Tech and the Johns Hopkins University, Dr. Jaffee and the university are entitled to milestone payments and royalty on sales of the vaccine product described in this article. We do not have any other relevant conflicts of interest to be disclosed.
References
- 1.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 2.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brahmer JR, Tykodi SS, Chow LQM, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pierantoni C, Pagliacci A, Scartozzi M, Berardi R, Bianconi M, Cascinu S. Pancreatic cancer: progress in cancer therapy. Crit Rev Oncol Hematol. 2008;67:27–38. doi: 10.1016/j.critrevonc.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Picozzi VJ, Pisters PW, Vickers SM, Strasberg SM. Strength of the evidence: adjuvant therapy for resected pancreatic cancer. J Gastrointest Surg. 2008;12:657–61. doi: 10.1007/s11605-007-0446-y. [DOI] [PubMed] [Google Scholar]
- 7.Jaffee EM, Hruban RH, Biedrzycki B, et al. Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J Clin Oncol. 2001;19:145–56. doi: 10.1200/JCO.2001.19.1.145. [DOI] [PubMed] [Google Scholar]
- 8.Laheru D, Lutz E, Burke J, et al. Allogeneic granulocyte macrophage colony-stimulating factor–secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clin Cancer Res. 2008;14:1455–63. doi: 10.1158/1078-0432.CCR-07-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lutz E, Yeo CJ, Lillemoe KD, et al. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma. A phase II trial of safety, efficacy, and immune activation. Ann Surg. 2011;253:328–35. doi: 10.1097/SLA.0b013e3181fd271c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas AM, Santarsiero LM, Lutz ER, et al. Mesothelin-specific CD8(+) T cell responses provide evidence of in vivo cross-priming by antigen-presenting cells in vaccinated pancreatic cancer patients. J Exp Med. 2004;200:297–306. doi: 10.1084/jem.20031435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grossman SA, Ye X, Lesser G, et al. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res. 2011;17:5473–80. doi: 10.1158/1078-0432.CCR-11-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sato H, Tsubosa Y, Kawano T. Correlation between the pre-therapeutic neutrophil to lymphocyte ratio and the pathologic response to neoadjuvant chemotherapy in patients with advanced esophageal cancer. World J Surg. 2012;36:617–22. doi: 10.1007/s00268-011-1411-1. [DOI] [PubMed] [Google Scholar]
- 13.Sarraf KM, Belcher E, Raevsky E, Nicholson AG, Goldstraw P, Lim E. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non–small cell lung cancer. J Thorac Cardiovasc Surg. 2009;137:425–8. doi: 10.1016/j.jtcvs.2008.05.046. [DOI] [PubMed] [Google Scholar]
- 14.Garcea G, Ladwa N, Neal CP, et al. Preoperative neutrophil-to-lymphocyte ratio (NLR) is associated with a reduced disease-free survival following curative resection of pancreatic adenocarcinoma. World J Surg. 2011;35:868–72. doi: 10.1007/s00268-011-0984-z. [DOI] [PubMed] [Google Scholar]
- 15.Fabris N, Piantanelli L. Differential effect of pancreatectomy on humoral and cell-mediated immune responses. Clin Exp Immunol. 1977;28:315–25. [PMC free article] [PubMed] [Google Scholar]
- 16.Takahashi K, Toyokawa H, Takai S, et al. Surgical influence of pancreatectomy on the function and count of circulating dendritic cells in patients with pancreatic cancer. Cancer Immunol Immunother. 2006;55:775–84. doi: 10.1007/s00262-005-0079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terando A, Mule JJ. On combining antineoplastic drugs with tumor vaccines. Cancer Immunol Immunother. 2003;52:680–5. doi: 10.1007/s00262-003-0426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Emens LA, Machiels JP, Reilly RT, Jaffee EM. Chemotherapy: friend or foe to cancer vaccines? Curr Opin Mol Ther. 2001;3:77–84. [PubMed] [Google Scholar]
- 19.Regine WF, Winter KA, Abrams RA, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA. 2008;299:1019–26. doi: 10.1001/jama.299.9.1019. [DOI] [PubMed] [Google Scholar]
- 20.Lissoni P, Brivio F, Fumagalli L, et al. Efficacy of cancer chemotherapy in relation to the pretreatment number of lymphocytes in patients with metastatic solid tumors. Int J Biol Markers. 2004;19:135–40. doi: 10.1177/172460080401900208. [DOI] [PubMed] [Google Scholar]
- 21.De Angulo G, Hernandez M, Morales-Arias J, et al. Early lymphocyte recovery as a prognostic indicator for high-risk Ewing sarcoma. J Pediatr Hematol Oncol. 2007;29:48–52. doi: 10.1097/MPH.0b013e31802d3e3e. [DOI] [PubMed] [Google Scholar]
- 22.Clark EJ, Connor S, Taylor MA, Madhavan KK, Garden OJ, Parks RW. Preoperative lymphocyte count as a prognostic factor in resected pancreatic ductal adenocarcinoma. HPB (Oxford) 2007;9:456–60. doi: 10.1080/13651820701774891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhatti I, Peacock O, Lloyd G, Larvin M, Hall RI. Preoperative hematologic markers as independent predictors of prognosis in resected pancreatic ductal adenocarcinoma: neutrophil–lymphocyte versus platelet–lymphocyte ratio. Am J Surg. 2010;200:197–203. doi: 10.1016/j.amjsurg.2009.08.041. [DOI] [PubMed] [Google Scholar]
- 24.Kanda M, Fujii T, Kodera Y, Nagai S, Takeda S, Nakao A. Nutritional predictors of postoperative outcome in pancreatic cancer. Br J Surg. 2011;98:268–74. doi: 10.1002/bjs.7305. [DOI] [PubMed] [Google Scholar]
- 25.Balmanoukian A, Ye X, Herman J, Laheru D, Grossman SA. The association between treatment-related lymphopenia and survival in newly diagnosed patients with resected adenocarcinoma of the pancreas. Cancer Invest. 2012;30:571–6. doi: 10.3109/07357907.2012.700987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Porrata LF, Markovic SN. Is absolute lymphocyte count just another prognostic factor in cancer? SRX Med. 2010 doi: 10.3814/2010/812304. [DOI] [Google Scholar]
- 27.Kimura F, Shimizu H, Yoshidome H, Ohtsuka M, Miyazaki M. Immunosuppression following surgical and traumatic injury. Surg Today. 2010;40:793–808. doi: 10.1007/s00595-010-4323-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tashiro T, Yamamori H, Takagi K, et al. Changes in immune function following surgery for esophageal carcinoma. Nutrition. 1999;15:760–6. doi: 10.1016/s0899-9007(99)00151-3. [DOI] [PubMed] [Google Scholar]
- 29.Corrigan M, Cahill RA, Redmond HP. The immunomodulatory effects of laparoscopic surgery. Surg Laparosc Endosc Percutan Tech. 2007;17:256–61. doi: 10.1097/SLE.0b013e318059b9c3. [DOI] [PubMed] [Google Scholar]
- 30.Hegarty N, Dasgupta P. Immunological aspects of minimally invasive oncologic surgery. Curr Opin Urol. 2008;18:129–33. doi: 10.1097/MOU.0b013e3282f517fc. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.