Abstract
Specialised chromatin in which canonical histone H3 is replaced by CENP-A, an H3 related protein, is a signature of active centromeres and provides the foundation for kinetochore assembly. The location of centromeres is not fixed since centromeres can be inactivated and new centromeres can arise at novel locations independently of specific DNA sequence elements. Therefore, the establishment and maintenance of CENP-A chromatin and kinetochores provide an exquisite example of genuine epigenetic regulation. The composition of CENP-A nucleosomes is contentious but several studies suggest that, like regular H3 particles, they are octamers. Recent analyses have provided insight into how CENP-A is recognised and propagated, identified roles for post-translational modifications and dissected how CENP-A recruits other centromere proteins to mediate kinetochore assembly.
Current Opinion in Cell Biology 2014, 26:41–50
This review comes from a themed issue on Cell architecture
Edited by Sue Biggins and Matthew D Welch
For a complete overview see the Issue and the Editorial
Available online 19th October 2013
0955-0674/$ – see front matter, © 2013 The Authors. Published by Elsevier Ltd. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/3.0/).
“Chaos is merely order waiting to be deciphered” — José de Sousa Saramago
Introduction
Centromeres are the sites at which the machinery, collectively known as the kinetochore, required to accurately segregate chromosomes is assembled. Following replication, the resulting sister-kinetochores on each sister-chromatid ensure that one chromatid from each chromosome is transmitted to each daughter nucleus. Kinetochores are an amalgamation of integrated functional modules: they include devices which ensure that sister-chromatids remain associated at centromeres (cohesion) [1], and sensors (the spindle assembly checkpoint) for detecting when all sister-kinetochores have attached to microtubules anchored at opposite spindle poles (bi-orientation). Once all sister-kinetochores are bi-oriented, this sensor throws a switch allowing the release of sister-centromeres and their separation into two new nuclei [2–4]. This separation and movement to opposite poles are mediated by the attachment of each kinetochore to microtubules utilising another apparatus that binds directly to microtubules [1,4–6].
The integration of these modules into a single unit allows the presence of an unattached kinetochore to be sensed and transduced via a signalling cascade that ultimately prevents the release of the tethers between all sister-centromeres and thereby halts the movement of chromosomes and the completion of both nuclear and cellular division until the problem is resolved. Gain or loss of chromosomes (aneuploidy) is one step on the path to forming cancerous cells [2–4,7]. Kinetochores therefore provide exquisite accuracy to the process of chromosome segregation so that over the course of thousands of cell division few detrimental chromosome segregation errors occur.
This view of the kinetochore as a highly honed and accurate piece of cellular engineering seems incompatible with the seemingly haphazard processes that in many organisms govern where kinetochores are assembled on chromosomes. Each chromosome must only assemble a single kinetochore, chromosomes with two kinetochores are intrinsically unstable (an exception being holocentric chromosomes). An effective way to ensure the assembly of only one kinetochore per chromosome would be to couple kinetochore assembly to a unique DNA sequence. Indeed, at budding yeast (Saccharomyces cerevisiae) centromeres, which have contributed greatly to our knowledge of centromere–kinetochore structure and function, specific centromere proteins bind to a DNA sequence motif which in turn ensures kinetochore assembly at that location [8]. Single base changes in a key centromere DNA element, or mutation of its DNA binding proteins, obliterate kinetochore assembly. This seems a completely logical template for centromere specification. However, in organisms with complex regional centromeres, the processes involved in centromere placement appear much more anarchic. In all organisms, the main driver of centromere specification appears to be the assembly of specialised chromatin containing the histone H3 variant generally known as CENP-A and called as CID (Drosophila), Cse4 (S. cerevisiae) and Cnp1 (Schizosaccharomyces pombe). The structure of CENP-A nucleosomes, their post-translational modifications (PTMs), the timing and mechanism of their deposition may all influence where kinetochores are assembled. Here we discuss recent developments that contribute to our understanding of CENP-A chromatin in addition to where and how it seeds kinetochore assembly.
Inducing new centromere formation
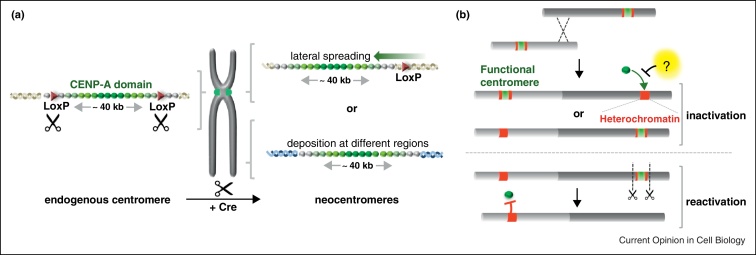
The inactivation of one centromere on dicentric chromosomes and the appearance of new centromeres at novel locations support the view that the establishment and propagation of centromeres are epigenetically regulated.
Regional centromeres in most organisms are restricted to the same single locus on each chromosome. These regions usually contain arrays of repetitive elements, such as alpha satellite repeat arrays at human centromeres, with divergent but related repeats found at each centromere [9], which may represent a preferred substrate directed by DNA binding factors (e.g. CENP-B) [10]. Thus DNA elements are involved in genetically specifying centromere placement; however, some regional centromeres are associated with unique DNA sequences. The lack of obvious common features suggests that epigenetic mechanisms direct formation of these centromeres. For example, new centromeres are formed on regions of horse, orang-utan and potato chromosomes that contain no satellite repeats [11–13]. Analyses of CENP-A distribution in chicken DT40 cells have shown that two of the 10 macro-chromosomes (Chr 5 and Z) lack repetitive DNA and display single CENP-A peaks that occupy ∼30 kb [14]. The induction of neocentromeres, as demonstrated in S. pombe and Candida albicans [15,16], has recently been applied in DT40 cells (Figure 1). Cre-induced deletion of 127 kb containing the Z centromere generated neocentromeres in 126 surviving colonies that retained the Z chromosome [17••]. This large number of novel neocentromeres can potentially be used to identify common features at these chromosomal locations that may promote CENP-A incorporation. Eighteen neocentromeres that formed on distinct Z chromosome sequences were further characterised. CENP-A peaks were confined to 35–47 kb regions, with no preference for the presence of repetitive elements. Most neocentromeres arose in regions flanking the original Z centromere, suggesting that a low level of CENP-A, resident in these regions at the time of centromere deletion, may seed new centromere formation. Examination of several induced neocentromeres in Candida also demonstrates that they frequently arise in close proximity to the original centromere [16,18]. Neocentromeres attract most centromere/kinetochore proteins (an exception being the satellite DNA-binding protein CENP-B) and allow efficient chromosome segregation. However, one human neocentromere was shown to inefficiently correct unsuitable spindle attachments, thus neocentromeres may not confer the same level of accuracy in segregation as natural centromeres [19].
Figure 1.
Establishment and propagation of centromeres are epigenetically regulated. (a) Centromere repositioning results in neocentromere formation at ectopic loci. In DT-40 cells, Cre-induced deletion of the centromere from chromosome Z generated surviving colonies able to retain chromosome Z and possessing neocentromeres. The majority of the characterised neocentromeres arose in region flanking the original chromosome Z suggesting that a low level of CENP-A may function to seed neocentromere formation. The same lateral spreading was also described in Candida. The remaining neocentromeres are localised on different regions of chromosome Z, suggesting that the original centromere is not unique in being able to attract CENP-A and assemble kinetochores. At each neocentromere, CENP-A occupies a ∼40 kb region, similar in size to original centromere. (b) In fission yeast, forced fusion of non-homologous chromosomes leads to formation of a dicentric chromosome. In some surviving cells, dicentric chromosomes are converted into monocentric chromosomes by deletion of one of the centromeres. In survivors in which DNA rearrangements have not occurred, the one of the centromeres has become inactivated and is coated in H3K9me-dependent heterochromatin. Although heterochromatin is associated with the inactive centromere, it is not required for centromere inactivation and other unknown mechanisms may be involved. However, heterochromatin does prevent reactivation of the inactive centromere, leading to neocentromere formation when the functional centromere on a dicentric is deleted.
Analyses in S. pombe and Drosophila indicate that heterochromatin influences the establishment of CENP-A chromatin and functional kinetochores [20–22]; however, H3K9 methylation, the key mark associated with heterochromatin, was not enriched at normal non-repetitive DT40 cell centromeres or induced neocentromeres. In Caenorhabditis elegans, CENP-A is deposited on chromosomal regions not transcribed in the germline and the lack of heterochromatin proteins (HP1) does not affect de novo centromere assembly on injected plasmids, suggesting that heterochromatin is not required [23,24]. Fission yeast heterochromatin acts as a platform to recruit many activities including several HDACs, chromatin remodelers, replication initiators and DNA repair proteins [25]. The concentration of associated activities may be responsible for promoting CENP-ACnp1 rather than heterochromatin itself. Such activities may be found elsewhere on chromosomes, without standard heterochromatin features, and alone may be sufficient to promote CENP-A assembly. Further analyses of multiple neocentromeres, generated on the same genetic background, should illuminate how CENP-A assembly is triggered at new chromosomal locations.
Preventing centromere formation
The formation of neocentromeres shows that the normal centromere locus on a chromosome is not unique in being able to attract CENP-A and assemble kinetochores. In addition, mechanisms exist that inactivate centromeres causing centromere protein loss, without affecting the DNA sequence itself, and that also prevent their reactivation [9]. Centromere inactivation has recently been shown to occur in fission yeast following the forced recombination between two non-homologous chromosomes to induce dicentric chromosome formation (Figure 1) [26••]. The dicentric state was deleterious but a proportion of surviving cells retained the dicentric chromosome with both centromere regions intact. However, CENP-ACnp1 was found to be lost from either centromere, and retained at the other. The CENP-ACnp1 negative centromere was not pulled to the spindle pole in anaphase, which is consistent with centromere inactivation. More survivors arose when dicentric formation was forced in cells with a defective kinetochore component, suggesting that kinetochore disassembly promotes centromere inactivation. Domains of H3K9me-dependent heterochromatin flank the central kinetochore domain at fission yeast centromeres. Heterochromatin was found to engulf the central domain at inactivated centromeres and prevent subsequent centromere reactivation. Surprisingly, however, heterochromatin itself is not required for centromere inactivation, but prevents the reactivation of dormant centromeres. Thus, when coated in heterochromatin, intact centromeric chromatin is epigenetically silenced and rendered unrecognisable so that it is unable to direct kinetochore assembly. The targeting of H3K9 methylation and heterochromatin to human α-satellite repeats also inhibits de novo CENP-A and kinetochore assembly [27]. However, heterochromatin also promotes de novo CENP-A and kinetochore assembly on naïve DNA templates in fission yeast [20,21]. The demarcation of heterochromatin domains relative to CENP-A domains, and their interplay, must influence whether nearby heterochromatin promotes or prevents CENP-A and kinetochore assembly. The details of how centromeres are inactivated and how heterochromatin prevents CENP-A deposition are currently unknown.
Directing CENP-A and kinetochore assembly
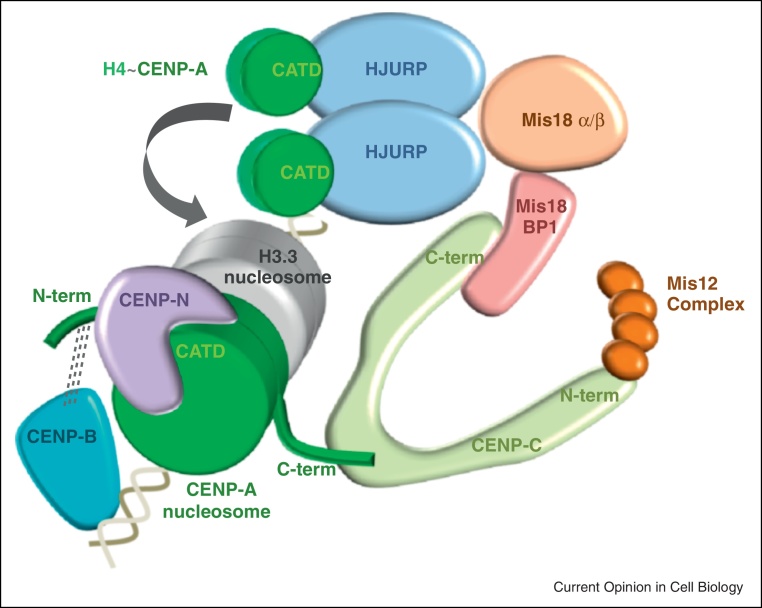
In vertebrate cells CENP-A is deposited at centromeres in early G1, independently of replication [28]. Deposition of CENP-A is dependent on its chaperone, HJURP [29,30]. HJURP associates with soluble CENP-A and is transiently recruited to centromeres in early G1. Recruitment of HJURP is dependent on the Mis18 Complex (Mis18α/β/Mis18BP1), which arrives at centromeres before HJURP in telophase [31•,32]. The centromere localisation of Mis18BP1 is inhibited by Cdk1/Cdk2-mediated phosphorylation from S phase to anaphase [33•]. CENP-C has been shown to discriminate CENP-A from H3 nucleosomes by binding directly to the distinct C-terminus of CENP-A and docking via acidic patches on histones H2A and H2B [34•,35]. CENP-N recognises CENP-A nucleosomes via the CENP-A targeting domain (CATD) within the histone-fold domain (HFD), even when transplanted into H3, and acts in conjunction with CENP-C to recruit other kinetochore components [36,37]. CENP-C directly binds Mis18BP1 and Mis18BP1/Mis18α/β is required to recruit HJURP to centromeres [32,38]. In fission yeast, Mis18 directly interacts with Scm3HJURP [39]. Interactions such as these create a loop where resident CENP-A recruits the chaperones that mediate the deposition of new CENP-A (Figure 2). During S phase, resident CENP-A at human and Drosophila centromeres is distributed equally to sister-centromeres, each inherits half the amount originally present at the fully replenished parental centromere [28,40•]. Where CENP-A is lost, the resulting gaps are perhaps temporarily filled by deposition of H3.3 as a placeholder in S phase until its replacement with CENP-A in G1 [41]. HJURP has been shown to self-dimerise, which may enable it to deposit two new CENP-A/H4 heterodimers, thus allowing CENP-A nucleosome assembly in place of H3.3 nucleosomes in G1 [42].
Figure 2.
CENP-A recognition and propagation at regional centromeres. CENP-C binds directly to the C-terminus of CENP-A in nucleosomes. The C-terminus of CENP-C recruits the Mis18 complex through Mis18BP1 (also known as Knl2). During replication the CENP-A nucleosomes are distributed equally to each sister-centromere so that CENP-A levels are halved and histone H3.3 is deposited as a placeholder. Free CENP-A/H4 heterodimers associate with a homodimer of HJURP which is recruited to centromeres via the Mis18 complex in telophase, allowing replacement of H3.3 with CENP-A in G1. Once assembled, the CATD within the HFD of CENP-A nuclesomes is recognised by CENP-N allowing recruitment of many other constitutive kinetochore components including the CENP-T/W/S/X complex (not shown). The CENP-B protein is known to bind directly to centromere repeats in mammals but is stabilised via the N-terminus of CENP-A and contributes to kinetochore integrity. The N-terminus of CENP-C associates with the Mis12 complex (see Figure 3). For simplicity interactions and nomenclature are only shown for vertebrate proteins.
Use of a human a cell line harbouring a conditional null allele of CENP-A demonstrated that one third of CENP-C was retained even when CENP-A was reduced to minimal levels (∼1%) and high levels of other kinetochore proteins persisted until CENP-A was undetectable [43••]. This suggests that kinetochore proteins can stabilise their own platform without CENP-A and only a small proportion of the normal amount of centromeric CENP-A is required to provide kinetochore function. These cells also allowed rigorous in vivo dissection of the distinct recognition modules within CENP-A and emphasise their two distinct roles in CENP-A propagation at a specific location and the assembly of kinetochores at that site. The CATD allows HJURP-mediated G1 deposition of CENP-A into, and its propagation in, distinct nucleosome particles. The N-termini and C-termini of CENP-A were found to be redundant with respect to promoting kinetochore assembly with CENP-C recruited by the C-terminus. CENP-B was shown to play a hitherto unrecognised role in kinetochore integrity by interactions via the N-terminus of CENP-A [43••]. This may relate to the finding that α-satellite DNA that binds CENP-B is a preferred substrate for de novo CENP-A and kinetochore assembly [10].
In Drosophila cultured cells, newly synthesised CENP-ACID is incorporated at centromeres at metaphase [40•]. In the somatic tissues of flies, the incorporation of new CENP-ACID at centromeres occurs from late telophase to early G1 [44•]. Drosophila lacks an HJURP ortholog but Cal1 performs the equivalent function and is recruited to centromeres via CENP-C [40•]. During meiosis, CENP-ACID is replenished twice, once during prophase of MI and also in spermatids following the completion of MII. Both Cal1 and CENP-C are required for CENP-ACID assembly at centromeres during meiosis. In mature Drosophila sperm most histones are replaced with protamines; however, as in vertebrates, CENP-ACID is retained in sperm chromatin. The retention of CENP-A on sperm chromatin is required to ensure that kinetochores are assembled and that their location is preserved following fertilisation [44•,45•].
Misguiding CENP-A assembly
To determine if CENP-A alone is sufficient to direct kinetochore assembly, CENP-A, or its chaperone HJURP, has been artificially tethered to DNA. In Drosophila S2 cells, tethering of LacI-CENP-ACID to LacO arrays inserted on a chromosome arm recruits kinetochore components and mediates association with microtubule fibres [46••]. Untethered endogenous CENP-ACID is also recruited to the tethering site and kinetochores persist after the initiating tethered LacI-CENP-ACID is removed. Moreover, tethered CENP-ACID conferred segregation function and mitotic stability to episomal plasmids. In human cells, tethering of HJURP to LacO sites also promoted the deposition of CENP-A and kinetochore assembly at an ectopic locus [31•]. These experiments demonstrate that CENP-A is indeed sufficient to specify centromeres and allow their propagation at that location.
Experimental overexpression of CENP-A may aid the identification of chromosomal features that promote its incorporation. Increased expression of CENP-ACID in Drosophila cells showed that CENP-ACID has a tendency to accumulate in proximity to heterochromatin [22]. In fission yeast, expression of additional CENP-ACnp1 led to its accumulation close to heterochromatic telomeres, where neocentromeres are known to form [47•]. Telomere repeats themselves are sufficient to direct CENP-ACnp1 incorporation nearby [48]. Defective transcription-coupled chromatin reassembly allows CENP-ACnp1 accumulation on transcription units where H3 loss is greatest and also facilitates de novo deposition of CENP-ACnp1 on fission yeast centromeric DNA, which is known to be transcribed [49]. Thus, alterations in the process of chromatin assembly during transcription can destabilise H3 nucleosomes and thereby allow CENP-ACnp1 to assemble in its place [47•]. Interestingly, in mice with reduced levels of H3.3, which normally replaces canonical H3 in transcribed genes, CENP-A is deposited broadly over the genome [50]. Such findings suggest that transcription-coupled modification and remodelling events can influence the incorporation of CENP-A.
CENP-A chromatin distinction
Canonical nucleosomes are octamers that contain two subunits each of the core histones H2A, H2B, H3 and H4. The substitution of H3 with CENP-A alone should be sufficient to allow recognition of these specialised nuclesomes. Indeed, structural analyses demonstrate that octameric CENP-A nucleosomes assembled in vitro are overall very similar to canonical H3 nucleosomes. One difference is that the αN-helical domain in CENP-A is shorter than that of H3, so less DNA is bound near the entry and exit sites [51•]. Consistent with this octameric structure, human centromeric CENP-A nucleosomes extracted from cells protect less DNA than H3 nucleosomes [52•]. AFM measurement of CENP-A particles extracted from Drosophila and human cells revealed that they have a reduced height relative to H3 nucleosomes and their height changes during the cell cycle [53,54,55•]. This height difference is central to the proposal that CENP-A particles are hemisomes (half-nucleosomes) rather than octamers. However, in vitro assembled octameric CENP-A nucleosomes also report a lower height relative to H3 nucleosomes [56•]. Thus, rather than indicating a different stoichiometry, the difference in height detected by AFM appears to be an intrinsic property of octameric CENP-A nucleosomes. Other analyses show that Drosophila CENP-ACID can be cross-linked as dimers in vivo, demonstrating that CENP-ACID nucleosomes contain two rather than one subunit of CENP-ACID [57•]. Moreover, counting the number of CENP-A signals in single nucleosomes released from human cellular chromatin using TIRF revealed the presence of two CENP-A subunits in the majority of particles [58•]. Thus the major difference in composition between CENP-A and H3 nucleosomes appears to be the replacement of both H3 subunits with CENP-A. As discussed above, the N-terminal and C-terminal regions, along with the CATD, mediate specific interactions that distinguish CENP-A from H3 nucleosomes.
Post-translational modifications on CENP-A
Histones are subject to a slew of PTMs that regulate the binding of specific proteins. Unlike H3, the N-terminal region of CENP-A is highly variable in sequence and length. Several CENP-A PTMs have been identified. S. cerevisae CENP-ACse4 was shown to be methylated on Arg37, acetylated on Lys49 and phosphorylated on Ser22, Ser33, Ser40 and Ser105 [59]. An Arg37Ala mutation in CENP-ACse4 resulted in reduced association of specific kinetochore components with centromeres [60]. Phosphorylation is mediated by Ipl1, the Aurora B kinase, and mutation of all 4 residues suggests a role for CENP-ACse4 phosphorylation in regulating sister-kinetochore bi-orientation [59]. Phosphorylation of human CENP-A on Ser7 by Aurora kinase plays a role in kinetochore function and cytokinesis [61]. Recent analyses suggest that any phosphorylation within the N-terminus of CENP-A (S7 of CENP-A or S10/S28 from H3 N-termini are sufficient) is required during mitosis to stabilise the CENP-C recruitment via phospho-binding 14-3-3 proteins [62]. Like other histones, the initiating methionine of human CENP-A is removed post-translationally, so that Gly1 is the first residue in nucleosomal CENP-A and is trimethylated on its primary amine [63•]. Simultaneous phosphorylation of both Ser16 and Ser18 was also detected at high levels on peptides that carried the Gly1me3 modification. The phosphorylation of Ser16 and Ser18 appears to affect kinetochore integrity. How this phosphorylation relates to Serine-7 phosphorylation and 14-3-3 recruitment is not known.
Other histone-related proteins at centromeres
Apart from CENP-A, four conserved histone-fold fold proteins (CENP-T/-W/-S/-X known as Cnn1/Wip1/Mhf1/Mhf2 in S. cerevisiae) have been shown to reside at vertebrate centromeres. CENP-T interacts directly with CENP-W and together these four proteins form a CENP-T/W/S/X heterotetrameric complex via their HFDs that assembles on DNA in a manner reminiscent of histone tetramers [64•]. Analyses of specific disruptive mutants in DT40 cells demonstrate that the formation of this tetramer is required to allow kinetochore assembly. CENP-T/W/S/X is therefore a second DNA binding module that operates alongside CENP-A to recruit kinetochore components. CENP-S and CENP-X are also known to associate with FANCM, a helicase that blocks cross-over formation as a result of homologous recombination by reversing D-loops [65,66]. FANCM might also be recruited to centromeres by CENP-S/-X where it could suppress potentially deleterious recombination events at centromeres, particularly those involving repetitive elements.
Bypassing CENP-A
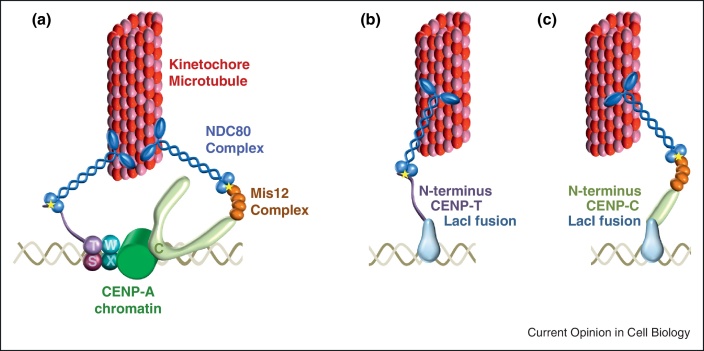
Kinetochores are essentially an elaborate ‘towbar’ that mediates and regulates the connection between microtubules and chromosomal DNA. Thus, the means of connecting microtubules to DNA may not be particularly important; provided the modules that bind DNA are linked to modules that can attach to microtubules, perhaps chromosome segregation can occur. A conserved N-terminal motif, several hundred residues from the HFD of CENP-T/Cnn1, directly associates with the Spc24/25 end of the NDC80 microtubule binding complex (Figure 3) [67•,68•]. Direct tethering of TetR-Cnn1 or LacI-CENP-T fusion proteins to TetO/LacO arrays can mediate chromosome segregation without CENP-A in human, DT40 and S. cerevisiae cells, but not when this NDC80 interaction motif is deleted [69••,70••]. Thus this N-terminal motif in CENP-T/Cnn1 plays a conserved role in connecting DNA to microtubules via the NDC80 complex. The NDC80 complex is also anchored at kinetochores through CENP-A via CENP-C and its interaction with the Mis12 complex (Mis12/Nnf1/Dsn1/Nsl1/) [71]. The Nsl1 component directly interacts with Spc24/25 of the NDC80 complex to allow kinetochore–microtubule interactions [72]. Consequently, tethering the N-terminal region of CENP-C (which associates directly the Mis12 complex, but does not recruit CENP-A) to LacO arrays also allows chromosome segregation, in the absence of CENP-A, in DT40 cells [69••]. Interestingly, Drosophila and C. elegans lack CENP-T along with other kinetochore proteins [73] and thus rely on the CENP-C/Mis12/Ndc80 pathway to connect with microtubules [74].
Figure 3.
Two pathways connect centromere DNA to kinetochore microtubules via CENP-A. (a) The four HFD proteins in the CENP-T/W/S/X complex form a heterotetramer that is recruited via other constitutive kinetochore components, such as CENP-N that binds CENP-A nucleosomes (not shown — see Figure 2). An extended structure in CENP-T separates the HFD near the C-terminus from the N-terminus. The N-terminus of CENP-T contains a short motif that directly associates with the RWD motif formed by Spc24/Spc25 of the NDC80 complex (star). CENP-C binds CENP-A nucleosomes and its N-terminus recruits the Mis12 complex. The Nsl1 subunit of the Mis12 complex directly associates with the RWD motif formed by Spc24/Spc25 of the NDC80 complex (star). The opposite end of NDC80 complex binds directly to microtubules. Fusion of the N-terminus of CENP-T (b), or the N-terminus of CENP-C (c), to LacI allows their artificial recruitment to arrays of LacO (LacI binding sites) at a non-centromeric locus where they can connect with microtubules and mediated chromosome segregation in the absence of CENP-A nucleosomes. The N-terminus of Cnn1, the S. cerevisiae ortholog of CENP-T, is also sufficient to mediate chromosomes segregation when tethered to plasmid DNA. For simplicity interactions and nomenclature are only shown for vertebrate proteins.
The tethering experiments discussed above demonstrate that the need for CENP-A chromatin can be bypassed when an alternative way of connecting kinetochore components to DNA is provided. It is therefore perhaps surprising that CENP-A has persisted through evolution as the major DNA binding unit at centromeres in eukaryotes. The continued use of a system that allows the epigenetic regulation of CENP-A deposition and plasticity in the placement of centromeres may have an important evolutionary role in permitting the survival of novel chromosome arrangements that ultimately may drive speciation. For organisms with genetically determined, DNA sequence dependent, centromeres, it is possible that the wrapping of centromeric DNA around specialised nucleosomes is required to resist the pulling forces exerted on centromeres during chromosome segregation and consequently CENP-A has been preserved. Intriguingly, so far no CENP-A related protein is evident within the sequenced genomes of kinetoplastids such as Trypanasoma, which occupy an ancient evolutionary niche [75]. Perhaps nature has also found ways of building kinetochores that do not involve CENP-A or chromatin and that connect with DNA more directly.
Conclusion
The identification of components that recognise different parts of CENP-A in nucleosomes, and consequently lead to the assembly of functional kinetochores has identified pivotal connections between CENP-A and constitutive kinetochore proteins. Tethering experiments have allowed the identification and dissection of components that link centromeric DNA with microtubules. It remains to be determined exactly how specific loading factors mediate the replication-independent deposition of CENP-A. The development of systems that promote neocentromere formation and centromere inactivation will continue to provide insights into the mechanisms that activate and repress centromeres. The features in chromatin that initiate de novo CENP-A deposition at specific chromosomal locations have yet to be identified.
“You need chaos in your soul to give birth to a dancing star” — Friedrich Nietzsche
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
We thank Alison Pidoux and Laxkmi Subramanian for comments on the manuscript. The Wellcome Trust supported the work of RCA [095021] and SC [086574]. Work in the Wellcome Trust Centre for Cell Biology is supported by the Wellcome Trust core funding [092076]. RCA is a Wellcome Trust Principal Research Fellow.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
References
- 1.Nasmyth K. Cohesin: a catenase with separate entry and exit gates? Nat Cell Biol. 2011;13:1170–1177. doi: 10.1038/ncb2349. [DOI] [PubMed] [Google Scholar]
- 2.Vleugel M., Hoogendoorn E., Snel B., Kops G.J.P.L. Evolution and function of the mitotic checkpoint. Dev Cell. 2012;23:239–250. doi: 10.1016/j.devcel.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 3.Lara-Gonzalez P., Westhorpe F.G., Taylor S.S. The spindle assembly checkpoint. Curr Biol. 2012;22:R966–R980. doi: 10.1016/j.cub.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Foley E.A., Kapoor T.M. Microtubule attachment and spindle assembly checkpoint signalling at the kinetochore. Nat Rev Mol Cell Biol. 2013;14:25–37. doi: 10.1038/nrm3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheeseman I.M., Desai A. Molecular architecture of the kinetochore–microtubule interface. Nat Rev Mol Cell Biol. 2008;9:33–46. doi: 10.1038/nrm2310. [DOI] [PubMed] [Google Scholar]
- 6.DeLuca J.G., Musacchio A. Structural organization of the kinetochore–microtubule interface. Curr Opin Cell Biol. 2012;24:48–56. doi: 10.1016/j.ceb.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pfau S.J., Amon A. Chromosomal instability and aneuploidy in cancer: from yeast to man. EMBO Rep. 2012;13:515–527. doi: 10.1038/embor.2012.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Westermann S., Drubin D.G., Barnes G. Structures and functions of yeast kinetochore complexes. Annu Rev Biochem. 2007;76:563–591. doi: 10.1146/annurev.biochem.76.052705.160607. [DOI] [PubMed] [Google Scholar]
- 9.Kalitsis P., Choo K.H.A. The evolutionary life cycle of the resilient centromere. Chromosoma. 2012;121:327–340. doi: 10.1007/s00412-012-0369-6. [DOI] [PubMed] [Google Scholar]
- 10.Okada T., Ohzeki J.-I., Nakano M., Yoda K., Brinkley W.R., Larionov V., Masumoto H. CENP-B controls centromere formation depending on the chromatin context. Cell. 2007;131:1287–1300. doi: 10.1016/j.cell.2007.10.045. [DOI] [PubMed] [Google Scholar]
- 11.Wade C.M., Giulotto E., Sigurdsson S., Zoli M., Gnerre S., Imsland F., Lear T.L., Adelson D.L., Bailey E., Bellone R.R. Genome sequence, comparative analysis, and population genetics of the domestic horse. Science. 2009;326:865–867. doi: 10.1126/science.1178158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Locke D.P., Hillier L.W., Warren W.C., Worley K.C., Nazareth L.V., Muzny D.M., Yang S.-P., Wang Z., Chinwalla A.T., Minx P. Comparative and demographic analysis of orang-utan genomes. Nature. 2011;469:529–533. doi: 10.1038/nature09687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gong Z., Wu Y., Koblízková A., Torres G.A., Wang K., Iovene M., Neumann P., Zhang W., Novák P., Buell C.R. Repeatless and repeat-based centromeres in potato: implications for centromere evolution. Plant Cell. 2012;24:3559–3574. doi: 10.1105/tpc.112.100511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shang W.-H., Hori T., Toyoda A., Kato J., Popendorf K., Sakakibara Y., Fujiyama A., Fukagawa T. Chickens possess centromeres with both extended tandem repeats and short non-tandem-repetitive sequences. Genome Res. 2010;20:1219–1228. doi: 10.1101/gr.106245.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishii K., Ogiyama Y., Chikashige Y., Soejima S., Masuda F., Kakuma T., Hiraoka Y., Takahashi K. Heterochromatin integrity affects chromosome reorganization after centromere dysfunction. Science. 2008;321:1088–1091. doi: 10.1126/science.1158699. [DOI] [PubMed] [Google Scholar]
- 16.Ketel C., Wang H.S.W., McClellan M., Bouchonville K., Selmecki A., Lahav T., Gerami-Nejad M., Berman J. Neocentromeres form efficiently at multiple possible loci in Candida albicans. PLoS Genet. 2009;5:e1000400. doi: 10.1371/journal.pgen.1000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang W.-H., Hori T., Martins N.M.C., Toyoda A., Misu S., Monma N., Hiratani I., Maeshima K., Ikeo K., Fujiyama A. Chromosome engineering allows the efficient isolation of vertebrate neocentromeres. Dev Cell. 2013;24:635–648. doi: 10.1016/j.devcel.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]; The formation of neocentromeres in chicken cells is analysed. Upon deletion of the endogenous centromere from Chromosome Z survivors are generated that possess neocentromeres located at different loci on chromosome Z. These neocentromeres are characterised using ChIP-seq analysis for CENP-A and histone modifications. Interestingly, low levels of CENP-A surrounding the endogenous centromere seem to be capable of seeding neocentromere formation.
- 18.Thakur J., Sanyal K. Efficient neocentromere formation is suppressed by gene conversion to maintain centromere function at native physical chromosomal loci in Candida albicans. Genome Res. 2013;23:638–652. doi: 10.1101/gr.141614.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bassett E.A., Wood S., Salimian K.J., Ajith S., Foltz D.R., Black B.E. Epigenetic centromere specification directs aurora B accumulation but is insufficient to efficiently correct mitotic errors. J Cell Biol. 2010;190:177–185. doi: 10.1083/jcb.201001035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Folco H.D., Pidoux A.L., Urano T., Allshire R.C. Heterochromatin and RNAi are required to establish CENP-A chromatin at centromeres. Science. 2008;319:94–97. doi: 10.1126/science.1150944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kagansky A., Folco H.D., Almeida R., Pidoux A.L., Boukaba A., Simmer F., Urano T., Hamilton G.L., Allshire R.C. Synthetic heterochromatin bypasses RNAi and centromeric repeats to establish functional centromeres. Science. 2009;324:1716–1719. doi: 10.1126/science.1172026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olszak A.M., van Essen D., Pereira A.J., Diehl S., Manke T., Maiato H., Saccani S., Heun P. Heterochromatin boundaries are hotspots for de novo kinetochore formation. Nat Cell Biol. 2011;13:799–808. doi: 10.1038/ncb2272. [DOI] [PubMed] [Google Scholar]
- 23.Gassmann R., Rechtsteiner A., Yuen K.W., Muroyama A., Egelhofer T., Gaydos L., Barron F., Maddox P., Essex A., Monen J. An inverse relationship to germline transcription defines centromeric chromatin in C. elegans. Nature. 2012;484 doi: 10.1038/nature10973. 534-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuen K.W.Y., Nabeshima K., Oegema K., Desai A. Rapid de novo centromere formation occurs independently of heterochromatin protein 1 in C. elegans embryos. Curr Biol. 2011;21:1800–1807. doi: 10.1016/j.cub.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grewal S.I. RNAi-dependent formation of heterochromatin and its diverse functions. Curr Opin Genet Dev. 2010;20:134–141. doi: 10.1016/j.gde.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H., Masuda F., Takayama Y., Takahashi K., Saitoh S. Epigenetic inactivation and subsequent heterochromatinization of a centromere stabilize dicentric chromosomes. Curr Biol. 2012;22:658–667. doi: 10.1016/j.cub.2012.02.062. [DOI] [PubMed] [Google Scholar]; The inactivation and reactivation of centromeres on a dicentric chromosome in S. pombe is demonstrated. Inactivation of a centromere is accompanied by the loss of CENP-ACnp1 and establishment of H3K9me heterochromatin. Although heterochromatin is not required for centromere inactivation, it is formed over the inactive centromere and prevents the reactivation of the inactive centromere.
- 27.Ohzeki J.-I., Bergmann J.H., Kouprina N., Noskov V.N., Nakano M., Kimura H., Earnshaw W.C., Larionov V., Masumoto H. Breaking the HAC barrier: histone H3K9 acetyl/methyl balance regulates CENP-A assembly. EMBO J. 2012;31:2391–2402. doi: 10.1038/emboj.2012.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jansen L.E.T., Black B.E., Foltz D.R., Cleveland D.W. Propagation of centromeric chromatin requires exit from mitosis. J Cell Biol. 2007;176:795–805. doi: 10.1083/jcb.200701066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foltz D.R., Jansen L.E.T., Bailey A.O., Yates J.R., Bassett E.A., Wood S., Black B.E., Cleveland D.W. Centromere-specific assembly of CENP-a nucleosomes is mediated by HJURP. Cell. 2009;137:472–484. doi: 10.1016/j.cell.2009.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dunleavy E.M., Roche D., Tagami H., Lacoste N., Ray-Gallet D., Nakamura Y., Daigo Y., Nakatani Y., Almouzni-Pettinotti G. HJURP is a cell-cycle-dependent maintenance and deposition factor of CENP-A at centromeres. Cell. 2009;137:485–497. doi: 10.1016/j.cell.2009.02.040. [DOI] [PubMed] [Google Scholar]
- Barnhart M.C., Kuich P.H.J.L., Stellfox M.E., Ward J.A., Bassett E.A., Black B.E., Foltz D.R. HJURP is a CENP-A chromatin assembly factor sufficient to form a functional de novo kinetochore. J Cell Biol. 2011;194:229–243. doi: 10.1083/jcb.201012017. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that tethering the CENP-A chaperone HJURP to an ectopic locus in a human cell line is sufficient to establish functional kinetochores. Tethering of LacI-HJURP to a LacO array inserted on a chromosome arm leads to the assembly of CENP-A chromatin and recruitment of other centromere specific proteins. HJURP recruitment is dependent on Mis18. In vitro reconstitution of CENP-A nucleosomes shows that an N-terminal fragment of HJURP is able to induce deposition of CENP-A nucleosomes.
- 32.Moree B., Meyer C.B., Fuller C.J., Straight A.F. CENP-C recruits M18BP1 to centromeres to promote CENP-A chromatin assembly. J Cell Biol. 2011;194:855–871. doi: 10.1083/jcb.201106079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva M.C.C., Bodor D.L., Stellfox M.E., Martins N.M.C., Hochegger H., Foltz D.R., Jansen L.E.T. Cdk activity couples epigenetic centromere inheritance to cell cycle progression. Dev Cell. 2011;22:52–63. doi: 10.1016/j.devcel.2011.10.014. [DOI] [PubMed] [Google Scholar]; The loading machinery for CENP-A in human cells is shown to be kept inactive by CDK activity during the cell cycle. CENP-A incorporation at centromeres initiates during metaphase and requires degradation of cyclin A. The cell cycle-dependent phosphorylation of Mis18BP1KNL2 plays a key role in the control of the timing of CENP-A assembly.
- Guse A., Carroll C.W., Moree B., Fuller C.J., Straight A.F. In vitro centromere and kinetochore assembly on defined chromatin templates. Nature. 2011;477:354–358. doi: 10.1038/nature10379. [DOI] [PMC free article] [PubMed] [Google Scholar]; An in vitro reconstitution system is utilised to investigate the contributions of different domains of CENP-A to kinetochore assembly. The authors show that just the C-terminal of CENP-A is both necessary and sufficient for centromere reconstitution and it is required for the recruitment of the kinetochore protein CENP-C.
- 35.Kato H., Jiang J., Zhou B.-R., Rozendaal M., Feng H., Ghirlando R., Xiao T.S., Straight A.F., Bai Y. A conserved mechanism for centromeric nucleosome recognition by centromere protein CENP-C. Science. 2013;340:1110–1113. doi: 10.1126/science.1235532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carroll C.W., Silva M.C.C., Godek K.M., Jansen L.E.T., Straight A.F. Centromere assembly requires the direct recognition of CENP-A nucleosomes by CENP-N. Nat Cell Biol. 2009;11:896–902. doi: 10.1038/ncb1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carroll C.W., Milks K.J., Straight A.F. Dual recognition of CENP-A nucleosomes is required for centromere assembly. J Cell Biol. 2010;189:1143–1155. doi: 10.1083/jcb.201001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dambacher S., Deng W., Hahn M., Sadic D., Fröhlich J., Nuber A., Hoischen C., Diekmann S., Leonhardt H., Schotta G. CENP-C facilitates the recruitment of M18BP1 to centromeric chromatin. Nucleus. 2012;3:101–110. doi: 10.4161/nucl.18955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pidoux A.L., Choi E.S., Abbott J.K.R., Liu X., Kagansky A., Castillo A.G., Hamilton G.L., Richardson W., Rappsilber J., He X. Fission yeast Scm3: a CENP-A receptor required for integrity of subkinetochore chromatin. Mol Cell. 2009;33:299–311. doi: 10.1016/j.molcel.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellone B.G., Grive K.J., Shteyn V., Bowers S.R., Oderberg I., Karpen G.H. Assembly of Drosophila centromeric chromatin proteins during mitosis. PLoS Genet. 2011;7:e1002068. doi: 10.1371/journal.pgen.1002068. [DOI] [PMC free article] [PubMed] [Google Scholar]; The cell cycle dependency of CENP-ACID assembly into chromatin is analysed in Drosophila cells. Pulse-chase experiments show that CENP-ACID and CENP-C are equally distributed to daughter cells whereas CAL1 possess higher turnover. Newly synthesised CENP-ACID is deposited at centromere during metaphase. CAL1 is recruited to centromeres just before CENP-ACID and physically interacts with pre-nucleosomal CENP-ACID. CENP-C is distributed during interphase and mitosis.
- 41.Dunleavy E.M., Almouzni G., Karpen G.H. H3.3 is deposited at centromeres in S phase as a placeholder for newly assembled CENP-A in G1 phase. Nucleus. 2011;2:146–157. doi: 10.4161/nucl.2.2.15211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zasadzińska E., Barnhart-Dailey M.C., Kuich P.H.J.L., Foltz D.R. Dimerization of the CENP-A assembly factor HJURP is required for centromeric nucleosome deposition. EMBO J. 2013;32:2113–2124. doi: 10.1038/emboj.2013.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fachinetti D., Diego Folco H., Nechemia-Arbely Y., Valente L.P., Nguyen K., Wong A.J., Zhu Q., Holland A.J., Desai A., Jansen L.E.T. A two-step mechanism for epigenetic specification of centromere identity and function. Nat Cell Biol. 2013;15:1056–1066. doi: 10.1038/ncb2805. [DOI] [PMC free article] [PubMed] [Google Scholar]; The role of CENP-A in centromere maintenance in human and in fission yeast is investigated. CENP-C and other kinetochore proteins are shown to be maintained at centromeres after the excision of a single remaining CENP-A gene from the genome. In addition, the N-terminal and C-terminal tails of CENP-A show redundancy in function. The C-terminus of CENP-A directly interacts with CENP-C. CENP-B is partially dependent on CENP-A and is shown to play a role in kinetochore assembly through the interaction with the N-terminal domain of CENP-A.
- Dunleavy E.M., Beier N.L., Gorgescu W., Tang J., Costes S.V., Karpen G.H. The cell cycle timing of centromeric chromatin assembly in Drosophila meiosis is distinct from mitosis yet requires CAL1 and CENP-C. PLoS Biol. 2012;10:e1001460. doi: 10.1371/journal.pbio.1001460. [DOI] [PMC free article] [PubMed] [Google Scholar]; The timing of CENP-ACID assembly is analysed in Drosophila mitotic tissues and in meiotic cells. CENP-ACID is deposited during late telophase and G1 in somatic tissue. In male and female meiotic cells, CENP-ACID is assembled in prophase of meiosis I and after exit from meiosis II in spermatids. Deposition of CENP-ACID in meiosis is dependent on CENP-C and CAL1 (see also [45•]).
- Raychaudhuri N., Dubruille R., Orsi G.A., Bagheri H.C., Loppin B., Lehner C.F. Transgenerational propagation and quantitative maintenance of paternal centromeres depends on Cid/Cenp-A presence in Drosophila sperm. PLoS Biol. 2012;10:e1001434. doi: 10.1371/journal.pbio.1001434. [DOI] [PMC free article] [PubMed] [Google Scholar]; Analysis of CENP-ACID deposition during spermatogenesis and following fertilisation in Drosophila. CENP-ACID is retained in mature Drosophila sperm while CENP-C and CAL1 are not. The presence of CENP-A on sperm chromatin is required to maintain the epigenetic mark on paternal chromosome. CENP-ACID is loaded during G2 before meiotic division and is dependent on CAL1 (see also [44•]).
- Mendiburo M.J., Padeken J., Fülöp S., Schepers A., Heun P. Drosophila CENH3 is sufficient for centromere formation. Science. 2011;334:686–690. doi: 10.1126/science.1206880. [DOI] [PubMed] [Google Scholar]; CENP-ACID is demonstrated to be sufficient to establish a functional centromere which is able to self-propagate in Drosophila S2 cells. Tethering of LacI-CENP-ACID to a LacO array inserted on a chromosome arm leads to the formation of a functional kinetochore. Untethered (not fused to LacI) CENP-ACID is also recruited to the LacO array and the kinetochore persists after the LacI-CENP-ACID is removed.
- Choi E.S., Strålfors A., Catania S., Castillo A.G., Svensson J.P., Pidoux A.L., Ekwall K., Allshire R.C. Factors that promote H3 chromatin integrity during transcription prevent promiscuous deposition of CENP-ACnp1 in fission yeast. PLoS Genet. 2012;8:e1002985. doi: 10.1371/journal.pgen.1002985. [DOI] [PMC free article] [PubMed] [Google Scholar]; A role for transcription-coupled chromatin reassembly in preventing spurious CENP-ACnp1 deposition in fission yeast is suggested. The authors demonstrate that defective Rpd3S/Clr6CII HDAC favours de novo CENP-A assembly on centromeric DNA. It is proposed that endogenous centromeres provide a chromatin environment which interferes with the normal transcription-coupled chromatin reassembly process thereby destabilising H3 nucleosomes in favour of CENP-ACnp1 nucleosome assembly and kinetochore formation.
- 48.Castillo A.G., Pidoux A.L., Catania S., Durand-Dubief M., Choi E.S., Hamilton G., Ekwall K., Allshire R.C. Telomeric repeats facilitate CENP-A(Cnp1) incorporation via telomere binding proteins. PLoS ONE. 2013;8:e69673. doi: 10.1371/journal.pone.0069673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Choi E.S., Strålfors A., Castillo A.G., Durand-Dubief M., Ekwall K., Allshire R.C. Identification of noncoding transcripts from within CENP-A chromatin at fission yeast centromeres. J Biol Chem. 2011;286:23600–23607. doi: 10.1074/jbc.M111.228510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bush K.M., Yuen B.T., Barrilleaux B.L., Riggs J.W., O’Geen H., Cotterman R.F., Knoepfler P.S. Endogenous mammalian histone H3.3 exhibits chromatin-related functions during development. Epigenetics Chromatin. 2013;6:7. doi: 10.1186/1756-8935-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachiwana H., Kagawa W., Shiga T., Osakabe A., Miya Y., Saito K., Hayashi-Takanaka Y., Oda T., Sato M., Park S.-Y. Crystal structure of the human centromeric nucleosome containing CENP-A. Nature. 2011;476:232–235. doi: 10.1038/nature10258. [DOI] [PubMed] [Google Scholar]; The structure of in vitro assembled human CENP-A nucleosomes is reported. The overall structure is similar to that of H3-containing nucleosomes. However, only 121 bp, rather than 147 bp, of DNA is wrapped around CENP-A nucleosomes. This is due to a shorter α-N helical region that makes less contact with DNA than in canonical H3 nucleosomes as it enters and exits CENP-A (see also [52•]).
- Hasson D., Panchenko T., Salimian K.J., Salman M.U., Sekulic N., Alonso A., Warburton P.E., Black B.E. The octamer is the major form of CENP-A nucleosomes at human centromeres. Nat Struct Mol Biol. 2013;20:687–695. doi: 10.1038/nsmb.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]; Nuclease digestion experiments show that CENP-A nucleosomes are octameric in vivo. The authors show that CENP-A nucleosomes protect a 110 bp fragment from MNase digestion that is consistent with the nucleosome structure [51•]. A range of fragment sizes are generated for CENP-A nucleosomes at neocentromeres suggesting that they are all generated from the same particles. The 110 bp fragment is larger than any fragments reconstituted on CENP-A/H4 tetrasomes suggesting that CENP-A nucleosomes are octameric in vivo. Similar results are obtained from analysis of neocentromeres suggesting that the properties of CENP-A in wrapping the DNA are independent of the DNA sequence.
- 53.Dalal Y., Wang H., Lindsay S., Henikoff S. Tetrameric structure of centromeric nucleosomes in interphase Drosophila cells. PLoS Biol. 2007;5:e218. doi: 10.1371/journal.pbio.0050218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dimitriadis E.K., Weber C., Gill R.K., Diekmann S., Dalal Y. Tetrameric organization of vertebrate centromeric nucleosomes. Proc Natl Acad Sci U S A. 2010;107:20317–20322. doi: 10.1073/pnas.1009563107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui M., Dimitriadis E.K., Hoischen C., An E., Quénet D., Giebe S., Nita-Lazar A., Diekmann S., Dalal Y. Cell-cycle-dependent structural transitions in the human CENP-A nucleosome in vivo. Cell. 2012;150:317–326. doi: 10.1016/j.cell.2012.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]; The stoichiometry of human CENP-A nucleosomes is analysed throughout the cell cycle. AFM measurements of immunopecipitated chromatin suggest that CENP-A nucleosomes are similar in height to H3 nucleosomes in S-phase but are halved in height from G2 to mitosis. The authors suggest that the structure of CENP-A nucleosomes is dynamic and oscillate from tetramers to octamers during the cell cycle.
- Miell M.D.D., Fuller C.J., Guse A., Barysz H.M., Downes A., Owen-Hughes T., Rappsilber J., Straight A.F., Allshire R.C. CENP-A confers a reduction in height on octameric nucleosomes. Nat Struct Mol Biol. 2013;20:763–765. doi: 10.1038/nsmb.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]; AFM measurements are performed on in vitro reconstituted octameric CENP-A and H3 nucleosomes (human and fission yeast) assembled on arrays of DNA. The analyses reveal that CENP-A nucleosomes have a lower height relative to H3 nucleosomes.
- Zhang W., Colmenares S.U., Karpen G.H. Assembly of drosophila centromeric nucleosomes requires CID dimerization. Mol Cell. 2012;45:263–269. doi: 10.1016/j.molcel.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]; Drosophila CENP-ACID can be cross-linked as dimers in vivo indicating that CENP-ACID nucleosomes contain two subunits of CENP-ACID. Mutations that affect the ability of CENP-ACID to dimerise result in CENP-ACID mislocalisation.
- Padeganeh A., Ryan J., Boisvert J., Ladouceur A.-M., Dorn J.F., Maddox P.S. Octameric CENP-A nucleosomes are present at human centromeres throughout the cell cycle. Curr Biol. 2013;23:764–769. doi: 10.1016/j.cub.2013.03.037. [DOI] [PubMed] [Google Scholar]; The stoichiometry of human CENP-A nucleosomes is assessed using a photo-bleaching-assisted method. The authors show that human centromeric nucleosomes contain CENP-A dimers together with histones H2B and H4. This octameric structure does not vary during the cell cycle.
- 59.Boeckmann L., Takahashi Y., Au W.C., Mishra P.K., Choy J.S., Dawson A.R., Szeto M.Y., Waybright T.J., Heger C., McAndrew C. Phosphorylation of centromeric histone H3 variant regulates chromosome segregation in Saccharomyces cerevisiae. Mol Biol Cell. 2013;24:2034–2044. doi: 10.1091/mbc.E12-12-0893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Samel A., Cuomo A., Bonaldi T., Ehrenhofer-Murray A.E. Methylation of CenH3 arginine 37 regulates kinetochore integrity and chromosome segregation. Proc Natl Acad Sci U S A. 2012;109:9029–9034. doi: 10.1073/pnas.1120968109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zeitlin S.G., Shelby R.D., Sullivan K.F. CENP-A is phosphorylated by Aurora B kinase and plays an unexpected role in completion of cytokinesis. J Cell Biol. 2001;155:1147–1157. doi: 10.1083/jcb.200108125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goutte-Gattat D., Shuaib M., Ouararhni K., Gautier T., Skoufias D.A., Hamiche A., Dimitrov S. Phosphorylation of the CENP-A amino-terminus in mitotic centromeric chromatin is required for kinetochore function. Proc Natl Acad Sci U S A. 2013;110:8579–8584. doi: 10.1073/pnas.1302955110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey A.O., Panchenko T., Sathyan K.M., Petkowski J.J., Pai P.-J., Bai D.L., Russell D.H., Macara I.G., Shabanowitz J., Hunt D.F. Posttranslational modification of CENP-A influences the conformation of centromeric chromatin. Proc Natl Acad Sci U S A. 2013;110:11827–11832. doi: 10.1073/pnas.1300325110. [DOI] [PMC free article] [PubMed] [Google Scholar]; Post-translational modifications of the N-terminal tail of human CENP-A are described. Using high-resolution MS, trimethylation of Gly1 is reported in pre-nucleosomal CENP-A and is catalysed by the N-terminal RCC1 methyltransferase in vitro. Phosphorylation of Serine 16 and Serine 18 forms intramolecular and intermolecular bridges that induce secondary structure of CENP-A N-termini affecting the chromatin conformation of in vitro reconstituted array.
- Nishino T., Takeuchi K., Gascoigne K.E., Suzuki A., Hori T., Oyama T., Morikawa K., Cheeseman I.M., Fukagawa T. CENP-T-W-S-X forms a unique centromeric chromatin structure with a histone-like fold. Cell. 2012;148:487–501. doi: 10.1016/j.cell.2011.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]; The structure of the CENP-T-W-S-X is reported. The histone-fold containing complexes CENP-T-W and CENP-S-X interact to form a stable heterotetramer that binds DNA and forms nucleosome-like structure. Mutations that impair the ability of the complex to bind DNA or heterotetramerise affect kinetochore integrity in vivo
- 65.Yan Z., Delannoy M., Ling C., Daee D., Osman F. A histone-fold complex and FANCM form a conserved DNA-remodeling complex to maintain genome stability. Mol Cell. 2010;37:865–878. doi: 10.1016/j.molcel.2010.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Singh T.R., Saro D., Ali A.M., Zheng X.-F., Du C.-H., Killen M.W., Sachpatzidis A., Wahengbam K., Pierce A.J., Xiong Y. MHF1–MHF2, a histone-fold-containing protein complex, participates in the fanconi anemia pathway via FANCM. Mol Cell. 2010;37:879–886. doi: 10.1016/j.molcel.2010.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino T., Rago F., Hori T., Tomii K., Cheeseman I.M., Fukagawa T. CENP-T provides a structural platform for outer kinetochore assembly. EMBO J. 2013;32:424–436. doi: 10.1038/emboj.2012.348. [DOI] [PMC free article] [PubMed] [Google Scholar]; The role of human CENP-T in kinetochore assembly is investigated. Using both in vivo and in vitro assays, the authors show that the N-terminus domain of CENP-T directly interacts with the Ndc80 complex and that this binding is stabilised by the phosphorylation of CENP-T. A high resolution structure for the CENP-T/Spc24/25 interface is provided. Mis12 and CENP-T compete for the same binding site in Ndc80 indicating that two independent pathways contribute to Ncd80 recruitment to centromeres. A similar model is described in budding yeast [68•].
- Malvezzi F., Litos G., Schleiffer A., Heuck A., Mechtler K., Clausen T., Westermann S. A structural basis for kinetochore recruitment of the Ndc80 complex via two distinct centromere receptors. EMBO J. 2013;32:409–423. doi: 10.1038/emboj.2012.356. [DOI] [PMC free article] [PubMed] [Google Scholar]; Structural analyses show that the N terminus of S. cerevisiae Cnn1 (CENP-T) binds Ncd80 through a conserved motif and the interaction is regulated by phosphorylation of Cnn1. A Cnn1-related motif is found in one of the subunit of the Mtw1 complex, Dsn1, and mediates the binding to Ndc80. Disruption of the interaction between Mtw1 and Ncd80 is essential for cell viability whereas Cnn1-Ncd80 interaction is not. However, these two distinct pathways are responsible for the recruitment of Ncd80 to the centromeres in budding yeast (see also [67•]).
- Hori T., Shang W.-H., Takeuchi K., Fukagawa T. The CCAN recruits CENP-A to the centromere and forms the structural core for kinetochore assembly. J Cell Biol. 2013;200:45–60. doi: 10.1083/jcb.201210106. [DOI] [PMC free article] [PubMed] [Google Scholar]; Tethering specific inner or outer kinetochore proteins to an ectopic locus in chicken cell line is shown to be sufficient to establish kinetochore structures. Tethering of LacI-HJURP, LacI-CENP-C (C terminus) or LacI-CENP-I to a LacO array inserted on a chromosome arm leads to the assembly of CENP-A chromatin. In contrast, the targeting of LacI-CENP-T or LacI-CENP-C (N-terminus) to the LacO arrays results in recruitment of Ndc80 and the chromosome passenger complex, but not CENP-A. Tethering CENP-T to a DNA plasmid containing LacO array is sufficient to faithfully segregate the plasmid to daughter cells.
- Schleiffer A., Maier M., Litos G., Lampert F., Hornung P., Mechtler K., Westermann S. CENP-T proteins are conserved centromere receptors of the Ndc80 complex. Nat Cell Biol. 2012;14:604–613. doi: 10.1038/ncb2493. [DOI] [PubMed] [Google Scholar]; Proteomic analysis of the CCAN network of budding yeast is reported. The authors describe the interaction between the N-terminal domain of CENP-T and Ndc80. This interaction competes with the binding of Mtw1 (Mis12) to Ndc80. Tethering of Cnn1 (CENP-T) is sufficient to induce segregation of a plasmid in the absence of centromere DNA. The CENP-T-Ndc80 interaction is conserved in vertebrates [67•,69••].
- 71.Screpanti E., De Antoni A., Alushin G.M., Petrovic A., Melis T., Nogales E., Musacchio A. Direct binding of Cenp-C to the Mis12 complex joins the inner and outer kinetochore. Curr Biol. 2011;21:391–398. doi: 10.1016/j.cub.2010.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Petrovic A., Pasqualato S., Dube P., Krenn V., Santaguida S., Cittaro D., Monzani S., Massimiliano L., Keller J., Tarricone A. The MIS12 complex is a protein interaction hub for outer kinetochore assembly. J Cell Biol. 2010;190:835–852. doi: 10.1083/jcb.201002070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Perpelescu M., Fukagawa T. The ABCs of CENPs. Chromosoma. 2011;120:425–446. doi: 10.1007/s00412-011-0330-0. [DOI] [PubMed] [Google Scholar]
- 74.Przewloka M.R., Venkei Z., Bolanos-Garcia V.M., Debski J., Dadlez M., Glover D.M. CENP-C is a structural platform for kinetochore assembly. Curr Biol. 2011;21:399–405. doi: 10.1016/j.cub.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 75.Akiyoshi B., Gull K. Evolutionary cell biology of chromosome segregation: insights from trypanosomes. Open Biol. 2013;3:130023. doi: 10.1098/rsob.130023. [DOI] [PMC free article] [PubMed] [Google Scholar]