Abstract
Hematopoietic stem cells can be procured from unrelated donors via either the bone marrow (BM) aspiration or peripheral blood stem cell (PBSC) collection methods. There is no evidence from prospective randomized trials in the unrelated donor setting about the relative health-related quality-of-life (HRQoL) benefits/costs to donors. The goals of this prospective longitudinal investigation were to describe and compare the donation-related HRQoL experiences of 332 BM and PBSC donors.
Donors were interviewed at pre-donation, 48 hours after donation, weekly until fully recovered and at 6 and 12 months post-donation.
Pre-donation, BM donors had lower confusion, fewer concerns, and were more prepared for donation. Shortly post-donation BM donors reported more physical side effects. BM donors also reported more donation-related impact on their social activities. However, BM donors reported somewhat better psychological status and were more likely to indicate that the donation made their lives more meaningful. There were virtually no longer-term differences in the experiences of the two donor groups including no recovery time difference beginning 3 weeks after donation.
Although BM donors may experience the process as more physically stressful and more psychologically beneficial in the short-term, the longer-term HRQoL consequences of BM and PBSC donors are similar.
Introduction
Hematopoietic stem cell (HSC) transplantation is increasingly used to treat leukemia or other blood-related diseases for which other forms of therapy are ineffective or would be less effective. Because a minority of patients requiring transplants can find a matched-related HSC donor, approximately 14,000 patients each year search international registries for unrelated donors (http://marrow.org/News/Media/Facts_and_Figures_(PDF).aspx).
When unrelated potential donors preliminarily match a patient, they are contacted to undergo additional testing to confirm eligibility. If selected, they then donate via either the traditional bone marrow (BM) aspiration procedure or the more recent peripheral blood stem cell (PBSC) procedure. Donors who donate BM undergo general/regional anesthesia and HSCs are collected percutaneously from the posterior iliac crests of the pelvis. Donors who donate PBSCs receive a five-day course of recombinant human granulocyte colony-stimulating factor (rhGCSF) after which HSCs are collected from the peripheral blood on one or two consecutive days in 4-5 hour apheresis sessions.
Rates of adverse events in donation vary across investigations, primarily depending on whether or not both related and unrelated donors are included.1-2 Strict guidelines mean that unrelated donors are on average younger and healthier than their related donor counterparts and therefore experience fewer adverse events. Published data indicate that for unrelated donors, both BM and PBSC donation are generally safe with a low incidence of serious adverse events – 1.34% in BM donors and 0.6% in PBSC donors.3-7 Therefore, the decision about which type of product is requested is left to the transplant physician managing the patient. In recent years the use of PBSC donation has increased due to more rapid hematopoietic engraftment, more reliable engraftment when reduced intensity conditioning is used, and the potential for greater graft versus tumor effect due to the larger number of immune cells in PBSC versus BM. PBSC now accounts for 75% of all adult-derived unrelated donations (National Marrow Donor Program statistics).8 Until recently, there was no evidence from prospective randomized trials in the unrelated donor setting that one product or the other conferred a survival advantage to recipients nor was there conclusive evidence about the relative health-related quality-of-life (HRQoL) benefits/costs to donors.
There have been several investigations of donor experiences of BM versus PBSC donation. These investigations focused on the physical experience and physical side-effects of the donation process and found that (a) both BM and PBSC donors experience side-effects of the donation process, most commonly pain and fatigue,9 (b) BM donors have a longer recovery time than do PBSC donors, 9,10 and (c) serious adverse events are very rare, but more common in BM donors.9 Other findings were mixed, including the questions of whether BM or PBSC donors experience similar pain severity and duration,9,10 overall symptom burden,9,11 and emotional stress related to donation.9,12 Many of these investigations were limited by small numbers of donors, inclusion of donors only from a single transplant center, non-randomized designs, and/or exclusive focus on physical rather than psychosocial factors.
To address questions about the relative advantages/disadvantages to both patients and donors of the two HSC collection and transplantation procedures, the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) recently completed a phase III trial randomizing patients to receive either marrow or PBSC grafts. Findings for transplant recipients indicated that there were no statistically significant differences in patient survival at two years between the two procedures.13 Here, we present findings from a planned subanalysis of the trial examining the physical and psychosocial experiences of the two types of donors. The goals were to describe the donation-related experiences of BM and PBSC donors and to determine whether there were group differences at pre- and multiple points post-donation.
Materials and Methods
Human subjects research protection
This investigation was approved by the Institutional Review Boards at the University of Pittsburgh, the National Marrow Donor Program (NMDP), and individual donor centers. All participants signed informed consent before completing the study interview.
Participants and study design
Our investigation was a pre-specified subgroup analysis of BMT CTN protocol 0201 (ClinicalTrials.gov Identifier: NCT00075816). The prospective, longitudinal investigation included NMDP donors enrolled in the parent randomized clinical trial (RCT) and randomized to donate PBSC or BM March, 2004 through October, 2009.
To be eligible, potential participants were required to (a) meet the standard NMDP requirements for donation (b) be selected for participation in the RCT, (c) consent to participate in both the RCT and the donor sub-study, and (d) be enrolled in the parent RCT. Potential participants were excluded from the study if they did not read, write, and speak English, were unable to complete a telephone interview due to cognitive or linguistic difficulties, or if they did not have access to a telephone. Department of Defense donors (after 02/06/07) and German Registry donors were also excluded from the donor sub-study on the basis of logistical issues and language respectively.
NMDP donor center coordinators consented participants for the study and passed contact information of enrolled donors to University of Pittsburgh staff. Interviewers from the University of Pittsburgh contacted participants by phone to complete data collection. Within four weeks prior to marrow donation or initiation of G-CSF administration for PBSC donors, participants completed a baseline interview. PBSC donors only were interviewed on day 4 of rhGCSF administration to assess current pain levels. All donors were interviewed again 48 hours after donation and weekly until symptom-free for three consecutive weeks. Participants were also interviewed 6 and 12 months post-donation. The baseline and 48 hours post-donation interviews required 20 minutes to complete – other interviews required 15 minutes. A Computer Assisted Telephone Interview (CATI) system was used to collect and enter interview data. Data were stored on a secure server in a proprietary data file.
Study measures
Four categories of participant characteristics were assessed: (1) socio-demographic, (2) physical status, (3) psychological status, and (4) donation-related. Measures were previously validated scales/items with established measurement properties either created for, or used in, other donation-related settings. Donor height and weight and experience of an adverse event (AE) or a serious adverse event (SAE) were collected directly from the NMDP.
Socio-demographic characteristics
age, sex, marital status, education level, employment status, and race.
Physical status
Overall physical status was assessed with the physical status summary scale of the SF-8 (Ware, 2001).14 Scores range from 0-100 with higher scores indicating better physical health. Current symptoms assessed as present/absent in the past 48 hours included tiredness, muscle aches, problems sleeping, bone pain, light headedness, pain where the needles were inserted, difficulty walking, bleeding, nausea, infection, chills, and fainting.11 Current pain was assessed with four items indicating highest pain intensity, average pain, amount of pain, and effect of pain on sleep during the past 48 hours. Pain was rated on a scale from 0-10 with a higher score indicating more pain. A composite pain index was also created by averaging the 4 item scores.9
Psychological status
Overall psychological status was assessed with the psychological status summary scale of the SF-8.14 Scores range from 0-100 with higher scores indicating better psychological health. Mood disturbance was assessed with the Profile of Mood States-Short Form (POMS-SF).15 The POMS-SF is a 30-item measure that produces scores on six subscales: depression, tension-anxiety, anger, confusion, fatigue, and vitality (range 0-4) and an overall distress score (calculated as the sum of the means of each of the POMS-SF subscales; range 0-24).16 For all POMS scales other than vitality, a higher score indicates greater distress – higher vitality scores indicate more vitality.
Donation-related
At the pre-donation interview, Interactions with others were assessed with four items asking whether donors consulted family/friends or professionals about donation and whether they had been encouraged/discouraged from donating (yes/no). 17-18 Concerns about donation were assessed with 13 concerns in three categories – medical, work/family, and other (yes/no).17-19 Preparedness for donation was assessed with three items asking whether donors felt informed about donation (1=not at all, 4= very well), prepared for donation (no, mostly, totally) and whether they felt they needed more information prior to donating (1=need much more information, 4=do not need any more information).11 All items were dichotomized to “totally prepared/informed” versus other categories. Health concerns were assessed with two items asking about worry about longer-term health impact of donation (1=definitely will have impact; 4=definitely will not have impact) and worry about never feeling 100% well again after donation (1=very often; 4=never).11 The two items were dichotomized to lowest concern about health impact (e.g., definitely will not have impact) versus other categories. Satisfaction with the donation decision was assessed with two items asking about overall satisfaction with the decision (1=not at all; 4=extremely) and whether they would volunteer/donate again if asked (1=no; 4=definitely), and an additional four items at post-donation time-points asking whether donors would encourage others to donate (1=discourage strongly; 5=encourage strongly), felt like a better person after donating (1=not at all; 3=a lot), felt proud about donating (1=not at all; 3=very), and whether donating made their life seem more meaningful (1=not at all; 4=very much). All items were dichotomized to highest satisfaction (e.g., extremely satisfied) versus other categories.20 At 48 hours post-donation only, the Social impact of donation was assessed with 6 items asking whether work/school/leisure activities were affected by donation, and whether the donor needed to make travel, childcare, financial, or home care arrangements (yes/no).9 Donation experience was assessed with two items asking about the physical/emotional stress of donation (1=not at all stressful; 4=very stressful).11 Items were dichotomized to no stress versus any stress. Recipient-related variables included whether the donor knew the recipient's health status (yes/no), frequency of thoughts about the recipient (≥ once per/day versus all other frequencies), whether they felt they had a special bond with (not at all versus a little, somewhat, and very much) and were worried about the recipient (very and pretty versus not very and not at all).11 Post-donation recovery was evaluated in two ways. Donors were assessed weekly post-donation for the presence/absence of 12 key symptoms11 and were considered fully recovered after 3 consecutive symptom-free weeks. Symptoms were assessed without asking donors to make an attribution of the symptoms specifically to donation. This is a conservative definition of recovery and necessarily means that no donor can be recognized as fully recovered until at least 3 weeks post-donation. Recovery was calculated both as the proportion of donors recovered versus not at 6 and 12 months and as a continuous variable (week of full recovery) across the 52 weeks following donation.
Clinical
Donor height (cm) and weight (kg) and the presence or absence of an AE or SAE.
Statistical analysis
Data were cleaned and exported from the CATI system to PASW Statistic 18, Release Version 18.0.3 (IBM Corporation, Somer, NY, USA) for analysis. Cross-sectional differences in donor HRQoL by donation type at each key time point were examined using odds-ratios for categorical variables and t-tests for continuous variables. To account for multiple comparisons, we applied the Holm-Bonferroni correction to each of the four classes of variables separately for each time point.21 When the correction was applied, comparisons significant at 0.001 remained significant, but those with p-values >0.001 no longer reached statistical significance. In the tables, we noted which comparisons were significant at 0.05, 0.01, and 0.001. In the text, we refer to comparisons with differences of p≤0.001 as significant and those with differences of p≤0.05 and p≤0.01 as marginally significant.
To examine longitudinal differences in physical and mental health (SF-8 physical and mental health summary scores) by donation type, we used linear mixed models analyses. Main effects for donation type and time and the donation type by time interaction were examined. We used Kaplan Meier (Log Rank Chi-squared) analyses to examine potential differences in recovery time by donation type using week of full recovery as the primary outcome.
Results
Participants
Of the 551 donors who participated in the parent RCT, 335 were eligible for the sub-study. The primary reason for ineligibility was registration in the Department of Defense (n=25) or German registries (n=154). An additional 34 potential donors were ineligible because of issues related to the recipient or because they were non-English speaking. Three donors declined participation. A total of 332 donors contributed data.
Table 1 lists interview completion rates by cross-sectional time point. A total panel of 236 (71%) completed the baseline, 48 hours post-donation, and 6 and 12 month post-donation interviews. There were no differences between panel and non-panel members by gender, race, age, marital status or employment status.
Table 1.
Interview completion rates at cross-sectional time points.
| Time Point | Interviews completed at time point | % of total N (332) |
|---|---|---|
| Pre-donation | 331 | 99% |
| 48 hours post-donation | 273 | 82% |
| Weekly until fully recovered | 318 | 96% |
| 6 months post-donation | 294 | 89% |
| 12 months post-donation | 288 | 87% |
Pre-donation
The pre-donation interview was completed by BM and PBSC donors an average of 10.6 and 11.5 days respectively prior to donation (BM and PBSC mode = 7 and 6 respectively). The majority of participants were in their 30s, white, male, married, and employed – about half had completed a bachelor's degree (Table 2). There were no group differences in demographic characteristics, clinical variables, physical/psychological status (although BM donors were marginally more likely to report infection and less likely to report confusion) or any of the variables related to interactions with others.
Table 2.
Pre-donation comparison of marrow and PBSC donors.
| Study Variable | Marrow (n=161) |
PBSC (n=170) |
Test Statistic (t or odds-ratio) |
p-value |
|---|---|---|---|---|
| Sociodemographic | ||||
| age (mean, sd) | 33.6 (9.18) | 35.1 (10.29) | -1.45 | 0.148 |
| % women | 34 | 34 | 1.03 (0.65-1.62) | 0.912 |
| % married | 63 | 53 | 0.67 (0.43-1.04) | 0.071 |
| % ≥ Bachelor's degree | 46 | 53 | 1.32 (0.86-2.04) | 0.204 |
| % employed | 93 | 91 | 1.32 (0.59-2.97) | 0.501 |
| Race | ||||
| % Caucasian/White | 86 | 83 | ||
| % Hispanic | 6 | 8 | ||
| % African American | 4 | 4 | ||
| % Asian American | 1 | 2 | ||
| % Other | 2 | 2 | ||
| Physical Status | ||||
| Overall physical status | ||||
| SF-8 physical health (mean, sd) (29-68)† | 56.47 (3.71) | 55.86 (4.79) | 0.78 | 0.436 |
| Current physical symptoms | ||||
| % tiredness | 49 | 52 | 1.10 (0.70-1.74) | 0.685 |
| % muscle aches | 17 | 24 | 1.54 (0.86-2.74) | 0.145 |
| % problems sleeping | 15 | 16 | 1.07 (0.57-2.00) | 0.845 |
| % bone pain | 6 | 8 | 1.49 (0.59-3.76) | 0.397 |
| % light headedness | 6 | 5 | 0.85 (0.32-2.27) | 0.747 |
| % difficulty walking | 4 | 4 | 0.97 (0.30-3.07) | 0.952 |
| % bleeding | 7 | 3 | 0.37 (0.11-1.21) | 0.087 |
| % nausea or vomiting | 1 | 4 | 6.00 (0.71-50.47) | 0.062 |
| % infection | 3 | 0 | 0.48 (0.43-0.55) | 0.041* |
| % chills | 2 | 2 | 0.97 (0.19-4.87) | 0.966 |
| % fainting | 1 | 1 | 0.97 (0.06-15.60) | 0.981 |
| Current physical symptoms | ||||
| Psychological Status | ||||
| Overall psychological status | ||||
| SF-8 mental health (mean, sd) (28-69) | 55.68 (4.83) | 55.12 (5.37) | 0.94 | 0.350 |
| Profile of mood states (POMS) | ||||
| depression (mean, sd) (0-4) | 0.10 (0.29) | 0.11 (0.24) | -0.28 | 0.784 |
| vitality (mean, sd) (0-4) | 2.40 (0.82) | 2.46 (0.67) | -0.60 | 0.548 |
| confusion (mean, sd) (0-4) | 0.23 (0.34) | 0.31 (0.40) | -2.04 | 0.043* |
| tension (mean, sd) (0-4) | 0.44 (0.51) | 0.45 (0.47) | -0.11 | 0.911 |
| anger (mean, sd) (0-4) | 0.22 (0.38) | 0.22 (0.32) | 0.06 | 0.951 |
| fatigue (mean, sd) (0-4) | 0.46 (0.61) | 0.55 (0.62) | -1.36 | 0.174 |
| total distress (mean, sd) (0-24) | 4.21 (1.91) | 4.19 (1.91) | 0.07 | 0.941 |
| Donation-related | ||||
| Interaction with others | ||||
| % consulted family/friends | 58 | 56 | 1.08 (0.68-1.72) | 0.738 |
| % consulted professionals | 29 | 35 | 0.75 (0.46-1.22) | 0.242 |
| % encouraged to donate | 26 | 30 | 0.80 (0.48-1.33) | 0.390 |
| % discouraged from donating | 26 | 27 | 0.91 (0.54-1.53) | 0.727 |
| Medical concerns | ||||
| % pain | 58 | 55 | 0.89 (0.56-1.40) | 0.604 |
| % anesthesia | 35 | 33 | 0.91 (0.56-1.48) | 0.709 |
| % damage health | 26 | 39 | 1.81 (1.10-2.97) | 0.018* |
| % use of needles | 13 | 16 | 1.33 (0.69-2.58) | 0.391 |
| Work/family concerns | ||||
| % family will worry | 56 | 49 | 0.78 (0.49-1.23) | 0.286 |
| % missing work/school | 43 | 41 | 0.92 (0.58-1.46) | 0.714 |
| % reimbursement for missed work | 19 | 19 | 1.00 (0.56-1.80) | 0.993 |
| % missing family activities | 15 | 15 | 1.06 (0.56-2.02) | 0.857 |
| % child/family care | 15 | 11 | 0.66 (0.33-1.32) | 0.239 |
| Other concerns | ||||
| % patient's chances are low | 43 | 38 | 0.83 (0.52-1.33) | 0.443 |
| % payment for medical treatment | 18 | 14 | 0.74 (0.40-1.38) | 0.343 |
| % transportation to donor center | 14 | 12 | 0.85 (0.43-1.67) | 0.629 |
| % against religious beliefs | 0 | 1 | 0.51 (0.45-0.57) | 0.164 |
| Overall health concerns | ||||
| % worry about longer-term health impact of donation | 63 | 66 | 1.13 (0.70-1.82) | 0.615 |
| % worry will never feel physically 100% again | 29 | 27 | 0.89 (0.54-1.49) | 0.658 |
| Preparation | ||||
| % feel very well informed | 84 | 79 | 0.70 (0.39-1.27) | 0.239 |
| % feel totally prepared | 85 | 75 | 0.55 (0.31-0.99) | 0.045* |
| % need much more information | 20 | 30 | 1.70 (0.99-2.91) | 0.051* |
| Satisfaction with donation decision | ||||
| % extremely satisfied with decision | 90 | 91 | 1.05 (0.48-2.30) | 0.894 |
| % would definitely volunteer again | 92 | 91 | 0.80 (0.35-1.82) | 0.590 |
| Clinical | ||||
| Weight (mean, sd) | 88.12 kg (18.64) | 85.07 kg (17.33) | 1.52 | 0.128 |
| Height (mean, sd) | 174.48 cm (9.15) | 173.92 cm (10.26) | 0.52 | 0.607 |
p ≤ .05;
p ≤ .01;
p ≤ .001
response range
The most prevalent concerns were that the procedure would be painful and that the donor's family would worry (>50% of donors for each concern). Groups differed marginally on concern about potential damage to the donors' health (BM=26% versus PBSC=39%). There were no group differences in health concerns.
Although the majority of donors felt well informed (81%) and fully prepared (80%), PBSC donors felt marginally less prepared (BM=85% versus PBSC=75%) and were marginally more likely to indicate that they needed more information (BM=20% versus PBSC=30%). More than 90% of donors were satisfied with the decision to donate and would volunteer again – there were no group differences in satisfaction.
48 hours post-donation
There were no differences in AE or SAE by donor type (Table 3). BM donors reported lower overall physical health at this time-point. The most commonly reported donation-related symptoms were tiredness and muscle aches and BM donors were significantly more likely to report experiencing multiple current symptoms. Table 3 reports pain for PBSC donors on day 4 of rhGCSF administration and for BM donors within 48 hours post-donation as these are the time points when the two groups are likely to experience the most pain. BM donors' worst pain was marginally higher than that of PBSC donors but the two groups did not differ on other pain indicators. BM donors reported better overall psychological status. There were no other group differences in psychological status.
Table 3.
48 hours post-donation comparison of marrow and PBSC donors.
| Study Variable | Marrow (n=131) |
PBSC (n=142) |
Test Statistic (t or odds ratio) |
p-value |
|---|---|---|---|---|
| Physical Status | ||||
| Overall physical status | ||||
| SF-8 physical health (mean, sd) (29-68)† | 35.38 (9.75) | 43.37 (9.75) | -6.59 | 0.000*** |
| Current physical symptoms | ||||
| % tiredness | 92 | 92 | 0.98 (0.40-2.40) | 0.972 |
| % muscle aches | 75 | 56 | 0.42 (0.25-0.71) | 0.421 |
| % problems sleeping | 50 | 39 | 0.66 (0.41-1.07) | 0.091 |
| % bone pain | 58 | 60 | 1.09 (0.67-1.77) | 0.717 |
| % light headedness | 50 | 34 | 0.50 (0.31-0.82) | 0.006** |
| % pain where needles inserted | 83 | 55 | 0.25 (0.14-0.44) | 0.000*** |
| % difficulty walking | 87 | 39 | 0.10 (0.05-0.18) | 0.000*** |
| % bleeding | 41 | 10 | 0.16 (0.08-0.30) | 0.000*** |
| % nausea or vomiting | 28 | 25 | 0.86 (0.51-1.48) | 0.590 |
| % infection | 0 | 0 | -- | -- |
| % chills | 20 | 9 | 0.41 (0.20-0.83) | 0.012* |
| % fainting | 3 | 0 | 0.47 (0.42-0.54) | 0.036* |
| Current pain1 | ||||
| pain index (mean, sd) (0-10) | 4.23 (1.86) | 3.84(1.94) | 1.68 | 0.094 |
| average pain (mean, sd) (0-10) | 4.04 (1.98) | 3.63(2.00) | 1.69 | 0.093 |
| worst pain (mean, sd) (0-10) | 5.55 (2.45) | 4.80(2.26) | 2.60 | 0.009** |
| time with pain (mean, sd) (0-10) | 4.77 (2.42) | 4.57(2.58) | 0.66 | 0.513 |
| interfered with sleep (mean, sd) (0-10) | 2.53 (2.40) | 2.35(2.34) | 0.62 | 0.535 |
| Psychological Status | ||||
| Overall psychological status | ||||
| SF-8 mental health (mean, sd) (28-69) | 55.40 (7.24) | 52.23 (6.24) | 3.85 | 0.000*** |
| Profile of mood states (POMS) | ||||
| depression (mean, sd) (0-4) | 0.10 (0.42) | 0.12 (0.24) | -0.61 | 0.541 |
| vitality (mean, sd) (0-4) | 1.23 (0.93) | 1.46 (0.88) | -2.12 | 0.035* |
| confusion (mean, sd) (0-4) | 0.26 (0.69) | 0.25 (0.42) | 0.13 | 0.900 |
| tension (mean, sd) (0-4) | 0.46 (0.69) | 0.47 (0.51) | -0.19 | 0.848 |
| anger (mean, sd) (0-4) | 0.12 (0.48) | 0.14 (0.25) | -0.50 | 0.615 |
| fatigue (mean, sd) (0-4) | 1.31 (1.02) | 1.23 (0.91) | 0.69 | 0.491 |
| total distress (mean, sd) (0-24) | 5.01 (2.75) | 4.75 (2.28) | 0.85 | 0.396 |
| Donation-related | ||||
| Donation experience | ||||
| % donation physically stressful | 76 | 75 | 0.91 (0.53-1.59) | 0.746 |
| % donation emotionally stressful | 55 | 58 | 1.12 (0.69-1.81) | 0.643 |
| Health concerns | ||||
| % worry about longer-term health impact of donation | 55 | 58 | 1.10 (0.68-1.78) | 0.695 |
| % worry will never feel physically 100% again | 27 | 32 | 1.30 (0.75-2.13) | 0.389 |
| Social impact | ||||
| % leisure/recreation affected | 87 | 51 | 6.46 (3.52-11.86) | 0.000*** |
| % work/school affected | 75 | 70 | 1.29 (0.75-2.20) | 0.359 |
| % transportation arrangements | 58 | 47 | 1.55 (0.96-2.50) | 0.073 |
| % child care arrangements | 28 | 16 | 2.04 (1.13-3.66) | 0.016* |
| % financial arrangements | 18 | 13 | 1.55 (0.80-3.00) | 0.197 |
| % home care help | 7 | 4 | 1.67 (0.58-4.83) | 0.338 |
| Satisfaction with donation decision | ||||
| % extremely satisfied with decision | 92 | 92 | 0.90 (0.37-2.15) | 0.804 |
| % would definitely donate again | 79 | 78 | 0.94 (0.53-1.68) | 0.843 |
| % would strongly encourage others | 82 | 82 | 1.00 (0.53-1.86) | 0.992 |
| % feel a lot like a better person | 76 | 76 | 1.02 (0.58-1.78) | 0.958 |
| % feel very proud | 63 | 65 | 1.06 (0.65-1.75) | 0.806 |
| % made life much more meaningful | 48 | 35 | 0.58 (0.35-0.94) | 0.026* |
| Recipient-related | ||||
| % know health status of recipient | 7 | 10 | 0.67 (0.28-1.60) | 0.365 |
| % think about recipient ≥ once/day | 79 | 78 | 0.92 (0.52-1.65) | 0.784 |
| % have special bond with recipient | 82 | 79 | 0.80 (0.43-1.46) | 0.457 |
| % very/pretty worried about recipient | 66 | 61 | 0.80 (0.49-1.32) | 0.385 |
| Clinical | ||||
| % adverse event | 15 | 16 | 1.12 (0.61-2.06) | 0.705 |
| % serious adverse event | 6 | 2 | 0.30 (0.08-1.13) | 0.061 |
p ≤ .05;
p ≤ .01;
p ≤ .001
response range
For PBSC donors, pain was assessed on the 4th day of rhGCSF administration. For BM donors, pain was assessed 48hours post-donation.
Two-thirds of donors reported that the donation was physically stressful and ∼60% reported that the donation was emotionally stressful. More than half were worried about longer-term health effects and approximately one-third were worried that they might never feel physically 100%. There were no group differences in these variables.
The most frequently endorsed social impacts of donation were effects on leisure/recreation and work/school. BM donors reported more impact on leisure/recreation activities and a marginally greater need to make child care arrangements. There were no other group differences in donation inconveniences.
The majority of donors were satisfied with the donation decision and there were no group differences in these variables. BM donors were marginally more likely to report that donation had made their lives more meaningful (BM=48% versus PBSC=35%).
Weekly assessments for the first 3 weeks post-donation
BM donors reported significantly more symptoms (p≤ 0.001) in each of the first three weeks following donation. Four key symptoms – pain at needle insertion sites, bone pain, muscle aches and difficulty walking – were reported by BM donors at significantly higher levels (p≤0.001) across each of the first three weeks post-donation (data not shown). Tiredness, lightheadedness, nausea, problems sleeping and chills were reported by BM donors at marginally higher rates (p≤0.05) in the first week following donation but these differences disappeared by the second week post-donation.
6 months post-donation
There were no group differences in overall physical or psychological status (Table 4). The most common current symptoms were tiredness (45%) and muscle aches (22%). The only marginally significant symptom differences were lightheadedness (BM=6% versus PBSC=1%) and pain where the needles entered (BM=6% versus PBSC=1%).
Table 4.
6 months post-donation comparison of marrow and PBSC donors.
| Study Variable | Marrow (n=141) |
PBSC (n=153) |
Test Statistic (t or odds ratio) |
p-value |
|---|---|---|---|---|
| Physical Status | ||||
| Overall physical status | ||||
| SF-8 physical health (mean, sd) (29-68)† | 65.18 (4.51) | 64.96 (3.85) | 0.45 | 0.651 |
| Current physical symptoms | ||||
| % tiredness | 42 | 48 | 1.30 (0.82-2.06) | 0.262 |
| % muscle aches | 24 | 20 | 0.80 (0.46-1.39) | 0.427 |
| % problems sleeping | 10 | 15 | 1.61 (0.79-3.26) | 0.187 |
| % bone pain | 9 | 5 | 0.54 (0.22-1.35) | 0.184 |
| % light headedness | 6 | 1 | 0.22 (0.05-1.06) | 0.039* |
| % pain where needles inserted | 6 | 1 | 0.12 (0.01-0.89) | 0.013* |
| % difficulty walking | 5 | 5 | 0.92 (0.31-2.69) | 0.876 |
| % bleeding | 1 | 2 | 2.80 (0.29-27.23) | 0.355 |
| % nausea or vomiting | 1 | 3 | 1.87 (0.34-10.35) | 0.469 |
| % infection | 0 | 2 | 0.52 (0.46-0.58) | 0.095 |
| % chills | 0 | 1 | 0.52 (0.47-0.58) | 0.336 |
| % fainting | 0 | 0 | - | - |
| Current pain | ||||
| pain index (mean, sd) (0-10) | 0.60 (1.38) | 0.67 (1.23) | -0.44 | 0.663 |
| average pain (mean, sd) (0-10) | 0.57 (1.38) | 0.61 (1.17) | -0.25 | 0.802 |
| worst pain (mean, sd) (0-10) | 0.87 (1.83) | 0.95 (1.64) | -0.41 | 0.685 |
| time with pain (mean, sd) (0-10) | 0.69 (1.58) | 0.82 (1.66) | -0.68 | 0.500 |
| interfered with sleep (mean, sd) (0-10) | 0.29 (1.12) | 0.31 (0.94) | -0.15 | 0.879 |
| Psychological Status | ||||
| Overall psychological status | ||||
| SF-8 mental health (mean, sd) (28-69) | 65.49 (5.81) | 65.03 (5.98) | 0.66 | 0.510 |
| Profile of mood states (POMS) | ||||
| depression (mean, sd) (0-4) | 0.10 (0.27) | 0.11 (0.25) | -0.37 | 0.716 |
| vitality (mean, sd) (0-4) | 2.16 (1.01) | 2.22 (0.98) | -0.59 | 0.555 |
| confusion (mean, sd) (0-4) | 0.26 (0.42) | 0.30 (0.80) | -0.44 | 0.660 |
| tension (mean, sd) (0-4) | 0.31 (0.45) | 0.36 (0.83) | -0.57 | 0.566 |
| anger (mean, sd) (0-4) | 0.19 (0.37) | 0.22 (0.78) | -0.42 | 0.676 |
| fatigue (mean, sd) (0-4) | 0.45 (0.63) | 0.23 (0.95) | -0.81 | 0.417 |
| total distress (mean, sd) (0-24) | 3.15 (2.24) | 3.10 (2.01) | 0.22 | 0.826 |
| Donation-related | ||||
| Donation experience | ||||
| % donation physically stressful | 74 | 75 | 1.04 (0.62-1.75) | 0.883 |
| % donation emotionally stressful | 63 | 69 | 1.28 (0.79-2.07) | 0.319 |
| Health concerns | ||||
| % worry about longer-term health impact of donation | 41 | 51 | 1.51 (0.95-2.40) | 0.81 |
| % worry will never feel physically 100% again | 14 | 9 | 0.65 (0.31-1.35) | 0.241 |
| Satisfaction with donation decision | ||||
| % extremely satisfied with decision | 92 | 88 | 0.70 (0.32-1.51) | 0.357 |
| % would defiitely donate again | 87 | 84 | 0.78 (0.40-1.51) | 0.461 |
| % would strongly encourage others | 82 | 78 | 0.79 (0.44-1.41) | 0.426 |
| % feel a lot like a better person | 75 | 75 | 0.97 (0.57-1.64) | 0.895 |
| % feel very proud | 56 | 62 | 1.27 (0.80-2.03) | 0.312 |
| % made life much more meaningful | 31 | 28 | 0.86 (0.52-1.42) | 0.561 |
| Recipient-related | ||||
| % know health status of recipient | 39 | 41 | 0.93 (0.58-1.48) | 0.756 |
| % think about recipient ≥ once/day | 16 | 14 | 0.85 (0.45-1.63) | 0.631 |
| % have special bond with recipient | 83 | 84 | 1.09 (0.59-2.03) | 0.776 |
| % very/pretty worried about recipient | 35 | 31 | 0.87 (0.53-1.41) | 0.563 |
| Recovery | ||||
| % recovered at 6 months | 81 | 82 | 1.11 (0.63-1.95) | 0.720 |
p ≤ .05;
p ≤ .01;
p ≤ .001
response range
Forty percent of donors knew the health status of their recipient and 84% said that they had a special bond with the recipient. Fifteen percent of donors indicated that they thought about the recipient more than once each day and 33% were worried about the recipient's health. There were no group differences in recipient-related variables.
Eighty-one percent of donors had reached three consecutive symptom-free weeks by 6 months post donation and there were no group differences in recovery.
12 months post-donation
PBSC donors had marginally better physical health status at this time-point than did BM donors (Table 5). However, this was due to 3 extreme outliers (SF-8 physical mean < 40) in the BM group. When we compared the distributions with extreme outliers removed (t = -1.28; df = 279; p = 0.20) and with Kruskal-Wallis nonparametric tests (p = 0.29) there was no significant difference in overall physical health. All three BM donors with very low physical status scores at 12 months post-donation were female aged 32-46. One donor had experienced an SAE but was fully recovered (defined as 3 symptom-free weeks) by 6 months post-donation. Both that donor and a second donor had higher SF-8 physical health summary scores at 6 months post-donation than at pre-donation and then declines between 6 and 12 months post-donation. There was no obvious link between donation and the lower physical scores at 12 months post-donation. The third donor reported ongoing pain where the needles entered, difficulty walking, problems sleeping, bone pain and muscle aches throughout the 52 week follow-up period and was still reporting symptoms at 12 months. This donor had consistently poorer physical health scores at 6 and 12 months post-donation.
Table 5.
12 months post-donation comparison of marrow and PBSC donors.
| Study Variable | Marrow (n=137) |
PBSC (n=151) |
Test Statistic (t or odds ratio) |
p-value |
|---|---|---|---|---|
| Physical Status | ||||
| Overall physical status | ||||
| SF-8 physical health (mean, sd) (29-68)† | 63.95 (6.41) | 65.21 (3.91) | -2.03 | .043* |
| Current physical symptoms | ||||
| % tiredness | 53 | 56 | 1.16 (0.73-1.85) | 0.525 |
| % muscle aches | 25 | 22 | 0.85 (0.49-1.46) | 0.552 |
| % problems sleeping | 19 | 21 | 1.15 (0.64-2.05) | 0.640 |
| % bone pain | 12 | 9 | 0.72 (0.34-1.53) | 0.391 |
| % light headedness | 4 | 7 | 1.87 (0.62-5.62) | 0.257 |
| % pain where needles inserted | 5 | 0 | 0.99 (0.96-1.01) | 0.742 |
| % difficulty walking | 11 | 5 | 0.46 (0.19-1.11) | 0.077 |
| % bleeding | 2 | 0 | 0.47 (0.42-0.53) | 0.136 |
| % nausea or vomiting | 4 | 4 | 1.09 (0.33-3.66) | 0.886 |
| % infection | 4 | 3 | 0.90 (0.26-3.19) | 0.876 |
| % chills | 2 | 2 | 0.91 (0.18-4.56) | 0.904 |
| % fainting | 1 | 0 | 0.48 (0.42-0.54) | 0.293 |
| Current pain | ||||
| pain index (mean, sd) (0-10) | 0.97 (1.72) | 0.88 (1.40) | 0.48 | 0.632 |
| average pain (mean, sd) (0-10) | 0.86 (1.60) | 0.84 (1.43) | 0.11 | 0.910 |
| worst pain (mean, sd) (0-10) | 1.24 (2.08) | 1.28 (2.01) | -0.18 | 0.855 |
| time with pain (mean, sd) (0-10) | 1.19 (2.12) | 1.01 (1.72) | 0.81 | 0.420 |
| interfered with sleep (mean, sd) (0-10) | 0.59 (1.64) | 0.40 (1.16) | 1.17 | 0.245 |
| Psychological Status | ||||
| Overall psychological status | ||||
| SF-8 mental health (mean, sd) (28-69) | 64.94 (6.16) | 64.86 (6.18) | 0.11 | 0.916 |
| Profile of mood states (POMS) | ||||
| depression (mean, sd) (0-4) | 0.11 (0.28) | 0.11 (0.29) | 0.11 | 0.912 |
| vitality (mean, sd) (0-4) | 2.19 (0.99) | 2.19 (0.86) | 0.02 | 0.986 |
| confusion (mean, sd) (0-4) | 0.28 (0.40) | 0.25 (0.39) | 0.61 | 0.545 |
| tension (mean, sd) (0-4) | 0.36 (0.45) | 0.34 (0.48) | 0.22 | 0.828 |
| anger (mean, sd) (0-4) | 0.19 (0.32) | 0.18 (0.36) | 0.40 | 0.691 |
| fatigue (mean, sd) (0-4) | 0.51 (0.71) | 0.55 (0.71) | -0.45 | 0.657 |
| total distress (mean, sd) (0-24) | 3.26 (2.38) | 3.24 (2.27) | 0.08 | 0.940 |
| Donation-related | ||||
| Donation experience | ||||
| % donation physically stressful | 66 | 68 | 1.05 (0.64-1.72) | 0.839 |
| % donation emotionally stressful | 60 | 58 | 0.94 (0.59-1.50) | 0.786 |
| Health concerns | ||||
| % worry about longer-term health impact of donation | 43 | 50 | 1.30 (0.82-2.08) | 0.264 |
| % worry will never feel physically 100% again | 17 | 10 | 0.55 (0.27-1.10) | 0.086 |
| Satisfaction with donation decision | ||||
| % extremely satisfied with decision | 91 | 91 | 1.03 (0.45-2.34) | 0.949 |
| % would definitely donate again | 88 | 85 | 0.74 (0.37-1.46) | 0.379 |
| % would strongly encourage others | 79 | 82 | 1.23 (0.69-2.21) | 0.481 |
| % feel a lot like a better person | 72 | 71 | 0.94 (0.56-1.58) | 0.823 |
| % feel very proud | 66 | 58 | 0.71 (0.44-1.14) | 0.155 |
| % made life much more meaningful | 31 | 29 | 0.93 (0.56-1.54) | 0.779 |
| Recipient-related | ||||
| % know health status of recipient | 43 | 40 | 1.14 (.71-1.82) | 0.598 |
| % think about recipient ≥ once/day | 10 | 11 | 1.05 (0.49-2.24) | 0.901 |
| % have special bond with recipient | 78 | 77 | 0.96 (0.55-1.67) | 0.876 |
| % very/pretty worried about recipient | 34 | 26 | 0.69 (0.41-1.16) | 0.160 |
| Recovery | ||||
| % recovered at 12 months | 87 | 87 | 0.95 (0.50-1.82) | 0.876 |
p ≤ .05;
p ≤ .01;
p ≤ .001
response range
Overall, the most common symptoms were tiredness (55% of all donors) and muscle aches (23% of all donors). There were no significant symptom differences. There were no group differences in overall psychological status or distress. Nearly half of donors remained concerned about the longer-term health effects of donation and 13% worried that they might never feel physically 100%. There were no group differences in these variables.
The majority of donors were extremely satisfied with their decision to donate (91%), would donate again if asked (86%), and would encourage others to donate (81%). There were no group differences in satisfaction variables.
Forty-one percent of donors knew the health status of their recipient. Eleven percent of donors indicated that they thought about the recipient more than once per day, 77% reported that they had a special bond with the recipient and 30% were worried about the recipient's health. There were no group differences in recipient-related variables.
Eighty-seven percent of donors had reached three consecutive symptom-free weeks by 12 months post-donation and there were no group differences in recovery. Among the group still reporting weekly symptoms at 12 months post donation, the most commonly reported symptoms across the entire post-donation period were tiredness (∼85% of the group consistently reported this symptom), muscle aches (∼50%), and problems sleeping (∼40%).
Longitudinal comparisons of physical/mental status and recovery
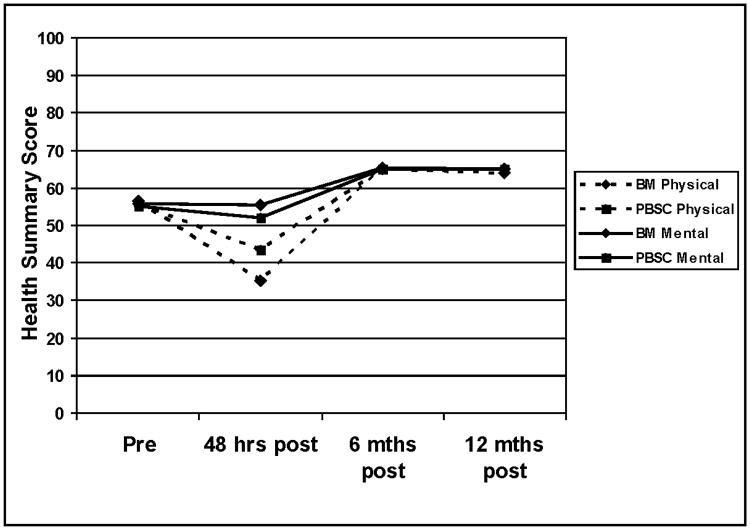
Mean values for the physical and mental status components of the SF-8 health survey are presented in Figure 1. Mixed models analyses indicated that there were main effect differences for donation type (Physical F=30.2; df=1, 589, p<.001; Mental F=8.9; df=1, 1,058, p=.003) and time (Physical F=678.8; df=3, 643, p<.001; Mental F=291.7; df=3, 539, p<.001). In addition, there were significant donation type by time interactions for both physical and mental status (Physical F=15.6; df=3, 643, p<.001; Mental F=3.2; df=3, 539, p=.023). Both groups experienced declines in physical status immediately following donation and then scores higher than baseline levels at 6 and 12 months. At 48 hours post-donation, BM donors reported poorer physical status and PBSC donors reported poorer mental status. Figure 2 presents the Kaplan Meier recovery curves for BM and PBSC donors. There was no significant group difference in recovery (Log Rank Chi-squared = 1.85; p = 0.18).
Figure 1.
Longitudinal pre- and post-donation physical and mental status for BM and PBSC donors. Physical and mental health summary scores were assessed with the SF-8; higher scores indicate better physical or mental health.
Figure 2.
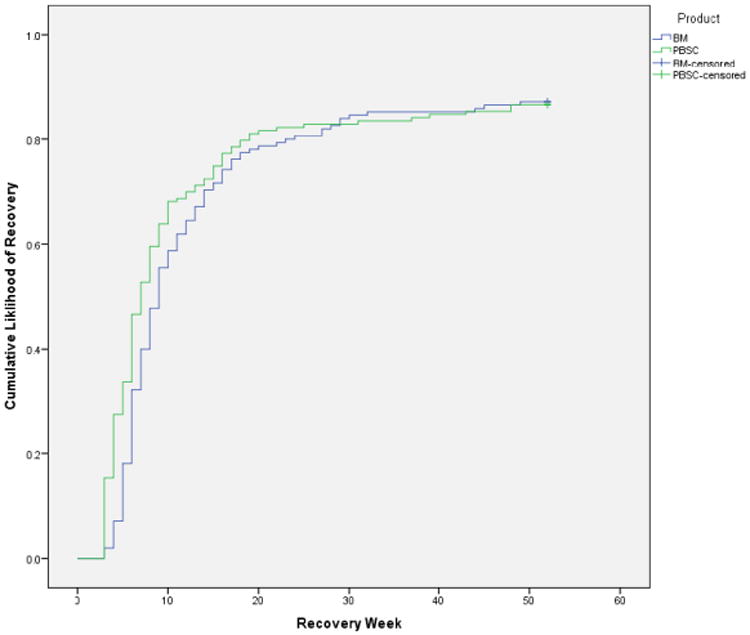
Time to full recovery by product donated.
Discussion
This investigation is one of the first to systematically and comprehensively examine HRQoL experiences of BM and PBSC donors in the context of a Phase III randomized trial. A central finding of this investigation is that there do not appear to be longer-term differences in the HRQoL experiences of the vast majority of BM and PBSC donors.
The pattern of findings at pre-donation indicate that BM donors are slightly advantaged over PBSC donors in terms of experiencing lower levels of confusion, fewer concerns about the donation procedure, and higher levels of preparedness for donation. Existing literature is mixed on whether there are pre-donation psychological and preparedness differences between BM and PBSC donors, but when such differences have been found – e.g., in pre-donation anxiety – they have also favored BM donors.22,23 It is possible that donor center staff were more thorough in preparing BM donors or that BM donors were more likely to seek out donation-related information on their own, given the more invasive nature of this HSC collection procedure. It is also possible that the administration of rhGCSF for which the longer-term risks are likely to be low but at the time of this investigation had not been fully examined in healthy donors may have heightened health-related concerns in the PBSC group. Regardless of the source of these concerns among PBSC donors, a review and potential revision of the pre-donation counseling process for PBSC donors may be warranted to ensure that all donors are fully informed about the donation and recovery process.
Findings from 48 hours post-donation indicate that BM donors had experienced more physical side effects and pain within the past 48 hours although the pain profiles of BM donors at 48 hours post-donation and PBSC donors on day 4 of rhGCSF administration were generally similar. BM donors also reported that the donation had a greater impact on their social activities. Despite these findings, BM donors were more likely to report better psychological status and that the donation had made their lives more meaningful at this time point. This finding may be due to the greater physical intensity of the BM donation process leading to more positive psychological outcomes shortly after donation. It is also possible that those close to BM donors may have viewed the process as entailing greater risk to the donor and may therefore have been more engaged with and congratulatory of the donor. Differences in physical side effects persisted for at least 3 weeks post-donation, with BM donors experiencing significantly greater adverse effects in these areas than PBSC donors.
There were few longer-term differences in the experiences of the two donor groups. At 6 months post-donation, BM donors were slightly more likely to report continued lightheadedness and pain where the needles entered than were PBSC donors and at 12 months, BM donors reported marginally poorer physical status. However, the former results are not statistically significant after correcting for multiple comparisons, and the latter was entirely due to three outliers in the BM group with very low physical status scores. For two of these outliers, there was no apparent link between donation and the lower 12 month physical scores. The third donor reported ongoing symptoms in addition to lower 6 and 12 month physical scores.
Nearly half of donors overall had at least some lingering concerns about the longer-term health impact of donation at 12 months post-donation. It is possible that this is a natural side-effect of any important non-required medical procedure, but it suggests that pre-donation information sessions and post-donation follow-up could better emphasize the very low probability that BM or PBSC donation will have longer-term health effects. In addition, in concordance with recommendations from the World Marrow Donor Association about longer-term donor follow-up, these residual health concerns should be the subject of ongoing assessment beyond the first year post-donation. 24
Longitudinal group differences in physical and psychological status were the result of declines in physical status – and psychological status for PBSC donors – immediately following donation, but then gains to levels above pre-donation levels at 6 and 12 months post-donation. Longitudinal group by time physical and psychological status interactions were primarily due to 48 hour post-donation differences when BM donors reported poorer physical status but better psychological status than did PBSC donors. There was no recovery time difference between the two groups as assessed starting 3 weeks post-donation, the first point at which a donor could be recorded as fully recovered.
It is interesting that approximately 13% of donors had not met our definition of recovery by 12 months post-donation. This is likely partly a result of our conservative definition of recovery – 3 consecutive symptom-free weeks and assessment of all symptoms regardless of whether they were attributable to donation – but may also indicate that there are other interesting characteristics about this subgroup. The most commonly reported symptoms by this group were fatigue, muscle aches, and problems sleeping which are less likely to be an ongoing result of donation than some other symptoms. We are currently analyzing predictors of recovery – including pre-donation physical and psychological status – for a separate manuscript. Other investigations that asked donors specifically about their recovery from the donation process – rather than reporting all symptoms regardless of whether they are attributable to donation – find that the majority of donors report feeling recovered within 4 weeks and virtually all report feeling recovered by 6 months post-donation. 25
Overall, these findings suggest that although BM donors may experience the process as more physically uncomfortable in the short-term, the physical impact of donation resolves relatively quickly and there are few longer-term differences, in aggregate, between the two groups. Conversely, BM donors may experience greater short-term psychological gains from donation – particularly in terms of feeling like the donation made their lives more meaningful – but there are no longer-term differences between the two groups in terms of psychological benefit.
A key limitation of the investigation is that donors were primarily white, and all were required to be able to write, read and speak English. Given the randomized study design and inclusion criteria, this was expected. However, it may limit generalizability to broader groups of donors. In addition, all participants were unrelated donors belonging to the NMDP registry. Their characteristics – young, self-selected, knowledgeable, healthy, and highly motivated – may make it difficult to extrapolate these findings to other groups of donors – particularly related donors. We are currently conducting an NIH-funded investigation that will compare the HRQoL experiences of donors who are related to the recipient and those who are unrelated.
Despite these limitations, this investigation improves our understanding of HRQoL issues involved in the HSC donation process, provides a stringent comparison of these two methods of donation, and indicates that the longer-term HRQoL consequences of BM and PBSC donation are similar in the vast majority of donors.
Key Points.
BM donation produces greater short term physical impact.
Few longer-term HRQoL differences between BM and PBSC donors.
Acknowledgments
Supported by a grant from the National Heart, Lung, and Blood Institute and the National Cancer Institute (U10HL069294), by the Office of Naval Research, and by the National Marrow Donor Program.
Footnotes
Contributions to the Donor Quality of Life ancillary study were made by The Health Resources and Services Administration Contract No. HHSH234200637020C. The authors of this publication alone are responsible for reporting and interpreting the data and all or part of the data used to compile this publication were collected pursuant to a contract with the Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transportation.
Authorship Contributions: All authors participated in the design of the research, selection of the analytic strategy, and manuscript preparation. JGB, and DH collected and managed data at the University of Pittsburgh, assisted with data analysis and contributed to the conceptual organization of the manuscript. RD, AF, and DC facilitated and managed sampling and participant recruitment at the NMDP. All authors interpreted data and reviewed and approved the final manuscript.
Conflict of Interest Disclosures: There are no conflicts to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Favre G, Beksac M, Bacigalupo A, et al. Differences between graft product and donor side effects following bone marrow or stem cell donation. Bone Marrow Transplantation. 2003;32:873–880. doi: 10.1038/sj.bmt.1704245. [DOI] [PubMed] [Google Scholar]
- 2.Halter J, Kodera Y, Ispizua A, et al. Severe events in donors after allogeneic hematopoietic stem cell donation. Haematologica. 2009;94(1):94–101. doi: 10.3324/haematol.13668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bolan CD, Hartzman RJ, Perry EH, et al. Donation activities and product integrity in unrelated donor allogeneic hematopoietic transplantation: experience of the National Marrow Donor Program. Biol Blood Marrow Transplant. 2008;14:23–28. doi: 10.1016/j.bbmt.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Holig K, Kramer M, Kroschinsky F, et al. Safety and efficacy of hematopoietic stem cell collection from mobilized peripheral blood in unrelated volunteers: 12 years of a single-center experience in 3928 donors. Blood. 2009;114:3757–3763. doi: 10.1182/blood-2009-04-218651. [DOI] [PubMed] [Google Scholar]
- 5.Pulsipher MA, Chitphakdithai P, Miller JP, et al. Adverse events among 2408 unrelated donors of peripheral blood stem cells: results of a prospective trial from the National Marrow Donor Program. Blood. 2009;113(15):3604–3611. doi: 10.1182/blood-2008-08-175323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rinaldi C, Savignano C, Pasca S, et al. Efficacy and safety of peripheral blood stem cell mobilization and collection: a single-center experience in 190 allogeneic donors. Transfusion. 2012;52(11):2387–2394. doi: 10.1111/j.1537-2995.2012.03619.x. [DOI] [PubMed] [Google Scholar]
- 7.Miller JP, Perry EH, Price TH, et al. Recovery and safety profiles of marrow and PBSC donors: experience of the National Marrow Donor Program. Biol Blood Marrow Transplant. 2008;14:29–36. doi: 10.1016/j.bbmt.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 8.National Marrow Donor Program data. www.marrow.org.
- 9.Rowley SD, Donaldson G, Lilleby K, et al. Experiences of donors enrolled in a randomized study of allogeneic bone marrow or peripheral blood stem cell transplantation. Blood. 2001;97:2541–2548. doi: 10.1182/blood.v97.9.2541. [DOI] [PubMed] [Google Scholar]
- 10.Karlsson L, Quinlan D, Guo D, et al. Mobilized blood cells vs bone marrow harvest: experience compared in 171 donors with particular reference to pain and fatigue. Bone Marrow Transplantation. 2004;33:709–713. doi: 10.1038/sj.bmt.1704418. [DOI] [PubMed] [Google Scholar]
- 11.Switzer GE, Goycoolea JM, Dew MA, et al. Donating stimulated peripheral blood stem cells vs bone marrow: do donors experience the procedures differently? Bone Marrow Transplantation. 2001;27:917–923. doi: 10.1038/sj.bmt.1703011. [DOI] [PubMed] [Google Scholar]
- 12.Auguier P, Macquart-Moulin G, Moatti JP, et al. Comparisons of anxiety, pain and discomfort in two procedures of hematopoietic stem cell collection: leukacytapheresis and bone marrow harvest. Bone Marrow Transplantation. 1995;16:541–547. [PubMed] [Google Scholar]
- 13.Anasetti C, Logan BR, Lee SJ, et al. Peripheral blood stem cells versus bone marrow from unrelated donors: results of Blood and Marrow Transplant Clinical Trials Network protocol 0201, a phase II, prosepective randomized trial. N Engl J Med. 2012;367(16):1487–1496. doi: 10.1056/NEJMoa1203517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ware JE, Kosinski M, Dewey JE, Gandek B. How to Score and Interpret Single-Item Health Status Measures: A Manual for Users of the SF-8™ Health Survey. Lincoln, RI: QualityMetric Incorporated; 2001. [Google Scholar]
- 15.Shacham S. A shortened version of the Profile of Mood States. J Pers Assess. 1983;47(3):305–306. doi: 10.1207/s15327752jpa4703_14. [DOI] [PubMed] [Google Scholar]
- 16.Curran SL, Andrykowski MA, Studts JL. Short form of the Profile of Mood States (POMS-SF): psychometric information. Psychol Assess. 1995;7(1):80–83. [Google Scholar]
- 17.Switzer GE, Simmons RG, Dew MA. Helping unrelated strangers: physical and psychological reactions to the bone marrow donation process among anonymous donors. J Appl Soc Psychol. 1996;26(6):469–490. [Google Scholar]
- 18.Switzer GE, Dew MA, Goycoolea JM, et al. Attrition of potential bone marrow donors at two key decision points leading to donation. Transplantation. 2004;77(10):1529–1534. doi: 10.1097/01.tp.0000122219.35928.d6. [DOI] [PubMed] [Google Scholar]
- 19.Switzer GE, Myaskovsky L, Goycoolea JM, et al. Factors associated with ambivalence about bone marrow donation among newly recruited unrelated potential donors. Transplantation. 2003;75(9):1517–1523. doi: 10.1097/01.TP.0000060251.40758.98. [DOI] [PubMed] [Google Scholar]
- 20.Switzer GE, Dew MA, Harrington DJ, et al. Ethnic differences in donation-related characteristics among potential hematopoietic stem cell donors. Transplantation. 2005;80(7):890–896. doi: 10.1097/01.tp.0000173648.60978.30. [DOI] [PubMed] [Google Scholar]
- 21.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 22.Bredeson C, Leger C, Couban S, et al. An evaluation of the donor experience in the Canadian multicenter randomized trial of bone marrow versus peripheral blood allografting. Biology of Blood and Marrow Transplantation. 2004;10:405–414. doi: 10.1016/j.bbmt.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Fortanier C, Kuentz M, Sutton L, et al. Healthy sibling donor anxiety and pain during bone marrow or peripheral blood stem cell harvesting for allogeneic transplantation: results of a randomised study. Bone Marrow Transplantation. 2002;29:145–149. doi: 10.1038/sj.bmt.1703338. [DOI] [PubMed] [Google Scholar]
- 24.Shaw BE, Ball L, Beksac M, et al. Donor safety: the role of the WMDA in ensuring the safety of volunteer unrelated donors: clinical and ethical considerations. Bone Marrow Transplantation. 2010;45:832–838. doi: 10.1038/bmt.2010.2. [DOI] [PubMed] [Google Scholar]
- 25.Pulsipher MA, Chitphakdithai P, Logan BR, et al. Acute toxicities of unrelated bone marrow versus peripheral blood stem cell donation: results of a prospective trial from the National Marrow Donor Program. Blood. 2013;121(1):197–206. doi: 10.1182/blood-2012-03-417667. [DOI] [PMC free article] [PubMed] [Google Scholar]