Abstract
Obesity is among the fastest growing diseases worldwide; treatment is inadequate and associated disorders, including gastrointestinal cancers, have high morbidity and mortality. An increased understanding of the mechanisms of obesity-induced carcinogenesis is required to develop methods to prevent or treat these cancers. We review the mechanisms of obesity-associated colorectal, esophageal, gastric, and pancreatic cancers and potential treatment strategies.
Keywords: obesity, colorectal cancer, esophageal cancer, gastric cancer, pancreatic cancer, gastrointestinal cancer, mechanisms
Introduction
The World Health Organization defines obesity as “abnormal or excessive fat accumulation to the extent that health is impaired.1” Obesity is the fastest growing lethal disease in the Western and developing worlds. People do not die from obesity itself but from its complications, which shorten life span.2 Up to 20% of all cancers can be attributed to obesity.3, 4 Since there are few effective therapies for obesity beside bariatric surgery, it is appropriate to define preventive measures for obesity-associated cancer. Obesity and increased waist circumference increase the risk of gastrointestinal (GI) cancers, which include colon, esophageal, gastric, and pancreatic cancers (Figure 1). We review possible mechanisms for the association between obesity and GI cancer initiation and progression, based on human and preclinical studies.
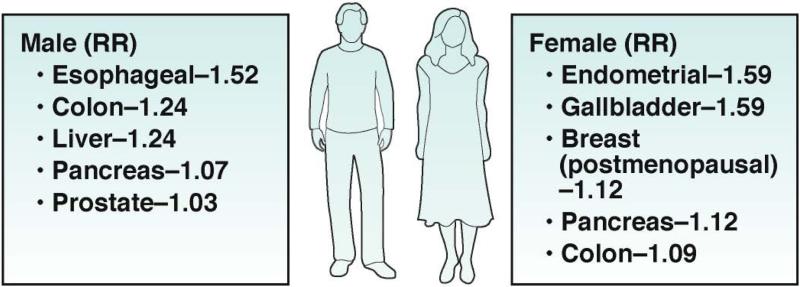
Figure 1. Relative Risks of GI Cancers in Obese Men and Women.
Obesity confers increased risk of several malignancies. Reductions in obesity rates should result in decreased cancer incidence and potentially mortality. In men, obesity-associated cancers include esophageal, colon, liver, pancreas and prostate. In women, obesity-associated cancers include endometrial, gallbladder, breast (postmenopausal women), pancreas and colon. Data presented as relative risk per 5 kg/m2 higher BMI. Adapted from [3].
A single mechanism is unlikely to be responsible for all obesity-associated tumors. Mechanisms that contribute to the multiple complications of obesity are likely to include the insulin and insulin-like growth factor (IGF) signaling pathways, adipokines, inflammation and immune responses, and the GI microbiota. However, different tissues have different mechanisms of obesity-associated carcinogenesis. Obesity is associated with the metabolic syndrome, which comprises insulin resistance, altered lipid levels, and increased blood pressure. Metabolic syndrome is more strongly associated with GI cancers than is obesity.
However, not all obese individuals, even those with severe disease, develop these complications,5 so it is important to identify those at risk. Increasing our understanding of the mechanisms for obesity complications should lead to the discovery of biomarkers of risk. Furthermore, obese patients also have a poor prognosis when diagnosed with GI cancers.6
Insulin and IGF1 Signaling
Insulin is a mitogenic hormone believed to affect cancer development. Since the discovery of insulin, researchers have proposed associations between type 2 diabetes and cancer, despite equivocal and controversial evidence.7, 8 Clarification of this association requires separation of the effects of concurrent hyperinsulinemia, hyperglycemia, their shared risk factors, and the IGF1 signaling pathway.
Obese patients frequently have hyperinsulinemia, independently of type 2 diabetes, presumably based on their increased need for energy regulation of metabolic processes.9 Insulin is anabolic in muscle, adipose tissue, and liver, to increase tissue mass, augment glucose uptake, and synthesize nutrients respectively. These anabolic effects are not directly related to carcinogenesis. Insulin-deficient diabetic animals are protected from cancer formation but this has not been reported in humans.10 Insulin-sensitive tissues such as liver, adipose tissue, and muscle infrequently develop malignancies, suggesting that insulin regulation of metabolic processes might protect against carcinogenesis.11 Hyperglycemia also can increase the availability of nutrients to cancer cells, which metabolize glucose via the Warburg effect, in which cancer cells produce energy via a high rate of glycolysis, followed by lactic acid fermentation in the cytosol, rather than by a comparatively low rate of glycolysis, followed by oxidation of pyruvate in mitochondria.12, 13 Furthermore, diabetes and cancer are common diseases that develop in adults and share risk factors, including inactivity and an unhealthy diet.14 Epidemiology studies have clearly associated type 2 diabetes with GI cancers. The molecular basis for this association is unclear, but could involve IGF1 signaling, which is mitogenic under conditions of hyperglycemia and/or hyperinsulinemia, independently of the metabolic features of type 2 diabetes.
IGF1 and GI Cancers
IGF1 production by the liver is stimulated by pituitary growth hormone, in response to hypothalamic integration of nutrient balance during periods of growth. IGF1 stimulates cell division and inhibits apoptosis and could therefore contribute to cancer development and metastasis.15 Excess production of IGF1 in patients with acromegaly increases their incidence to aggressive colon cancer,16 whereas individuals with IGF1 deficiency (Laron syndrome) are protected from cancer development.17, 18 Obese patients have been reported with higher circulating levels of IGF1 than non-obese individuals, in the presence of hyperinsulinemia.19
Human liver produces multiple isoforms of IGF; IGF1 is the most highly abundant isoform in circulation. IGF1 is transported in blood via IGF binding proteins (IGFBPs), predominantly bound by IGFBP3 in humans. The IGFs bind IGF receptors (IGFRs) and the insulin receptor (IR) as well as alternatively spliced combinations of their respective subunits. For the purposes of this review we shall focus on IGF1 action on IGF1R
IGF1R expression and activity vary among human tissues. The GI expresses IGF1R, and this expression is altered in the mucosa and submucosa of involved intestinal areas of patients with Crohn's disease.20 In the gut, IGF1 appears to modulate nutrient uptake through endocrine and neural pathways.21 The remarkable ability of the intestine to regenerate enterocytes depends on IGF1 and other signals.
IGF1R expression varies among tumor cells, and can have paracrine and autocrine effects that promote tumorigenesis and metastasis.22 IGF1 signals via its receptor to insulin receptor substrate 1, phosphatidylinositol 3-kinase (PI3K), AKT, and mammalian target of rapamycin (mTOR) to stimulate cell proliferation.23 These shared features of IGF1 and insulin signaling provide evidence for conservation of proliferation signaling pathways in response to nutrient excess. Divergent effects of insulin and IGF1 signaling result from heterogeneity in IGFR expression during development (prenatal vs postnatal) and in different tissues.24 In insulin-sensitive tissues, IGF1 signals through the Ras–mitogen activated kinase-like protein (MAPK) pathway to stimulate proliferation, rather than inducing FOXO1 transcriptional activity, which regulates metabolism.23, 24 Continuous exposure of cells to IGF1 could promote tumorigenesis through the Ras–MAPK pathway.25, 26
The cellular actions of IGF1 can be inhibited by hormones such as somatostatin, by scavenging circulating IGF1 or blocking IGF1R. Monoclonal antibodies against IGF1 have been shown to inhibit colorectal cancer (CRC) stem cells in mice.27 Studies are underway to test the effects of monoclonal antibodies that block circulating IGF1 or IGF1R in patients with cancer and high serum levels of IGF.
Ligand-targeting agents, such as octreotide, have been limited by the large reservoir of circulating IGF1 bound to IGFBP3 and other proteins.28 The antibody CP-751781, which blocks IGF1R, was tested in a phase 1 dose-escalation study of 6 patients with colorectal cancer with acceptable tolerability, expected side effects of hyperglycemia and preliminary evidence of efficacy at the highest dose of 20 mg/kg.29, 30 Subsequent studies showed modest success in patients with non-small cell lung cancers that express IGF1.31
Adipokines
Adipose tissues synthesize and secrete many polypeptide growth factors and cytokines known as adipokines. Adipokines are produced mainly by white adipose tissue preadipocytes and mature adipocytes. Numerous adipokines have been reported to alter metabolic cellular function in rodents and humans.32 Altered of leptin and adiponectin have been associated with GI cancers; changes have also been reported in levels of resistin, although these findings are unconvincing.33
Leptin is a 16-KDa non-glycosylated protein encoded by LEP; it was originally described as a regulator of energy balance via the hypothalamus.34 Leptin is secreted by adipocytes, in proportion to white adipose tissue mass; more is produced by subcutaneous than visceral tissue.35 Concentrations of circulating leptin, bound to plasma proteins, vary from 5 to 10 ng/ml in normal-weight individuals, and also vary with obesity and pregnancy.36
Factors that alter leptin production and action include insulin, estrogen, and inflammatory mediators such as IL1B, IL6, and TNFα.37 Lipopolysaccharides (LPS) (membrane components of gram-negative bacteria) also increase leptin expression in white adipose tissue.38 Agents that block the β3 adrenergic receptor, free fatty acids, growth hormone, and peroxisome proliferator-activated receptor (PPAR) agonists reduce leptin secretion. Leptin binds to transmembrane receptors on stomach and colon cancer cells, resulting in activation of the JAK–STAT, MAPK, PI3K–AKT, insulin receptor substrate, and mTOR signaling pathways.39
Many GI tissues, cancer cell lines, and immune cells express a functional leptin receptor (LEPR or OB-R).40 Some tumors even express leptin and its receptor, to allow for autocrine signaling.41 Leptin stimulates cellular proliferation, migration, and invasion of tumor cells and inhibits apoptosis. Leptin also increases cytokine release from macrophages and increases insulin resistance.42 Leptin produced by adipose tissue can affect adjacent tumors.43 Leptin is involved in angiogenesis and can activate aromatase in adipose tissue.44 Furthermore, since leptin inhibits the activity of T regulatory (Treg) cells, it can regulate immune surveillance of GI cancers.45 Clearly, leptin's diverse biologic functions make it a good candidate for a mediator of cancer development and progression.
Adiponectin is a 30 kDa polypeptide with a C-terminal globular domain similar to TNFα. Circulating concentrations of adiponectin are 3–30ng/ml. Adiponectin occurs as a monomer that can form low- and high-molecular weight multimeric oligomers with biologic activities.46 Multiple circulating active forms of this hormone complicate analysis of adiponectin concentrations in obesity. These forms show an integrated pulsatile diurnal rhythm which is paralleled by leptin-binding protein concentrations.47 Adiponectin is expressed in differentiated adipocytes, at higher concentrations in subcutaneous than visceral adipose tissue, and in an inverse association with total body fat mass.48 Transcription of ADIPOQ and secretion of the protein are stimulated by IGF1 and PPARγ agonists, and inhibited by TNFα, IL6, or glucocorticoids.49, 50
Adiponectin interacts with its receptors 1 and 2 to increase insulin sensitivity.51 Adiponectin also has anti-proliferative and angiogenic effects.52 Many cancers express adiponectin receptors, including gastric, colon, and pancreatic tumors.40, 53, 54 In rodents, adiponectin prevents NFκB-dependent expression of the inflammatory cytokines TNFα, interferon-γ, and IL6.55 Adiponectin inhibits IL6 and increases IL10 and TIMP1 activity mediated by the AMPK pathway.56 Overall, the actions of adiponectin and leptin on cell functions tend to balance each other out.57
The anti-inflammatory effects of adiponectin are supported by an observed inverse correlation between plasma levels of adiponectin and c-reactive protein in obese diabetic and non-diabetic individuals.46 Adiponectin also blocks LPS-stimulated production of TNFα by macrophage, inhibits toll-like receptor-mediated activation of NFκB,58 and increases M2-type macrophage, while reducing markers of M1 types.59 Since chronic inflammation promotes carcinogenesis in many GI organs, these anti-inflammatory effects of adiponectin may be important in limiting cancer risk.
Sex Hormones
Epidemiologic studies suggest differences in complications from obesity in men vs women, potentially due to altered distribution of adipose tissue mass between the sexes, which determines differences in plasma levels of sex hormones (Figure 1). For example, the increased incidence of post-menopausal breast cancer in obese women (compared with non-obese women) could be explained in part by their higher circulating levels of estrogen. This results from greater aromatization of androgenic precursors to estradiol via increased adipose tissue activity of cytochrome P450 aromatase.60 Men have a higher incidence of CRC than women of the same age; post-menopausal estrogen replacement therapy reduces the incidence of CRC—particularly of estrogen receptor 3 tumors.61, 62
Genetic Factors
Sixty five to 80% of the variation in body mass index (BMI) is determined by genetic factors.63 Gene polymorphisms that affect insulin signaling have been associated with body size.64 Potential genetic factors predisposing to obesity also may enhance some cancers.65 Genome-wide association studies have described altered “macrophage-enriched metabolic network genes”, 66, 67 and have explored mutual candidate genes in selected areas of the genome. To our knowledge, genome-wide epigenetic studies have not revealed changes pertinent to mechanisms of neoplasia involved in obesity. The most powerfully associated single-nucleotide polymorphism associated with increased body mass is the fat mass and obesity-related (FTO) gene, which may function through nutrient sensing but with no relation to GI cancers.68
Inflammation
Inflammatory responses may be beneficial, limited with minimal impact, or pathologic with dysregulated immune function. Obesity is associated with chronic low-grade inflammation (also called meta-inflammation), characterized by abnormal cytokine production, immune activation, and increased inflammatory signaling. This chronic inflammation is more pronounced in the visceral than subcutaneous fat compartments. In obese individuals, adipocytes, preadipocytes, and surrounding stromal macrophages release inflammatory cytokines and chemokines, including IL17, IL22, TNFα, IL6, and the chemoattractant MCP-1 (Figure 2).69 These factors have a role in cancer.70 Although the NLRP-inflammosome is involved in obesity and cancer in rodents, it might not be in humans.71 It is not clear these immune and inflammatory proteins act on GI tissues through the circulation. Increased levels of leukocyte calprotectin in the feces of obese otherwise healthy individuals indicate the presence of low-grade intestinal inflammation.72 Reduced levels of IL8, TNFα, MCP1, and T-cell infiltration were observed in colonic biopsies of obese patients after weight loss.73
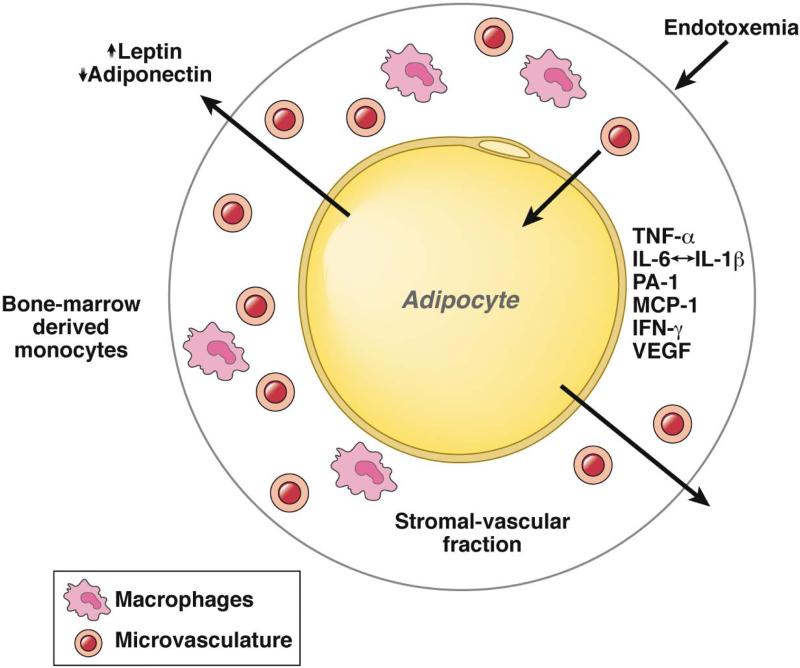
Figure 2. Inflammation and Adipocytes.
Interactions among circulating and local factors contribute to obesity-associated inflammation. Adipocytes are cells specialized for storage of nutrients in the form of triglyceride and cholesterol. Leptin and adiponectin are examples of adipokines produced in response to adipocyte size and overall energy balance. After reaching an undefined trigger (adipocyte size, hypoxia, insulin resistance), immune cells, including macrophages, are recruited to adipocytes. This activates an inflammatory signaling response that leads to secretion of TNFα, IL6, PA1, MCP1, IFNγ and VEGF. Resulting chronic inflammation leads to insulin resistance, cardiovascular disease, and different cancers.
In obese patients, chronic inflammation appears in the GI tract, liver (steatohepatitis), vasculature, and pancreas.74 Obesity increases the size of adipocytes, leading to their necrosis and subsequent accumulation of activated macrophages that appear, histologically, as crown-like structures.75 These secrete inflammatory mediators, including IL6, MCP1, and TNFα, which have been implicated in development of insulin resistance.76, 77 This inflammation is accompanied by upregulation of the anti-inflammatory factors IL10, IL4, and TGFβ. Adipose tissue in obese patients has a restricted T-cell receptor repertoire, containing fewer Treg cells than adipose tissue in lean individuals.78 The altered activity of Treg cells might increase inflammation in the adipose tissue to promote carcinogenesis.
If inflammation is an important determinant for obesity–associated cancer, could reducing inflammation lower cancer risk? Inflammation might be reduced by weight loss,79, 80 exercise, drugs, or diets designed to reduce inflammation—such as those high in omega-3 fatty acids81. Reducing adipose tissue inflammation reduces insulin resistance and could alter adipokine concentrations or the intestinal microbiota in obese individuals, but no study has examined such effects on GI carcinogenesis.
It is important to recognize that not all obese individuals have these inflammatory complications, and to ask whether macrophage activation is an initiator or effector of adipose inflammation. Some individuals appear to have metabolically benign obesity,82 assisted by a protective effect of abdominal superficial fat.83 Whether such individuals are relatively protected from GI cancers is unknown.
Endotoxemia
Endotoxemia is an important component of obesity-associated inflammation, resulting in uptake across the intestinal epithelium of LPS. In humans a high-fat diet increases, whereas a prudent low-fat diet reduces, endotoxemia.84 Endotoxemia can result from increased intestinal permeability, increased levels of LPS-containing bacteria in the intestine, or both. Researchers have not clearly determined the mechanisms of endotoxemia in obese individuals, and it is not clear whether endotoxemia affects GI carcinogenesis.
Intestinal Microbiota
High-fat diets do not lead to obesity or insulin resistance in germ-free mice, whereas transfer of fecal contents from obese to germ-free mice increased their fat cell mass, indicating a role for the intestinal microbiota in obesity.85 Furthermore, transfer of microbiota from human twins, discordant for obesity, to mice altered metabolism in the mice.86 The intestinal microbiota might contribute to obesity via its capacity to increase caloric salvage of indigestible dietary polysaccharides, or by regulating intestinal genes that increase fat storage in adipose tissues.87 A high-fat diet might also induce GI inflammation88—partially through induction of toll-like receptor 4,89 accompanied by LPS uptake and endotoxemia,90 which could contribute to obesity-associated GI carcinogenesis.
Although the fecal microbiota can differ between obese and lean individuals,91, 92 the changes are inconsistent. The abundance of bacteroides species and the ratio of bacteroides to firmacutes are reduced with weight loss93 and bariatric surgery94 in obese subjects, but this observation has been inconsistent.95 Local host factors are important, because experimentally induced GI inflammation can alter gut microbiota.96 Bacterial transformation of bile acids in the colonic lumen could act on mucosal epithelia and host metabolism. It is not clear if these changes can be used in strategies to prevent or treat CRC.
Overall, human studies have not clarified the role of microbiota in the development or maintenance of obesity. Transfer of fecal microbiota from non-diabetic subjects into the small intestine of diabetics induced a small but significant change in insulin resistance, indicating that the microbiota might be manipulated to treat diabetes.97 Fecal transplantation is frequently used to treat patients with chronic relapsing C difficile infection.98 The composition of the intestinal microbiota affects tissues other than the small and large intestine, and could be involved in development of esophageal, gastric, and even pancreatic neoplasms.
CRC
Approximately 1.2 million new cases of CRC are diagnosed annually worldwide, with almost 600,000 deaths.99 CRC has the highest incidence and mortality among GI cancers. Epidemiologic studies have correlated obesity with CRC. A meta-analysis showed that in men, an increase of 5kg/m2 in BMI confers a relative risk (RR) of 1.24 for colon cancer.3 However, in women, the relationship between BMI and cancer risk is complicated by difference in fat distribution. A pooled analysis associated waist circumference and waist—hip ratio with sex-related differences in risk of colon cancer.100 There is a statistically significant difference in the effects of waist circumference on cancer risk (stronger association for men than women), but not for waist-hip-ratio. In men, BMI > 35 increases the risk of dying from hepato-digestive cancers. However, the effects of obesity on the early stages of colon carcinogenesis require clarification. In animal models of colon cancer, obesity and energy restriction affect development of aberrant crypt foci (ACF).101 The mechanisms that mediate the association between obesity and colorectal cancer are complex and are likely to involve insulin and IGF signaling, adipokines, and inflammation (Figure 3).
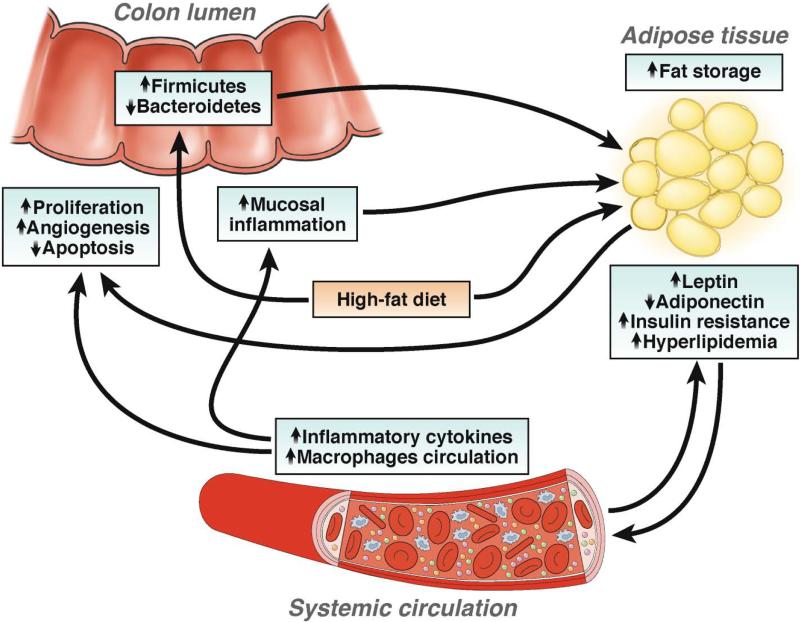
Figure 3. Obesity-induced Factors Contribute to Colorectal Carcinogenesis Mechanisms by which obesity could contribute to development of CRC.
Western-style diets lead to increased adiposity and changes in the microbiota, to adapt to the increased energy supply. Adipokines such as leptin and adiponectin could allow for establishment of a tumor microenvironment. In obese individuals, hyperlipidemia and insulin resistance lead to low-grade systemic inflammation, which promotes tumor cell proliferation and angiogenesis and reduces apoptosis. These mechanisms are likely to vary among individuals and little is known about their interactions.
Insulin and IGF Signaling
IGFRs are expressed in the mucosal and muscular layers of the normal colon, and are important for colonocyte metabolism. Insulin stimulates proliferation of cultured colonocytes and CRC cells directly, by binding IGFR to activate signaling via the MAPK pathway, or indirectly, by increasing concentrations of IGF1.25, 102, 103
Colon cancer cells overexpress IGFIR104; binding of IGF1 to its receptor inhibits apoptosis, increases proliferation, and contributes to the development, progression, and metastatic potential of CRC.26, 105-107
In ApcMin/+ mice, IGF1 showed a stage-specific effect on colorectal carcinogenesis, increasing proliferation in preneoplastic, but not in normal epithelial cells.108 Increased plasma levels of IGF1 and hyperinsulinemia appeared also to induce development of dysplastic ACF.109
Adipokines
Leptin stimulates the proliferation, migration, and invasion of tumor cells. The proliferative and survival effects of leptin are mediated by the Ob-R–signal transducer and activator of transcription 3 (STAT3) pathway in colon cancers in mice.110 Moreover, leptin can stimulate invasive activity of cell lines derived from colonic adenomas, increasing their metastatic potential.111 Leptin induces production of inflammatory cytokines by colonocytes.112-114
Most data from humans on the effects of leptin relate to CRC risk and prognosis, but epidemiologic data are unconvincing. Leptin concentrations were associated with increased risk of CRC in men but not women in 2 nested control studies.115, 116 However, leptin levels were associated with increased risk in women in other studies,117, 118 although these did not account for lifestyle factors, body fat distribution, and metabolic markers. A European Prospective Investigation into Cancer and nutrition (EPIC) cohort nested case-control study found no relation between leptin concentrations and CRC risk, but a strong inverse relationship between soluble leptin receptor (OB-R) concentrations and colon cancer in men.119 A study from the Middle East reported higher levels of OB-R in adenomas and carcinomas than healthy colon tissues, which surprisingly were associated with increased survival.120 Another study correlated high circulating levels of soluble OB-R with advanced-stage colon cancer, indicating an effect on cancer progression.121 In contrast, in a recent large nested case-control study, plasma level of OB-R was not associated with overall risk of CRC.122 Overall, serum levels of leptin have not been associated with progression-free survival of patients with CRC. Most of these studies appeared to be limited in design and small patient numbers; data associating colonic adenoma incidence and leptin concentrations were weak.123
Adiponectin–/– mice fed high-fat diets gained more weight and developed larger orthotopic colon tumors than control mice; subsequent administration of adiponectin reduced colon tumor growth.124 In mice, lower levels of adiponectin and adiponectin receptor 1 expression were associated with a higher incidence of polyps under high-fat diet.125 Furthermore, plasma levels of adiponectin were inversely related to numbers of preneoplastic ACF in patients,109 whereas low levels of adiponectin were frequently observed in obese individuals and those with type 2 diabetes.126 These lower levels are an independent risk reduction factor for the development of several tumors including colon cancer. A large prospective nested case–control study found an inverse association between plasma level of adiponectin and risk for CRC in men but not women.122 These findings were confirmed by a meta-analysis showing a 2% decreased risk of colorectal neoplasms for each 1 μg/ml increment in adiponectin level, only in men.127
Different molecular weight adiponectins could have varying effects on development of colorectal neoplasia in obese individuals. In the EPIC cohort, a nested case-control study found that high-molecular weight adiponectin was not associated with increased CRC risk whereas non-nigh molecular weight adiponectin was inversely associated with CRC, even after adjusting for BMI and waist circumference.128 The mechanisms for these contrasting observations remain to be explained. Adiponectin polymorphisms have not clearly been related to colorectal neoplasia.
Inflammatory Factors
Studies addressing a direct relation between circulating inflammatory markers and CRC showed equivocal results. Patients with colorectal adenomas or CRC have increased levels of IL6 levels and may parallel tumor size.129, 130 IL6-deficient mice develop smaller size and numbers of colonic adenomas following administration of azoxymethane and dextran sodium sulphate.131 Furthermore, in the same model, a weekly dose of a neutralizing antibody against the IL6 receptor reduced average tumor numbers and size compared to control mice.132
In a study of a large cohort of CRC patients matched with healthy controls, Chan et al. associated increased risk of CRC with baseline plasma level soluble tumor necrosis factor receptor-2 (sTNFR2, a surrogate marker for TNFα), but not levels of IL6 nor c-reactive protein.133 Interestingly, sTNFR2 is associated with increased insulin resistance, and in this study it was also related to increased BMI. Consumption of anti-inflammatory agents (aspirin and non-steroidal anti-inflammatory drugs) reduced risk of CRC among women with higher baseline levels of sTNFR-2.133
The inflammatory leukotriene D4 (LTD4) has also been proposed to be involved in development of CRC. LTD4 stimulates proliferation and reduces apoptosis in several non-transformed intestinal epithelial cell lines.134, 135 Higher BMI is accompanied by higher levels of prostaglandin, whereas physical activity lowers the levels.136
Obesity and Molecular Pathways
Type II diabetes and obesity are influenced by genetic and functional variations in the WNT signaling pathway, important for energy metabolism.137 Altered WNT signaling, mutations in RAS and RAF, DNA epigenetic changes and loss of function of p53 are all involved in development of CRC. Among other things, p53 and p21 regulate cell metabolism and energy balance. Loss of p53 function is a late event in carcinogenesis, leading to abnormalities in the regulation of cell energy and metabolism, including mTOR signaling.138
An analysis of 2 large databases (the Nurses’ Health Study and the Health Professionals Follow-up Study) showed that tumors with 50% or more cells that stained positive for nuclear p53 were associated with significantly shorter cancer-specific survival in non-obese patients (BMIs<30), but were not associated with longer survival of patients with BMIs>30.139 Obesity was significantly associated with increased mortality among patients with tumor cells that were negative for nuclear p53. The authors of this study subsequently associated loss of p21 from tumor cells with increased cancer-specific mortality among patients under 60 y of age.140 In patients with BMIs>30, p21 expression reduced survival, as observed for p53. These findings associate p53 and p21 regulation with cancer cell metabolism and obesity.
Loss of 18q frequently occurs during late stages colorectal carcinogenesis; it is inversely associated with MSI and CpG island methylation. Data from 532 CRC samples without high MSI independently associated loss of 18q with BMIs>30.141
Furthermore, higher BMIs and physical inactivity were associated only with CRCs that were negative for β-catenin activation.142 However, activation of β-catenin significantly increased cancer-specific and overall survival only in patients with BMIs ≥ 30. Physical activity after a diagnosis of CRC correlated with increased cancer-specific survival of patients with tumors that were negative for β-catenin activation.143 The progression of colorectal tumors that are negative for β-catenin activation appears to be dependent on the patient's energy balance after diagnosis is made. Tumors with active β-catenin may progress regardless of metabolic status—β-catenin might affect influence cell sensitivity to obesity, as well as to physical activity, during colon carcinogenesis. It is important to note that obesity-associated molecular changes in the colon could determine outcomes of patients with cancer, and be used to direct therapy. Finally, a recent increase in BMI (by 5 kg/m2 increments) was associated with microsatellite stable and MSI-low, but not with MSI-high tumors.
Diet
The Western diet and life style are risk factors for CRC, although there is no direct proof for this association. Case-control studies have generally associated energy intake with colorectal cancer risk whereas cohort studies have not.144, 145 Dietary glycemic load and CRC risk have been positively associated in cohort and case-control studies,146 but the relationship between dietary fat and CRC have not been substantiated by epidemiology studies.147 In obese individuals, hyperlipidermia could feed tumor anabolism. If obesity increases the risk for cancers of the GI tract, along with morbidity and mortality after diagnosis, what do we know about the effect of weight loss or other interventions on these complications?
In human weight loss studies, intentional weight loss must be distinguished from antecedent weight loss produced by the cancer itself. The IOWA Health Study showed that intentional weight loss greater than 20 lbs in women followed for 7 y reduced total cancers by 11%, breast cancer by 19%, CRC by 9%, and all obesity-related cancers by 14%.148 Another study also showed that recent weight loss reduced CRC incidence.149 Fiber intake is negatively related to CRC incidence, but the effects on weight loss are controversial.150
Studies of obese patients who underwent bariatric surgery have reported reductions in cancer incidence.151, 152 Among patients followed for more than 10 y, the incidence of breast cancer was reduced by 45%, but bariatric surgery had no effect on overall cancer development,153, 154 and surprisingly, increased the incidence of CRC.155 This increase might result from the increased concentrations of colonic bile acids or fatty acids in these patients.
Esophageal Cancer
Esophageal cancer is the eighth most common cancer and is the sixth most common cause of cancer death. 156 Incidences of esophageal squamous cell carcinoma (ESC) and adenocarcinoma (EAC) vary among countries. Though ESC is predominant worldwide, the incidence of EAC has been increasing—particularly in western countries, accounting for 50% of esophageal cancers.157 This likely is due in part due to increasing BMIs.
Many studies have identified obesity as a risk factor for EAC in men and women.158 A meta-analysis of prospective observational studies identified a RR of 1.52 and 1.51 per 5Kg/m2 increase in BMI in men and women, respectively.3 (Figure 1) The prospective NIH-AARP Diet and Health Study found EAC risk was highest for those with BMIs > 35 kg/m2.159
Obesity and Progression to EAC
Obese individuals have a higher prevalence of gastroesophgeal reflux disease, which can lead to Barrett's esophagus (BE) and intestinal metaplasia, which are precursors to EAC.160-162 A meta-analysis showed significant and dose-dependent associations between obesity and gastroesophgeal reflux disease, with RRs increasing from 1.43 to 1.94 for BMIs 25–30 kg/m2.163 Abdominal obesity is also a risk factor for BE (adjusted RR, 2.9);164 levels of risk are similar to those of increasing BMI.165, 166
It is unclear what stage of EAC development adiposity affects (Figure 4). The most recent data indicate that obesity early in life increases risk for EAC. In the Factors Influencing the Barrett's/Adenocarcinoma Relationship (FINBAR) population-based case-control study, EAC occurred more commonly among subjects who were overweight or obese 5 y before diagnosis.167 Similarly, in a hospital-based, case-control study, EAC cases were more likely to have been overweight (BMI > 25) at age 20 or 10 y before diagnosis, indicating an effect of early-life obesity.168
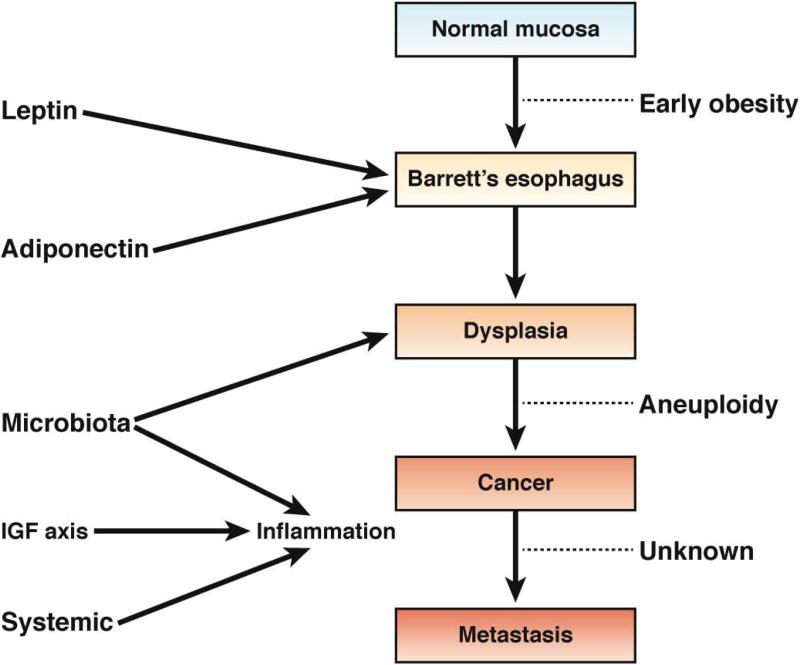
Figure 4. Obesity and Esophageal Cancer Mechanisms by which obesity could contribute to carcinogenesis in the esophagus.
In early stages of obesity, leptin and adiponectin contribute to the frequency and development of BE. The transition from benign metaplasia to dysplasia is thought to be partially mediated by changes in the microbiota, in addition to an acid environment. Development of aneuploidy and subsequent cancer have been associated with chronic low-grade inflammation and altered levels of IGF1. It is not clear whether obesity contributes to metastasis of esophageal cancer.
The Seattle BE Project associated increasing waist to hip ratio with risk of abnormal esophageal histology, aneuploidy, increase in the percentage of cells with 4N DNA content, and other genetic defects (loss of heterozygosity at 9p and 17p), which occur in premalignant lesions.169 Other markers of malignancy include DNA methylation, oxidative DNA damage, telomere length, and altered levels of specific microRNAs—these require further investigation. 170-173
Preliminary data from human studies have associated EAC progression with changes in the miRNA profile in the affected epithelium.174 There are few data on the contribution of epigenetic alterations to the metaplasia–dysplasia–EAC pathway. The biologic and diagnostic implications of epigenetic changes and altered metabolism remain to be explored and may provide novel targets for the prevention and treatment of BE and EAC.
Insulin and IGF1 Signaling
Adipose tissue is metabolically active, secreting numerous biologically active products 175-177 that promote inflammation and insulin resistance. 178, 179 Insulin and IGF signaling have been shown to promote BE and EAC 180, 181 Gene expression analyses of EAC samples from viscerally obese subjects with increased total and free levels of IGF1 showed an association between obesity and increased tumor expression of IGF1R. Patients whose tumors did not express IGF1R survived longer than patients with IGF1R-positive tumors. However, in a longitudinal study of patients with BE, concentrations of IGF1 and IGFBP3 were not associated with EAC risk.182 Aneuploidy was 3-fold greater among participants with higher levels of IGFBP3, indicating that IGF signaling could mediate the association between obesity and EAC.
Adipokines
Serum levels adiponectin levels are low in patients with EAC (but not ESC), and decrease significantly with tumor progression and metastasis.183-185 Recently, using data from the Seattle BE Study, Duggan et. al185 found that among patients with BE, levels of adiponectin had a nonlinear inverse association with risk of developing EAC. Other studies have also demonstrated how adiponectin may influence the development of EAC, although more data are needed to validate these findings.186-188 Further translational research is needed to determine how to increase adiponectin signaling, such as by upregulating adiponectin receptors, providing adiponectin receptor agonists, or administering human recombinant adiponectin.189
Leptin could also have a role in BE and EAC development190, 191—it stimulates cell proliferation and inhibits apoptosis in EAC cell lines.188, 192, 193 Increased leptin levels have been significantly associated with risk of progression from BE to EAC.185 The relationship between leptin and EAC warrants further study. Leptin receptor signaling might be inhibited with epidermal growth factor receptor or extracellular signal-regulated kinase inhibitors.188
Obesity and Development of EAC
The homeobox transcription factors CDX1 and CDX2 are determinants of cell fate that are required for GI tract development. CDX1 and 2 are not normally expressed in the adult esophagus; their overexpression in mice promoted intestinal metaplasia.194 Variants at the CDX2 binding site of VDR, which encodes the human vitamin D receptor, have been associated with obesity and fat mass.195 Altered activities of CDX1 and 2 have been shown to promote the epithelial to mesenchymal transition in GI cancer cells. This process can be mediated by epidermal growth factor, fibroblast growth factors, and transforming growth factors; levels of these factors are increased in obese individuals. Retinoic acid regulates an intracellular network of signaling pathways that lead to expression of CDX1 and 2, which also is modulated by thyroxine and its receptors. The thyroxine signaling mechanisms are linked to activation of farnesoid X nuclear receptor (FXR) and the G protein-coupled bile acid receptor 1 (GPBAR1 or TGR5).196 TGR5 is expressed in colon and activated by bile acids, diet, and microbiome-derived ligands, which release incretins with multiple effects on systemic cellular processes. Obese mice have been reported to have altered Tgr5 signaling; 197 studies are needed in humans. The TGF5 pathway could provide a link between the intestinal microbiome and EAC in obesity.
Intestinal Microbiota
The intestinal microbiota has been linked to activation of the inflammasome and inflammation in the intestinal epithelia. Inflammasomes help regulate aerobic glycolysis, which is involved in cell transformation, via the Warburg effect.198 It is not clear how obesity might alter the esophageal microbiome. Microbiota that colonize the esophagus are predominantly composed of Streptococcus species, but may be altered in esophagitis and BE patients to move gram-negative anaerobes and microaerophile.199 An altered esophageal microbiome might be involved in genesis of EAC after Helicobacter pylori eradication or in obese individuals, but further studies are needed.
Gastric Adenocarcinoma (GCA)
Gastric cancer ranks fourth in incidence and second in cancer-related death.156 About 90% of gastric cancers are GCAs, which are further categorized as distal or non-cardia GCAs and proximal or cardia-GCAs. Although the incidence of non-cardia GCA has decreased, that of cardia-GCA has increased. Like EAC, this is in part due to increasing BMIs and obesity.158, 159, 200 In a population-based cohort study, Chow et. al found a 2-fold increase for development of cardia-GCA with increased BMI. Additional studies showed that obesity increased the incidence of cardia-GCA.201 A meta-analysis of 4 cohort studies found no association between non-cardia GCA and obesity.202 Subgroup analysis also showed no differences for total GCA between obese and normal weight patients, after adjusting for sex, but did find a 1.5-fold increase in cardia-GCA among obese individuals.
Insulin and IGF1 Signaling
It is unclear how obesity might increase risk for EAC or GCA. The diseases share proposed mechanisms.203 Healthy gastric mucosa, hyperplastic polyps, intraepithelial neoplasia, and adenocarcinomas all express IGF1;204 levels increase progressively from benign proliferating lesions to cancer, indicating its role in tumor progression. Another study reported an increasing percentage of IGF1R-positive cells from healthy stomach, to hyperplastic tissue adjacent to carcinomas, to cancer.205 This study also associated IGF1R expression with metastasis and invasion. Low levels of IGF1R mRNA were associated with increased rate of overall survival in patients with GCA.206 Strategies to block IGF1R might therefore be developed to treat GCA.
Adipokines
Three small case-controlled studies reported varying levels of adiponectin in patients with GCA.54, 207, 208 One found lower plasma levels of adiponectin in patients with GCA than controls, but the relationship between BMI and adiponectin was stronger in controls than in patients. Another study made similar findings, but found a strong correlation between BMI and leptin in controls and patients. A small cohort study found no correlation between plasma level of adiponectin and BMI, but did associate level of adiponectin with tumor grade, so this protein might reduce GCA progression.
GCAs express levels of leptin and its receptor, especially during invasion.209-213 One study reported higher concentrations of leptin in patients with intestinal metaplasia. Another associated higher levels of leptin with stage and histologic features of GCA, Borrmann classification, metastasis to lymph nodes, and poor outcome. It is unclear if leptin activation of JAK–STAT signaling directly promotes or facilitates pathogenesis.
Inflammatory Factors
Obesity-induced inflammation is believed to promote development of GCA, via TNFα, IL6, and MCP1. In vitro and in vivo studies have shown that TNFα, IL6, IL17, and MCP1 stimulate proliferation and inhibit apoptosis of human gastric cancer cell lines.214, 215 Strategies to target these pathways could reduce GCA growth and invasion.
Pancreatic Adenocarcinoma (PAC)
Pancreatic cancer is the thirteenth most-common cancer and the eighth-leading cause of cancer-related death.156 The most common and deadly form of pancreatic cancer is pancreatic adenocarcinoma (PAC), which accounts for more than 90% of cases. Based on data from 23 cohort and 13 case-controlled studies, The Word Cancer Research Fund Panel concluded that there is “convincing increased risk” of PAC related to body adiposity and a “probable increased risk” with abdominal adiposity.216 A large meta-analysis found RRs of 1.07 and 1.12 for PAC per 5 kg/m2 increase in BMI in men and women, respectively.3 This was also observed in a pooled analysis of 7 prospective cohorts, with a RR of 1.06 for every 5kg/m2 increment of BMI.217
BMI has been associated with risk of PAC, age at onset, and overall survival. A case-control study reported an increased risk for PAC, and an earlier age of onset, among individuals who were overweight or obese in early adulthood (ORs, 1.67–2.58).218 Overweight or obese older patients with PAC were less likely to survive by an average of 4.5 months, with a RRs of 1.26 and 1.86, respectively.
Insulin and IGF1 Signaling
Several prospective studies associated increased circulating levels of glucose, insulin, and plasma C-peptide with increased risk for PAC.219-222 Epidemiology studies found no significant associations between circulating IGF1 or its binding proteins with PAC, but other studies associated lower levels of IGFBP1 with increased risk for PAC.223-225 A case–control study found that subjects in the lowest quartile of plasma IGFBP1 level had an odds ratio for PAC of 2.07, compared to the 3 highest quartiles; the effects of low plasma IGFBP1 became progressively stronger with time. IGF1 and IGF1R are highly expressed in pancreatic cell lines; IGF1 signaling promotes their proliferation, invasion, expression of angiogenic factors, and reduces apoptosis.226-229
Adipokines
There have been few studies of leptin and adiponectin levels in PAC patients. In obese rats injected with human PAC cells, no associations were made between leptin levels and cell proliferation or tumor progression. However, these processes did have an inverse relationship with adiponectin level. Other studies produced contradictory data. Most patients with PAC patients have lost weight by the time they receive their diagnosis, which could lower levels of leptin and increase levels of adiponectin; circulating adiponectin has been inversely associated with tumor progression. 53, 230-232 Future studies should clarify the relationships among adipokine levels, obesity, and PAC. It will be interesting to investigate the effects of metformin on the AMP-activated protein kinase pathway, via adiponectin receptors 1 and 2.189
Genetic associations between obesity and PAC
Genetic factors that contribute to obesity might also increase susceptibility to PAC. A genome-wide association study identified NR5A2 as a susceptibility gene for PAC.233 In a case-control study, carries of a specific NR5A2 variant had a lower risk of PAC compared to carries of a common allele, regardless of BMI or diabetes.234, 235 The association between PAC and polymorphisms in PPARG is controversial.
CONCLUSIONS
Our understanding of how obesity contributes to the pathogenesis and development of GI cancers is increasing; greater insight into the molecular mechanisms might lead to identification of new therapeutic targets. Given the lack of current effective therapies for weight control or reduction beyond bariatric surgery, research and clinical efforts will focus on the connections between increase in adipose cell mass and GI carcinogenesis. Further studies of the hormonal, inflammatory, genetic, and dietary factors that contribute to development and progression of GI cancers will help us understand the role of obesity in these processes.
Acknowledgments
Supported in part by:
R01 DK 081410 and T32 DK 007150 (AJS)
Italian Association for Cancer Research (Milan, Italy, IG 10216), the European Community's Seventh Framework Program FP7-KBBE-2012-2016 under grant agreement 311876 (Pathway-27) (LR)
Center for Basic and Translational Research on Disorders of the Digestive System through the generosity of the Leona M. and Harry B Helmsley Charitable Trust; and Clinical Translational Science Award 5UL1TR000043-08 from the National Center for Advancing Translational Sciences (JOA and PRH)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: Luigi Ricciardiello received an unrestricted scientific grant from SLA Pharma AG. José O. Alemán, Leonardo H. Eusebi, Kavish Patidar, Arun J. Sanyal and Peter R. Holt report no conflicts of interest.
Authors involvement
José O. Alemán: drafting of the manuscript, manuscript editing, figures preparation
Leonardo H. Eusebi: drafting and editing of the manuscript
Kavish Patidar: drafting of the manuscript
Luigi Ricciardiello: drafting and editing of the manuscript; manuscript concept and design
Arun J. Sanyal: drafting of the manuscript; manuscript concept and design
Peter R. Holt: drafting of the manuscript, manuscript editing, figures preparation; manuscript concept and design
REFERENCES
- 1.Ogden CL, Yanovski SZ, Carroll MD, et al. The epidemiology of obesity. Gastroenterology. 2007;132:2087–102. doi: 10.1053/j.gastro.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 2.Fontaine KR, Redden DT, Wang C, et al. Years of life lost due to obesity. Jama. 2003;289:187–93. doi: 10.1001/jama.289.2.187. [DOI] [PubMed] [Google Scholar]
- 3.Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–78. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 4.Wolin KY, Carson K, Colditz GA. Obesity and cancer. Oncologist. 2013;15:556–65. doi: 10.1634/theoncologist.2009-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kloting N, Fasshauer M, Dietrich A, et al. Insulin-sensitive obesity. Am J Physiol Endocrinol Metab. 2010;299:E506–15. doi: 10.1152/ajpendo.00586.2009. [DOI] [PubMed] [Google Scholar]
- 6.Demark-Wahnefried W, Platz EA, Ligibel JA, et al. The role of obesity in cancer survival and recurrence. Cancer Epidemiol Biomarkers Prev. 2012;21:1244–59. doi: 10.1158/1055-9965.EPI-12-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.YALOW RS, BERSON SA. Immunoassay of endogenous plasma insulin in man. The Journal of clinical investigation. 1960;39:1157–75. doi: 10.1172/JCI104130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.JOSLIN EP, LOMBARD HL, BURROWS RE, et al. Diabetes and cancer. The New England journal of medicine. 1959;260:486–8. doi: 10.1056/NEJM195903052601007. [DOI] [PubMed] [Google Scholar]
- 9.Lakka HM, Salonen JT, Tuomilehto J, et al. Obesity and weight gain are associated with increased incidence of hyperinsulinemia in non-diabetic men. Horm Metab Res. 2002;34:492–8. doi: 10.1055/s-2002-34788. [DOI] [PubMed] [Google Scholar]
- 10.Heuson JC, Legros N. Influence of insulin deprivation on growth of the 7,12-dimethylbenz(a)anthracene-induced mammary carcinoma in rats subjected to alloxan diabetes and food restriction. Cancer research. 1972;32:226–32. [PubMed] [Google Scholar]
- 11.DeBerardinis RJ, Thompson CB. Cellular metabolism and disease: what do metabolic outliers teach us? Cell. 2012;148:1132–44. doi: 10.1016/j.cell.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warburg O, Wind F, Negelein E. THE METABOLISM OF TUMORS IN THE BODY. The Journal of general physiology. 1927;8:519–30. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science (New York, N.Y.) 2009;324:1029–33. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jenab M, Riboli E, Cleveland RJ, et al. Serum C-peptide, IGFBP-1 and IGFBP-2 and risk of colon and rectal cancers in the European Prospective Investigation into Cancer and Nutrition. International journal of cancer. Journal international du cancer. 2007;121:368–76. doi: 10.1002/ijc.22697. [DOI] [PubMed] [Google Scholar]
- 15.Finkelstein JW, Kream J, Ludan A, et al. Sulfation factor (somatomedin): an explanation for continued growth in the absence of immunoassayable growth hormone in patients with hypothalamic tumors. The Journal of clinical endocrinology and metabolism. 1972;35:13–7. doi: 10.1210/jcem-35-1-13. [DOI] [PubMed] [Google Scholar]
- 16.Orme SM, McNally RJ, Cartwright RA, et al. Mortality and cancer incidence in acromegaly: a retrospective cohort study. United Kingdom Acromegaly Study Group. J Clin Endocrinol Metab. 1998;83:2730–4. doi: 10.1210/jcem.83.8.5007. [DOI] [PubMed] [Google Scholar]
- 17.Laron Z. The essential role of IGF-I: lessons from the long-term study and treatment of children and adults with Laron syndrome. The Journal of clinical endocrinology and metabolism. 1999;84:4397–404. doi: 10.1210/jcem.84.12.6255. [DOI] [PubMed] [Google Scholar]
- 18.Shevah O, Laron Z. Patients with congenital deficiency of IGF-I seem protected from the development of malignancies: a preliminary report. Growth Horm IGF Res. 2007;17:54–7. doi: 10.1016/j.ghir.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Brick DJ, Gerweck AV, Meenaghan E, et al. Determinants of IGF1 and GH across the weight spectrum: from anorexia nervosa to obesity. European journal of endocrinology / European Federation of Endocrine Societies. 2010;163:185–91. doi: 10.1530/EJE-10-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El Yafi F, Winkler R, Delvenne P, et al. Altered expression of type I insulin-like growth factor receptor in Crohn's disease. Clinical and experimental immunology. 2005;139:526–33. doi: 10.1111/j.1365-2249.2004.02724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Goudoever JB, Corpeleijn W, Riedijk M, et al. The Impact of Enteral Insulin-Like Growth Factor 1 and Nutrition on Gut Permeability and Amino Acid Utilization. J. Nutr. 2008;138:1829S–1833. doi: 10.1093/jn/138.9.1829S. [DOI] [PubMed] [Google Scholar]
- 22.Peyrat JP, Bonneterre J, Beuscart R, et al. Insulin-like growth factor 1 receptors in human breast cancer and their relation to estradiol and progesterone receptors. Cancer research. 1988;48:6429–33. [PubMed] [Google Scholar]
- 23.Gallagher EJ, LeRoith D. Minireview: IGF, Insulin, and Cancer. Endocrinology. 2011;152:2546–51. doi: 10.1210/en.2011-0231. [DOI] [PubMed] [Google Scholar]
- 24.Butler AA, LeRoith D. Minireview: tissue-specific versus generalized gene targeting of the igf1 and igf1r genes and their roles in insulin-like growth factor physiology. Endocrinology. 2001;142:1685–8. doi: 10.1210/endo.142.5.8148. [DOI] [PubMed] [Google Scholar]
- 25.Giovannucci E. Nutrition, insulin, insulin-like growth factors and cancer. Horm Metab Res. 2003;35:694–704. doi: 10.1055/s-2004-814147. [DOI] [PubMed] [Google Scholar]
- 26.Giovannucci E. Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. Am J Clin Nutr. 2007;86:s836–42. doi: 10.1093/ajcn/86.3.836S. [DOI] [PubMed] [Google Scholar]
- 27.Hart LS, Dolloff NG, Dicker DT, et al. Human colon cancer stem cells are enriched by insulin-like growth factor-1 and are sensitive to figitumumab. Cell cycle (Georgetown, Tex.) 2011;10:2331–8. doi: 10.4161/cc.10.14.16418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pollak M. NCIC-CTG MA14 Trial: Tamoxifen (tam) vs. tam + octreotide (oct) for adjuvant treatment of stage I or II postmenopausal breast cancer -- Pollak et al. 26 (15 Supplement): 532 - - ASCO Meeting Abstracts. ASCO Meeting Abstracts. 2008:532. [Google Scholar]
- 29.Haluska P, Shaw HM, Batzel GN, et al. Phase I dose escalation study of the anti insulin-like growth factor-I receptor monoclonal antibody CP-751,871 in patients with refractory solid tumors. Clin Cancer Res. 2007;13:5834–40. doi: 10.1158/1078-0432.CCR-07-1118. [DOI] [PubMed] [Google Scholar]
- 30.Haluska P, Worden F, Olmos D, et al. Safety, tolerability, and pharmacokinetics of the anti-IGF-1R monoclonal antibody figitumumab in patients with refractory adrenocortical carcinoma. Cancer chemotherapy and pharmacology. 2010;65:765–73. doi: 10.1007/s00280-009-1083-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jassem J, Langer CJ, Karp DD, et al. Randomized, open label, phase III trial of figitumumab in combination with paclitaxel and carboplatin versus paclitaxel and carboplatin in patients with non-small cell lung cancer (NSCLC). ASCO Meeting Abstracts. 2010;28:7500. [Google Scholar]
- 32.MacDougald OA, Burant CF. The rapidly expanding family of adipokines. Cell Metab. 2007;6:159–61. doi: 10.1016/j.cmet.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 33.Luo R, Li X, Jiang R, et al. Serum concentrations of resistin and adiponectin and their relationship to insulin resistance in subjects with impaired glucose tolerance. J Int Med Res. 2012;40:621–30. doi: 10.1177/147323001204000224. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Proenca R, Maffei M, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 35.Fain JN, Madan AK, Hiler ML, et al. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145:2273–82. doi: 10.1210/en.2003-1336. [DOI] [PubMed] [Google Scholar]
- 36.Houseknecht KL, Mantzoros CS, Kuliawat R, et al. Evidence for leptin binding to proteins in serum of rodents and humans: modulation with obesity. Diabetes. 1996;45:1638–43. doi: 10.2337/diab.45.11.1638. [DOI] [PubMed] [Google Scholar]
- 37.Margetic S, Gazzola C, Pegg GG, et al. Leptin: a review of its peripheral actions and interactions. Int J Obes Relat Metab Disord. 2002;26:1407–33. doi: 10.1038/sj.ijo.0802142. [DOI] [PubMed] [Google Scholar]
- 38.Grunfeld C, Zhao C, Fuller J, et al. Endotoxin and cytokines induce expression of leptin, the ob gene product, in hamsters. J Clin Invest. 1996;97:2152–7. doi: 10.1172/JCI118653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garofalo C, Surmacz E. Leptin and cancer. J Cell Physiol. 2006;207:12–22. doi: 10.1002/jcp.20472. [DOI] [PubMed] [Google Scholar]
- 40.Drew JE, Farquharson AJ, Padidar S, et al. Insulin, leptin, and adiponectin receptors in colon: regulation relative to differing body adiposity independent of diet and in response to dimethylhydrazine. Am J Physiol Gastrointest Liver Physiol. 2007;293:G682–91. doi: 10.1152/ajpgi.00231.2007. [DOI] [PubMed] [Google Scholar]
- 41.Hoda MR, Keely SJ, Bertelsen LS, et al. Leptin acts as a mitogenic and antiapoptotic factor for colonic cancer cells. Br J Surg. 2007;94:346–54. doi: 10.1002/bjs.5530. [DOI] [PubMed] [Google Scholar]
- 42.Capel F, Klimcakova E, Viguerie N, et al. Macrophages and adipocytes in human obesity: adipose tissue gene expression and insulin sensitivity during calorie restriction and weight stabilization. Diabetes. 2009;58:1558–67. doi: 10.2337/db09-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.White PB, True EM, Ziegler KM, et al. Insulin, leptin, and tumoral adipocytes promote murine pancreatic cancer growth. J Gastrointest Surg. 2010;14:1888–93. doi: 10.1007/s11605-010-1349-x. discussion 1893-4. [DOI] [PubMed] [Google Scholar]
- 44.Rose DP, Vona-Davis L. The cellular and molecular mechanisms by which insulin influences breast cancer risk and progression. Endocr Relat Cancer. 2012;19:R225–41. doi: 10.1530/ERC-12-0203. [DOI] [PubMed] [Google Scholar]
- 45.Sennello JA, Fayad R, Morris AM, et al. Regulation of T cell-mediated hepatic inflammation by adiponectin and leptin. Endocrinology. 2005;146:2157–64. doi: 10.1210/en.2004-1572. [DOI] [PubMed] [Google Scholar]
- 46.Ouchi N, Kihara S, Funahashi T, et al. Obesity, adiponectin and vascular inflammatory disease. Curr Opin Lipidol. 2003;14:561–6. doi: 10.1097/00041433-200312000-00003. [DOI] [PubMed] [Google Scholar]
- 47.Gavrila A, Peng CK, Chan JL, et al. Diurnal and ultradian dynamics of serum adiponectin in healthy men: comparison with leptin, circulating soluble leptin receptor, and cortisol patterns. J Clin Endocrinol Metab. 2003;88:2838–43. doi: 10.1210/jc.2002-021721. [DOI] [PubMed] [Google Scholar]
- 48.Motoshima H, Wu X, Sinha MK, et al. Differential regulation of adiponectin secretion from cultured human omental and subcutaneous adipocytes: effects of insulin and rosiglitazone. J Clin Endocrinol Metab. 2002;87:5662–7. doi: 10.1210/jc.2002-020635. [DOI] [PubMed] [Google Scholar]
- 49.Kern PA, Di Gregorio GB, Lu T, et al. Adiponectin expression from human adipose tissue: relation to obesity, insulin resistance, and tumor necrosis factor-alpha expression. Diabetes. 2003;52:1779–85. doi: 10.2337/diabetes.52.7.1779. [DOI] [PubMed] [Google Scholar]
- 50.Ziemke F, Mantzoros CS. Adiponectin in insulin resistance: lessons from translational research. Am J Clin Nutr. 2010;91:258S–261S. doi: 10.3945/ajcn.2009.28449C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Storgaard H, Poulsen P, Ling C, et al. Relationships of plasma adiponectin level and adiponectin receptors 1 and 2 gene expression to insulin sensitivity and glucose and fat metabolism in monozygotic and dizygotic twins. J Clin Endocrinol Metab. 2007;92:2835–9. doi: 10.1210/jc.2006-1812. [DOI] [PubMed] [Google Scholar]
- 52.Shibata R, Ouchi N, Kihara S, et al. Adiponectin stimulates angiogenesis in response to tissue ischemia through stimulation of amp-activated protein kinase signaling. J Biol Chem. 2004;279:28670–4. doi: 10.1074/jbc.M402558200. [DOI] [PubMed] [Google Scholar]
- 53.Dalamaga M, Migdalis I, Fargnoli JL, et al. Pancreatic cancer expresses adiponectin receptors and is associated with hypoleptinemia and hyperadiponectinemia: a case-control study. Cancer Causes Control. 2009;20:625–33. doi: 10.1007/s10552-008-9273-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ishikawa M, Kitayama J, Kazama S, et al. Plasma adiponectin and gastric cancer. Clin Cancer Res. 2005;11:466–72. [PubMed] [Google Scholar]
- 55.Wu B, Fukuo K, Suzuki K, et al. Relationships of systemic oxidative stress to body fat distribution, adipokines and inflammatory markers in healthy middle-aged women. Endocr J. 2009;56:773–82. doi: 10.1507/endocrj.k08e-332. [DOI] [PubMed] [Google Scholar]
- 56.Yamauchi T, Kamon J, Minokoshi Y, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–95. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 57.Vansaun MN. Molecular pathways: adiponectin and leptin signaling in cancer. Clin Cancer Res. 2013;19:1926–32. doi: 10.1158/1078-0432.CCR-12-0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yokota T, Oritani K, Takahashi I, et al. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood. 2000;96:1723–32. [PubMed] [Google Scholar]
- 59.Ohashi K, Parker JL, Ouchi N, et al. Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J Biol Chem. 2010;285:6153–60. doi: 10.1074/jbc.M109.088708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Derby CA, Zilber S, Brambilla D, et al. Body mass index, waist circumference and waist to hip ratio and change in sex steroid hormones: the Massachusetts Male Ageing Study. Clin Endocrinol (Oxf) 2006;65:125–31. doi: 10.1111/j.1365-2265.2006.02560.x. [DOI] [PubMed] [Google Scholar]
- 61.Lin JH, Giovannucci E. Sex hormones and colorectal cancer: what have we learned so far? J Natl Cancer Inst. 2010;102:1746–7. doi: 10.1093/jnci/djq444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rudolph A, Toth C, Hoffmeister M, et al. Colorectal cancer risk associated with hormone use varies by expression of estrogen receptor-beta. Cancer Res. 2013;73:3306–15. doi: 10.1158/0008-5472.CAN-12-4051. [DOI] [PubMed] [Google Scholar]
- 63.Bouchard C, Tremblay A. Genetic influences on the response of body fat and fat distribution to positive and negative energy balances in human identical twins. J Nutr. 1997;127:943S–947S. doi: 10.1093/jn/127.5.943S. [DOI] [PubMed] [Google Scholar]
- 64.Lee RK, Hittel DS, Nyamandi VZ, et al. Unconventional microarray design reveals the response to obesity is largely tissue specific: analysis of common and divergent responses to diet-induced obesity in insulin-sensitive tissues. Appl Physiol Nutr Metab. 2012;37:257–68. doi: 10.1139/h11-159. [DOI] [PubMed] [Google Scholar]
- 65.Gunter MJ, Leitzmann MF. Obesity and colorectal cancer: epidemiology, mechanisms and candidate genes. J Nutr Biochem. 2006;17:145–56. doi: 10.1016/j.jnutbio.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 66.Yang X, Deignan JL, Qi H, et al. Validation of candidate causal genes for obesity that affect shared metabolic pathways and networks. Nat Genet. 2009;41:415–23. doi: 10.1038/ng.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gulati P, Cheung MK, Antrobus R, et al. Role for the obesity-related FTO gene in the cellular sensing of amino acids. Proc Natl Acad Sci U S A. 2013;110:2557–62. doi: 10.1073/pnas.1222796110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vongsuvanh R, George J, Qiao L, et al. Visceral adiposity in gastrointestinal and hepatic carcinogenesis. Cancer Lett. 2013;330:1–10. doi: 10.1016/j.canlet.2012.11.038. [DOI] [PubMed] [Google Scholar]
- 69.Balistreri CR, Caruso C, Candore G. The role of adipose tissue and adipokines in obesity-related inflammatory diseases. Mediators Inflamm. 2010;2010:802078. doi: 10.1155/2010/802078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ben-Neriah Y, Karin M. Inflammation meets cancer, with NF-kappaB as the matchmaker. Nat Immunol. 2011;12:715–23. doi: 10.1038/ni.2060. [DOI] [PubMed] [Google Scholar]
- 71.Goossens GH, Blaak EE, Theunissen R, et al. Expression of NLRP3 inflammasome and T cell population markers in adipose tissue are associated with insulin resistance and impaired glucose metabolism in humans. Mol Immunol. 2012;50:142–9. doi: 10.1016/j.molimm.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 72.Poullis A, Foster R, Shetty A, et al. Bowel inflammation as measured by fecal calprotectin: a link between lifestyle factors and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2004;13:279–84. doi: 10.1158/1055-9965.epi-03-0160. [DOI] [PubMed] [Google Scholar]
- 73.Pendyala S, Neff LM, Suarez-Farinas M, et al. Diet-induced weight loss reduces colorectal inflammation: implications for colorectal carcinogenesis. Am J Clin Nutr. 2011;93:234–42. doi: 10.3945/ajcn.110.002683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121:2111–7. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cinti S, Mitchell G, Barbatelli G, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–55. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 76.Strissel KJ, Stancheva Z, Miyoshi H, et al. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007;56:2910–8. doi: 10.2337/db07-0767. [DOI] [PubMed] [Google Scholar]
- 77.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–46. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 78.Feuerer M, Herrero L, Cipolletta D, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–9. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cancello R, Henegar C, Viguerie N, et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54:2277–86. doi: 10.2337/diabetes.54.8.2277. [DOI] [PubMed] [Google Scholar]
- 80.Imayama I, Ulrich CM, Alfano CM, et al. Effects of a caloric restriction weight loss diet and exercise on inflammatory biomarkers in overweight/obese postmenopausal women: a randomized controlled trial. Cancer research. 2012;72:2314–26. doi: 10.1158/0008-5472.CAN-11-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Spencer M, Finlin BS, Unal R, et al. Omega-3 Fatty Acids Reduce Adipose Tissue Macrophages in Human Subjects With Insulin Resistance. Diabetes. 2013 doi: 10.2337/db12-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stefan N, Kantartzis K, Machann J, et al. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med. 2008;168:1609–16. doi: 10.1001/archinte.168.15.1609. [DOI] [PubMed] [Google Scholar]
- 83.Golan R, Shelef I, Rudich A, et al. Abdominal superficial subcutaneous fat: a putative distinct protective fat subdepot in type 2 diabetes. Diabetes Care. 2012;35:640–7. doi: 10.2337/dc11-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pendyala S, Walker JM, Holt PR. A high-fat diet is associated with endotoxemia that originates from the gut. Gastroenterology. 2012;142:1100–1101. e2. doi: 10.1053/j.gastro.2012.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Backhed F, Ding H, Wang T, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101:15718–23. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ridaura VK, Faith JJ, Rey FE, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341:1241214. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–4. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ding S, Chi MM, Scull BP, et al. High-fat diet: bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLoS One. 2010;5:e12191. doi: 10.1371/journal.pone.0012191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.de La Serre CB, Ellis CL, Lee J, et al. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am J Physiol Gastrointest Liver Physiol. 2010;299:G440–8. doi: 10.1152/ajpgi.00098.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–72. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 91.Ley RE, Backhed F, Turnbaugh P, et al. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102:11070–5. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ley RE, Turnbaugh PJ, Klein S, et al. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–3. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 93.Musso G, Gambino R, Cassader M. Interactions between gut microbiota and host metabolism predisposing to obesity and diabetes. Annu Rev Med. 2011;62:361–80. doi: 10.1146/annurev-med-012510-175505. [DOI] [PubMed] [Google Scholar]
- 94.Furet JP, Kong LC, Tap J, et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes. 2010;59:3049–57. doi: 10.2337/db10-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Duncan SH, Lobley GE, Holtrop G, et al. Human colonic microbiota associated with diet, obesity and weight loss. Int J Obes (Lond) 2008;32:1720–4. doi: 10.1038/ijo.2008.155. [DOI] [PubMed] [Google Scholar]
- 96.Arthur JC, Perez-Chanona E, Muhlbauer M, et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338:120–3. doi: 10.1126/science.1224820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vrieze A, Van Nood E, Holleman F, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913–6. e7. doi: 10.1053/j.gastro.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 98.van Nood E, Dijkgraaf MG, Keller JJ. Duodenal infusion of feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:2145. doi: 10.1056/NEJMc1303919. [DOI] [PubMed] [Google Scholar]
- 99.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 100.Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies. Am J Clin Nutr. 2007;86:556–65. doi: 10.1093/ajcn/86.3.556. [DOI] [PubMed] [Google Scholar]
- 101.Koch TC, Briviba K, Watzl B, et al. Obesity-related promotion of aberrant crypt foci in DMH-treated obese Zucker rats correlates with dyslipidemia rather than hyperinsulinemia. Eur J Nutr. 2008;47:161–70. doi: 10.1007/s00394-008-0711-1. [DOI] [PubMed] [Google Scholar]
- 102.Bruce WR, Wolever TM, Giacca A. Mechanisms linking diet and colorectal cancer: the possible role of insulin resistance. Nutr Cancer. 2000;37:19–26. doi: 10.1207/S15327914NC3701_2. [DOI] [PubMed] [Google Scholar]
- 103.Komninou D, Ayonote A, Richie JP, Jr., et al. Insulin resistance and its contribution to colon carcinogenesis. Exp Biol Med (Maywood) 2003;228:396–405. doi: 10.1177/153537020322800410. [DOI] [PubMed] [Google Scholar]
- 104.Ouban A, Muraca P, Yeatman T, et al. Expression and distribution of insulin-like growth factor-1 receptor in human carcinomas. Hum Pathol. 2003;34:803–8. doi: 10.1016/s0046-8177(03)00291-0. [DOI] [PubMed] [Google Scholar]
- 105.Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 106.Guo YS, Narayan S, Yallampalli C, et al. Characterization of insulinlike growth factor I receptors in human colon cancer. Gastroenterology. 1992;102:1101–8. [PubMed] [Google Scholar]
- 107.Singh P, Rubin N. Insulinlike growth factors and binding proteins in colon cancer. Gastroenterology. 1993;105:1218–37. doi: 10.1016/0016-5085(93)90971-e. [DOI] [PubMed] [Google Scholar]
- 108.Fenton JI, Hord NG, Lavigne JA, et al. Leptin, insulin-like growth factor-1, and insulin-like growth factor-2 are mitogens in ApcMin/+ but not Apc+/+ colonic epithelial cell lines. Cancer Epidemiol Biomarkers Prev. 2005;14:1646–52. doi: 10.1158/1055-9965.EPI-04-0916. [DOI] [PubMed] [Google Scholar]
- 109.Takahashi H, Takayama T, Hosono K, et al. Correlation of the plasma level of insulin-like growth factor-1 with the number of aberrant crypt foci in male individuals. Mol Med Rep. 2009;2:339–43. doi: 10.3892/mmr_00000105. [DOI] [PubMed] [Google Scholar]
- 110.Endo H, Hosono K, Uchiyama T, et al. Leptin acts as a growth factor for colorectal tumours at stages subsequent to tumour initiation in murine colon carcinogenesis. Gut. 2011;60:1363–71. doi: 10.1136/gut.2010.235754. [DOI] [PubMed] [Google Scholar]
- 111.Attoub S, Noe V, Pirola L, et al. Leptin promotes invasiveness of kidney and colonic epithelial cells via phosphoinositide 3-kinase-, rho-, and rac-dependent signaling pathways. Faseb J. 2000;14:2329–38. doi: 10.1096/fj.00-0162. [DOI] [PubMed] [Google Scholar]
- 112.Drew JE. Molecular mechanisms linking adipokines to obesity-related colon cancer: focus on leptin. Proc Nutr Soc. 2012;71:175–80. doi: 10.1017/S0029665111003259. [DOI] [PubMed] [Google Scholar]
- 113.Padidar S, Farquharson AJ, Williams LM, et al. Leptin up-regulates pro-inflammatory cytokines in discrete cells within mouse colon. J Cell Physiol. 2011;226:2123–30. doi: 10.1002/jcp.22546. [DOI] [PubMed] [Google Scholar]
- 114.Park EJ, Lee JH, Yu GY, et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stattin P, Lukanova A, Biessy C, et al. Obesity and colon cancer: does leptin provide a link? Int J Cancer. 2004;109:149–52. doi: 10.1002/ijc.11668. [DOI] [PubMed] [Google Scholar]
- 116.Stattin P, Palmqvist R, Soderberg S, et al. Plasma leptin and colorectal cancer risk: a prospective study in Northern Sweden. Oncol Rep. 2003;10:2015–21. [PubMed] [Google Scholar]
- 117.Tamakoshi K, Toyoshima H, Wakai K, et al. Leptin is associated with an increased female colorectal cancer risk: a nested case-control study in Japan. Oncology. 2005;68:454–61. doi: 10.1159/000086988. [DOI] [PubMed] [Google Scholar]
- 118.Ho GY, Wang T, Gunter MJ, et al. Adipokines linking obesity with colorectal cancer risk in postmenopausal women. Cancer Res. 2012;72:3029–37. doi: 10.1158/0008-5472.CAN-11-2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Aleksandrova K, Boeing H, Jenab M, et al. Leptin and soluble leptin receptor in risk of colorectal cancer in the European Prospective Investigation into Cancer and Nutrition cohort. Cancer Res. 2012;72:5328–37. doi: 10.1158/0008-5472.CAN-12-0465. [DOI] [PubMed] [Google Scholar]
- 120.Uddin S, Bavi PP, Hussain AR, et al. Leptin receptor expression in Middle Eastern colorectal cancer and its potential clinical implication. Carcinogenesis. 2009;30:1832–40. doi: 10.1093/carcin/bgp145. [DOI] [PubMed] [Google Scholar]
- 121.Tutino V, Notarnicola M, Guerra V, et al. Increased soluble leptin receptor levels are associated with advanced tumor stage in colorectal cancer patients. Anticancer Res. 2011;31:3381–3. [PubMed] [Google Scholar]
- 122.Song M, Zhang X, Wu K, et al. Plasma adiponectin and soluble leptin receptor and risk of colorectal cancer: a prospective study. Cancer Prev Res (Phila) 2013;6:875–85. doi: 10.1158/1940-6207.CAPR-13-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chia VM, Newcomb PA, Lampe JW, et al. Leptin concentrations, leptin receptor polymorphisms, and colorectal adenoma risk. Cancer Epidemiol Biomarkers Prev. 2007;16:2697–703. doi: 10.1158/1055-9965.EPI-07-0467. [DOI] [PubMed] [Google Scholar]
- 124.Moon HS, Liu X, Nagel JM, et al. Salutary effects of adiponectin on colon cancer: in vivo and in vitro studies in mice. Gut. 2013;62:561–70. doi: 10.1136/gutjnl-2012-302092. [DOI] [PubMed] [Google Scholar]
- 125.Fujisawa T, Endo H, Tomimoto A, et al. Adiponectin suppresses colorectal carcinogenesis under the high-fat diet condition. Gut. 2008;57:1531–8. doi: 10.1136/gut.2008.159293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ohashi K, Ouchi N, Matsuzawa Y. Adiponectin and hypertension. Am J Hypertens. 2011;24:263–9. doi: 10.1038/ajh.2010.216. [DOI] [PubMed] [Google Scholar]
- 127.Xu XT, Xu Q, Tong JL, et al. Meta-analysis: circulating adiponectin levels and risk of colorectal cancer and adenoma. J Dig Dis. 2011;12:234–44. doi: 10.1111/j.1751-2980.2011.00504.x. [DOI] [PubMed] [Google Scholar]
- 128.Aleksandrova K, Boeing H, Jenab M, et al. Total and high-molecular weight adiponectin and risk of colorectal cancer: the European Prospective Investigation into Cancer and Nutrition Study. Carcinogenesis. 2012;33:1211–8. doi: 10.1093/carcin/bgs133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chung YC, Chang YF. Serum interleukin-6 levels reflect the disease status of colorectal cancer. J Surg Oncol. 2003;83:222–6. doi: 10.1002/jso.10269. [DOI] [PubMed] [Google Scholar]
- 130.Kim S, Keku TO, Martin C, et al. Circulating levels of inflammatory cytokines and risk of colorectal adenomas. Cancer Res. 2008;68:323–8. doi: 10.1158/0008-5472.CAN-07-2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Grivennikov S, Karin E, Terzic J, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–13. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Becker C, Fantini MC, Wirtz S, et al. IL-6 signaling promotes tumor growth in colorectal cancer. Cell Cycle. 2005;4:217–20. [PubMed] [Google Scholar]
- 133.Chan AT, Ogino S, Giovannucci EL, et al. Inflammatory markers are associated with risk of colorectal cancer and chemopreventive response to anti-inflammatory drugs. Gastroenterology. 2011;140:799–808. doi: 10.1053/j.gastro.2010.11.041. quiz e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Paruchuri S, Broom O, Dib K, et al. The pro-inflammatory mediator leukotriene D4 induces phosphatidylinositol 3-kinase and Rac-dependent migration of intestinal epithelial cells. J Biol Chem. 2005;280:13538–44. doi: 10.1074/jbc.M409811200. [DOI] [PubMed] [Google Scholar]
- 135.Sikalidis AK, Varamini B. Roles of hormones and signaling molecules in describing the relationship between obesity and colon cancer. Pathol Oncol Res. 2011;17:785–90. doi: 10.1007/s12253-010-9352-9. [DOI] [PubMed] [Google Scholar]
- 136.Martinez ME, Heddens D, Earnest DL, et al. Physical activity, body mass index, and prostaglandin E2 levels in rectal mucosa. J Natl Cancer Inst. 1999;91:950–3. doi: 10.1093/jnci/91.11.950. [DOI] [PubMed] [Google Scholar]
- 137.Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008;8:387–98. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- 138.Levine AJ, Feng Z, Mak TW, et al. Coordination and communication between the p53 and IGF-1-AKT-TOR signal transduction pathways. Genes Dev. 2006;20:267–75. doi: 10.1101/gad.1363206. [DOI] [PubMed] [Google Scholar]
- 139.Morikawa T, Kuchiba A, Liao X, et al. Tumor TP53 expression status, body mass index and prognosis in colorectal cancer. Int J Cancer. 2012;131:1169–78. doi: 10.1002/ijc.26495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ogino S, Nosho K, Shima K, et al. p21 expression in colon cancer and modifying effects of patient age and body mass index on prognosis. Cancer Epidemiol Biomarkers Prev. 2009;18:2513–21. doi: 10.1158/1055-9965.EPI-09-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ogino S, Nosho K, Irahara N, et al. Prognostic significance and molecular associations of 18q loss of heterozygosity: a cohort study of microsatellite stable colorectal cancers. J Clin Oncol. 2009;27:4591–8. doi: 10.1200/JCO.2009.22.8858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Morikawa T, Kuchiba A, Lochhead P, et al. Prospective analysis of body mass index, physical activity, and colorectal cancer risk associated with beta-catenin (CTNNB1) status. Cancer Res. 2013;73:1600–10. doi: 10.1158/0008-5472.CAN-12-2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Morikawa T, Kuchiba A, Yamauchi M, et al. Association of CTNNB1 (beta-catenin) alterations, body mass index, and physical activity with survival in patients with colorectal cancer. Jama. 2011;305:1685–94. doi: 10.1001/jama.2011.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Boutron-Ruault MC, Senesse P, Meance S, et al. Energy intake, body mass index, physical activity, and the colorectal adenoma-carcinoma sequence. Nutr Cancer. 2001;39:50–7. doi: 10.1207/S15327914nc391_7. [DOI] [PubMed] [Google Scholar]
- 145.Slattery ML, Potter J, Caan B, et al. Energy balance and colon cancer--beyond physical activity. Cancer Res. 1997;57:75–80. [PubMed] [Google Scholar]
- 146.Michaud DS, Fuchs CS, Liu S, et al. Dietary glycemic load, carbohydrate, sugar, and colorectal cancer risk in men and women. Cancer Epidemiol Biomarkers Prev. 2005;14:138–47. [PubMed] [Google Scholar]
- 147.Kushi L, Giovannucci E. Dietary fat and cancer. Am J Med. 2002;113(Suppl 9B):63S–70S. doi: 10.1016/s0002-9343(01)00994-9. [DOI] [PubMed] [Google Scholar]
- 148.Parker ED, Folsom AR. Intentional weight loss and incidence of obesity-related cancers: the Iowa Women's Health Study. Int J Obes Relat Metab Disord. 2003;27:1447–52. doi: 10.1038/sj.ijo.0802437. [DOI] [PubMed] [Google Scholar]
- 149.Rapp K, Klenk J, Ulmer H, et al. Weight change and cancer risk in a cohort of more than 65,000 adults in Austria. Ann Oncol. 2008;19:641–8. doi: 10.1093/annonc/mdm549. [DOI] [PubMed] [Google Scholar]
- 150.Papathanasopoulos A, Camilleri M. Dietary fiber supplements: effects in obesity and metabolic syndrome and relationship to gastrointestinal functions. Gastroenterology. 2010;138:65–72. e1–2. doi: 10.1053/j.gastro.2009.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753–61. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 152.Sjostrom L, Gummesson A, Sjostrom CD, et al. Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): a prospective, controlled intervention trial. Lancet Oncol. 2009;10:653–62. doi: 10.1016/S1470-2045(09)70159-7. [DOI] [PubMed] [Google Scholar]
- 153.Derogar M, Hull MA, Kant P, et al. Increased risk of colorectal cancer after obesity surgery. Ann Surg. 2013;258:983–8. doi: 10.1097/SLA.0b013e318288463a. [DOI] [PubMed] [Google Scholar]
- 154.Ostlund MP, Lu Y, Lagergren J. Risk of obesity-related cancer after obesity surgery in a population-based cohort study. Ann Surg. 2010;252:972–6. doi: 10.1097/SLA.0b013e3181e33778. [DOI] [PubMed] [Google Scholar]
- 155.Derogar M, Hull MA, Kant P, et al. Increased Risk of Colorectal Cancer After Obesity Surgery. Ann Surg. 2013 doi: 10.1097/SLA.0b013e318288463a. [DOI] [PubMed] [Google Scholar]
- 156.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 157.Bird-Lieberman EL, Fitzgerald RC. Early diagnosis of oesophageal cancer. Br J Cancer. 2009;101:1–6. doi: 10.1038/sj.bjc.6605126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kubo A, Corley DA. Body mass index and adenocarcinomas of the esophagus or gastric cardia: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:872–8. doi: 10.1158/1055-9965.EPI-05-0860. [DOI] [PubMed] [Google Scholar]
- 159.Abnet CC, Freedman ND, Hollenbeck AR, et al. A prospective study of BMI and risk of oesophageal and gastric adenocarcinoma. Eur J Cancer. 2008;44:465–71. doi: 10.1016/j.ejca.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Solaymani-Dodaran M, Logan RF, West J, et al. Risk of oesophageal cancer in Barrett's oesophagus and gastro-oesophageal reflux. Gut. 2004;53:1070–4. doi: 10.1136/gut.2003.028076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Falk GW. Barrett's esophagus. Gastroenterology. 2002;122:1569–91. doi: 10.1053/gast.2002.33427. [DOI] [PubMed] [Google Scholar]
- 162.Chak A, Falk G, Grady WM, et al. Assessment of familiality, obesity, and other risk factors for early age of cancer diagnosis in adenocarcinomas of the esophagus and gastroesophageal junction. Am J Gastroenterol. 2009;104:1913–21. doi: 10.1038/ajg.2009.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hampel H, Abraham NS, El-Serag HB. Meta-analysis: obesity and the risk for gastroesophageal reflux disease and its complications. Ann Intern Med. 2005;143:199–211. doi: 10.7326/0003-4819-143-3-200508020-00006. [DOI] [PubMed] [Google Scholar]
- 164.Edelstein ZR, Farrow DC, Bronner MP, et al. Central adiposity and risk of Barrett's esophagus. Gastroenterology. 2007;133:403–11. doi: 10.1053/j.gastro.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 165.Corley DA, Kubo A, Levin TR, et al. Abdominal obesity and body mass index as risk factors for Barrett's esophagus. Gastroenterology. 2007;133:34–41. doi: 10.1053/j.gastro.2007.04.046. quiz 311. [DOI] [PubMed] [Google Scholar]
- 166.Beddy P, Howard J, McMahon C, et al. Association of visceral adiposity with oesophageal and junctional adenocarcinomas. Br J Surg. 2010;97:1028–34. doi: 10.1002/bjs.7100. [DOI] [PubMed] [Google Scholar]
- 167.Anderson LA, Watson RG, Murphy SJ, et al. Risk factors for Barrett's oesophagus and oesophageal adenocarcinoma: results from the FINBAR study. World J Gastroenterol. 2007;13:1585–94. doi: 10.3748/wjg.v13.i10.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.de Jonge PJ, Steyerberg EW, Kuipers EJ, et al. Risk factors for the development of esophageal adenocarcinoma in Barrett's esophagus. Am J Gastroenterol. 2006;101:1421–9. doi: 10.1111/j.1572-0241.2006.00626.x. [DOI] [PubMed] [Google Scholar]
- 169.Vaughan TL, Kristal AR, Blount PL, et al. Nonsteroidal anti-inflammatory drug use, body mass index, and anthropometry in relation to genetic and flow cytometric abnormalities in Barrett's esophagus. Cancer Epidemiol Biomarkers Prev. 2002;11:745–52. [PubMed] [Google Scholar]
- 170.Jin Z, Hamilton JP, Yang J, et al. Hypermethylation of the AKAP12 promoter is a biomarker of Barrett's-associated esophageal neoplastic progression. Cancer Epidemiol Biomarkers Prev. 2008;17:111–7. doi: 10.1158/1055-9965.EPI-07-0407. [DOI] [PubMed] [Google Scholar]
- 171.Olliver JR, Hardie LJ, Gong Y, et al. Risk factors, DNA damage, and disease progression in Barrett's esophagus. Cancer Epidemiol Biomarkers Prev. 2005;14:620–5. doi: 10.1158/1055-9965.EPI-04-0509. [DOI] [PubMed] [Google Scholar]
- 172.Risques RA, Vaughan TL, Li X, et al. Leukocyte telomere length predicts cancer risk in Barrett's esophagus. Cancer Epidemiol Biomarkers Prev. 2007;16:2649–55. doi: 10.1158/1055-9965.EPI-07-0624. [DOI] [PubMed] [Google Scholar]
- 173.Ali AS, Ali S, Ahmad A, et al. Expression of microRNAs: potential molecular link between obesity, diabetes and cancer. Obes Rev. 2011;12:1050–62. doi: 10.1111/j.1467-789X.2011.00906.x. [DOI] [PubMed] [Google Scholar]
- 174.Zhao BS, Liu SG, Wang TY, et al. Screening of microRNA in patients with esophageal cancer at same tumor node metastasis stage with different prognoses. Asian Pac J Cancer Prev. 2013;14:139–43. doi: 10.7314/apjcp.2013.14.1.139. [DOI] [PubMed] [Google Scholar]
- 175.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–56. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 176.Weinsier RL, Hunter GR, Gower BA, et al. Body fat distribution in white and black women: different patterns of intraabdominal and subcutaneous abdominal adipose tissue utilization with weight loss. Am J Clin Nutr. 2001;74:631–6. doi: 10.1093/ajcn/74.5.631. [DOI] [PubMed] [Google Scholar]
- 177.Vega GL, Adams-Huet B, Peshock R, et al. Influence of body fat content and distribution on variation in metabolic risk. J Clin Endocrinol Metab. 2006;91:4459–66. doi: 10.1210/jc.2006-0814. [DOI] [PubMed] [Google Scholar]
- 178.Yudkin JS. Adipose tissue, insulin action and vascular disease: inflammatory signals. Int J Obes Relat Metab Disord. 2003;27(Suppl 3):S25–8. doi: 10.1038/sj.ijo.0802496. [DOI] [PubMed] [Google Scholar]
- 179.van Kruijsdijk RC, van der Wall E, Visseren FL. Obesity and cancer: the role of dysfunctional adipose tissue. Cancer Epidemiol Biomarkers Prev. 2009;18:2569–78. doi: 10.1158/1055-9965.EPI-09-0372. [DOI] [PubMed] [Google Scholar]
- 180.Donohoe CL, Doyle SL, McGarrigle S, et al. Role of the insulin-like growth factor 1 axis and visceral adiposity in oesophageal adenocarcinoma. Br J Surg. 2012;99:387–96. doi: 10.1002/bjs.8658. [DOI] [PubMed] [Google Scholar]
- 181.Doyle SL, Donohoe CL, Finn SP, et al. IGF-1 and its receptor in esophageal cancer: association with adenocarcinoma and visceral obesity. The American journal of gastroenterology. 2012;107:196–204. doi: 10.1038/ajg.2011.417. [DOI] [PubMed] [Google Scholar]
- 182.Siahpush SH, Vaughan TL, Lampe JN, et al. Longitudinal study of insulin-like growth factor, insulin-like growth factor binding protein-3, and their polymorphisms: risk of neoplastic progression in Barrett's esophagus. Cancer Epidemiol Biomarkers Prev. 2007;16:2387–95. doi: 10.1158/1055-9965.EPI-06-0986. [DOI] [PubMed] [Google Scholar]
- 183.Howard JM, Beddy P, Ennis D, et al. Associations between leptin and adiponectin receptor upregulation, visceral obesity and tumour stage in oesophageal and junctional adenocarcinoma. Br J Surg. 2010;97:1020–7. doi: 10.1002/bjs.7072. [DOI] [PubMed] [Google Scholar]
- 184.Yildirim A, Bilici M, Cayir K, et al. Serum adiponectin levels in patients with esophageal cancer. Jpn J Clin Oncol. 2009;39:92–6. doi: 10.1093/jjco/hyn143. [DOI] [PubMed] [Google Scholar]
- 185.Duggan C, Onstad L, Hardikar S, et al. Association Between Markers of Obesity and Progression From Barrett's Esophagus to Esophageal Adenocarcinoma. Clin Gastroenterol Hepatol. 2013;11:934–43. doi: 10.1016/j.cgh.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Konturek PC, Burnat G, Rau T, et al. Effect of adiponectin and ghrelin on apoptosis of Barrett adenocarcinoma cell line. Dig Dis Sci. 2008;53:597–605. doi: 10.1007/s10620-007-9922-1. [DOI] [PubMed] [Google Scholar]
- 187.Kendall BJ, Macdonald GA, Hayward NK, et al. Leptin and the risk of Barrett's oesophagus. Gut. 2008;57:448–54. doi: 10.1136/gut.2007.131243. [DOI] [PubMed] [Google Scholar]
- 188.Beales IL, Ogunwobi OO. Leptin synergistically enhances the anti-apoptotic and growth-promoting effects of acid in OE33 oesophageal adenocarcinoma cells in culture. Mol Cell Endocrinol. 2007;274:60–8. doi: 10.1016/j.mce.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 189.Barb D, Williams CJ, Neuwirth AK, et al. Adiponectin in relation to malignancies: a review of existing basic research and clinical evidence. Am J Clin Nutr. 2007;86:s858–66. doi: 10.1093/ajcn/86.3.858S. [DOI] [PubMed] [Google Scholar]
- 190.Ogunwobi OO, Beales IL. Leptin stimulates the proliferation of human oesophageal adenocarcinoma cells via HB-EGF and Tgfalpha mediated transactivation of the epidermal growth factor receptor. Br J Biomed Sci. 2008;65:121–7. doi: 10.1080/09674845.2008.11732814. [DOI] [PubMed] [Google Scholar]
- 191.Somasundar P, Riggs D, Jackson B, et al. Leptin stimulates esophageal adenocarcinoma growth by nonapoptotic mechanisms. Am J Surg. 2003;186:575–8. doi: 10.1016/j.amjsurg.2003.07.017. [DOI] [PubMed] [Google Scholar]
- 192.Ogunwobi O, Mutungi G, Beales IL. Leptin stimulates proliferation and inhibits apoptosis in Barrett's esophageal adenocarcinoma cells by cyclooxygenase-2-dependent, prostaglandin-E2-mediated transactivation of the epidermal growth factor receptor and c-Jun NH2-terminal kinase activation. Endocrinology. 2006;147:4505–16. doi: 10.1210/en.2006-0224. [DOI] [PubMed] [Google Scholar]
- 193.Thompson OM, Beresford SA, Kirk EA, et al. Serum leptin and adiponectin levels and risk of Barrett's esophagus and intestinal metaplasia of the gastroesophageal junction. Obesity (Silver Spring) 2010;18:2204–11. doi: 10.1038/oby.2009.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Silberg DG, Sullivan J, Kang E, et al. Cdx2 ectopic expression induces gastric intestinal metaplasia in transgenic mice. Gastroenterology. 2002;122:689–96. doi: 10.1053/gast.2002.31902. [DOI] [PubMed] [Google Scholar]
- 195.Gu JM, Xiao WJ, He JW, et al. Association between VDR and ESR1 gene polymorphisms with bone and obesity phenotypes in Chinese male nuclear families. Acta Pharmacol Sin. 2009;30:1634–42. doi: 10.1038/aps.2009.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Chen X, Lou G, Meng Z, et al. TGR5: a novel target for weight maintenance and glucose metabolism. Exp Diabetes Res. 2011;2011:853501. doi: 10.1155/2011/853501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Watanabe M, Houten SM, Mataki C, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–9. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- 198.Wen H, Ting JP, O'Neill LA. A role for the NLRP3 inflammasome in metabolic diseases--did Warburg miss inflammation? Nat Immunol. 2012;13:352–7. doi: 10.1038/ni.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Yang L, Lu X, Nossa CW, et al. Inflammation and intestinal metaplasia of the distal esophagus are associated with alterations in the microbiome. Gastroenterology. 2009;137:588–97. doi: 10.1053/j.gastro.2009.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Chow WH, Blot WJ, Vaughan TL, et al. Body mass index and risk of adenocarcinomas of the esophagus and gastric cardia. J Natl Cancer Inst. 1998;90:150–5. doi: 10.1093/jnci/90.2.150. [DOI] [PubMed] [Google Scholar]
- 201.Lagergren J, Bergstrom R, Nyren O. Association between body mass and adenocarcinoma of the esophagus and gastric cardia. Ann Intern Med. 1999;130:883–90. doi: 10.7326/0003-4819-130-11-199906010-00003. [DOI] [PubMed] [Google Scholar]
- 202.Yang P, Zhou Y, Chen B, et al. Overweight, obesity and gastric cancer risk: results from a meta-analysis of cohort studies. Eur J Cancer. 2009;45:2867–73. doi: 10.1016/j.ejca.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 203.Wijnhoven BP, Siersema PD, Hop WC, et al. Adenocarcinomas of the distal oesophagus and gastric cardia are one clinical entity. Rotterdam Oesophageal Tumour Study Group. Br J Surg. 1999;86:529–35. doi: 10.1046/j.1365-2168.1999.01082.x. [DOI] [PubMed] [Google Scholar]
- 204.Wang HB, Zhou CJ, Song SZ, et al. Evaluation of Nrf2 and IGF-1 expression in benign, premalignant and malignant gastric lesions. Pathol Res Pract. 2011;207:169–73. doi: 10.1016/j.prp.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 205.Liu WD, Yu R, Zhou GR. [Expression and significance of IGF-1R and VEGF in gastric carcinoma]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2009;25:529–30. [PubMed] [Google Scholar]
- 206.Matsubara J, Yamada Y, Nakajima TE, et al. Clinical significance of insulin-like growth factor type 1 receptor and epidermal growth factor receptor in patients with advanced gastric cancer. Oncology. 2008;74:76–83. doi: 10.1159/000139127. [DOI] [PubMed] [Google Scholar]
- 207.Nakajima TE, Yamada Y, Hamano T, et al. Adipocytokine levels in gastric cancer patients: resistin and visfatin as biomarkers of gastric cancer. J Gastroenterol. 2009;44:685–90. doi: 10.1007/s00535-009-0063-5. [DOI] [PubMed] [Google Scholar]
- 208.Seker M, Bilici A, Sonmez B, et al. The association of serum adiponectin levels with histopathological variables in gastric cancer patients. Med Oncol. 2010;27:1319–23. doi: 10.1007/s12032-009-9382-x. [DOI] [PubMed] [Google Scholar]
- 209.Friedman JM. Leptin and the regulation of body weigh. Keio J Med. 2010;60:1–9. doi: 10.2302/kjm.60.1. [DOI] [PubMed] [Google Scholar]
- 210.Pai R, Lin C, Tran T, et al. Leptin activates STAT and ERK2 pathways and induces gastric cancer cell proliferation. Biochem Biophys Res Commun. 2005;331:984–92. doi: 10.1016/j.bbrc.2005.03.236. [DOI] [PubMed] [Google Scholar]
- 211.Shida D, Kitayama J, Mori K, et al. Transactivation of epidermal growth factor receptor is involved in leptin-induced activation of janus-activated kinase 2 and extracellular signal-regulated kinase 1/2 in human gastric cancer cells. Cancer Res. 2005;65:9159–63. doi: 10.1158/0008-5472.CAN-05-0598. [DOI] [PubMed] [Google Scholar]
- 212.Capelle LG, de Vries AC, Haringsma J, et al. Serum levels of leptin as marker for patients at high risk of gastric cancer. Helicobacter. 2009;14:596–604. doi: 10.1111/j.1523-5378.2009.00728.x. [DOI] [PubMed] [Google Scholar]
- 213.Zhao X, Huang K, Zhu Z, et al. Correlation between expression of leptin and clinicopathological features and prognosis in patients with gastric cancer. J Gastroenterol Hepatol. 2007;22:1317–21. doi: 10.1111/j.1440-1746.2007.04941.x. [DOI] [PubMed] [Google Scholar]
- 214.Kai H, Kitadai Y, Kodama M, et al. Involvement of proinflammatory cytokines IL-1beta and IL-6 in progression of human gastric carcinoma. Anticancer Res. 2005;25:709–13. [PubMed] [Google Scholar]
- 215.Kuroda T, Kitadai Y, Tanaka S, et al. Monocyte chemoattractant protein-1 transfection induces angiogenesis and tumorigenesis of gastric carcinoma in nude mice via macrophage recruitment. Clin Cancer Res. 2005;11:7629–36. doi: 10.1158/1078-0432.CCR-05-0798. [DOI] [PubMed] [Google Scholar]
- 216.Thompson R. Preventing cancer: the role of food, nutrition and physical activity. J Fam Health Care. 2010;20:100–2. [PubMed] [Google Scholar]
- 217.Jiao L, Berrington de Gonzalez A, Hartge P, et al. Body mass index, effect modifiers, and risk of pancreatic cancer: a pooled study of seven prospective cohorts. Cancer Causes Control. 2010;21:1305–14. doi: 10.1007/s10552-010-9558-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Li D, Morris JS, Liu J, et al. Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. Jama. 2009;301:2553–62. doi: 10.1001/jama.2009.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Stattin P, Bjor O, Ferrari P, et al. Prospective study of hyperglycemia and cancer risk. Diabetes Care. 2007;30:561–7. doi: 10.2337/dc06-0922. [DOI] [PubMed] [Google Scholar]
- 220.Pisani P. Hyper-insulinaemia and cancer, meta-analyses of epidemiological studies. Arch Physiol Biochem. 2008;114:63–70. doi: 10.1080/13813450801954451. [DOI] [PubMed] [Google Scholar]
- 221.Stolzenberg-Solomon RZ, Graubard BI, Chari S, et al. Insulin, glucose, insulin resistance, and pancreatic cancer in male smokers. Jama. 2005;294:2872–8. doi: 10.1001/jama.294.22.2872. [DOI] [PubMed] [Google Scholar]
- 222.Michaud DS, Wolpin B, Giovannucci E, et al. Prediagnostic plasma C-peptide and pancreatic cancer risk in men and women. Cancer Epidemiol Biomarkers Prev. 2007;16:2101–9. doi: 10.1158/1055-9965.EPI-07-0182. [DOI] [PubMed] [Google Scholar]
- 223.Stolzenberg-Solomon RZ, Limburg P, Pollak M, et al. Insulin-like growth factor (IGF)-1, IGF-binding protein-3, and pancreatic cancer in male smokers. Cancer Epidemiol Biomarkers Prev. 2004;13:438–44. [PubMed] [Google Scholar]
- 224.Wolpin BM, Michaud DS, Giovannucci EL, et al. Circulating insulin-like growth factor axis and the risk of pancreatic cancer in four prospective cohorts. Br J Cancer. 2007;97:98–104. doi: 10.1038/sj.bjc.6603826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–7. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 226.Bergmann U, Funatomi H, Yokoyama M, et al. Insulin-like growth factor I overexpression in human pancreatic cancer: evidence for autocrine and paracrine roles. Cancer Res. 1995;55:2007–11. [PubMed] [Google Scholar]
- 227.Ohmura E, Okada M, Onoda N, et al. Insulin-like growth factor I and transforming growth factor alpha as autocrine growth factors in human pancreatic cancer cell growth. Cancer Res. 1990;50:103–7. [PubMed] [Google Scholar]
- 228.Stoeltzing O, Liu W, Reinmuth N, et al. Regulation of hypoxia-inducible factor-1alpha, vascular endothelial growth factor, and angiogenesis by an insulin-like growth factor-I receptor autocrine loop in human pancreatic cancer. Am J Pathol. 2003;163:1001–11. doi: 10.1016/s0002-9440(10)63460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Zeng H, Datta K, Neid M, et al. Requirement of different signaling pathways mediated by insulin-like growth factor-I receptor for proliferation, invasion, and VPF/VEGF expression in a pancreatic carcinoma cell line. Biochem Biophys Res Commun. 2003;302:46–55. doi: 10.1016/s0006-291x(03)00107-4. [DOI] [PubMed] [Google Scholar]
- 230.Zyromski NJ, Mathur A, Pitt HA, et al. Obesity potentiates the growth and dissemination of pancreatic cancer. Surgery. 2009;146:258–63. doi: 10.1016/j.surg.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 231.Chang MC, Chang YT, Su TC, et al. Adiponectin as a potential differential marker to distinguish pancreatic cancer and chronic pancreatitis. Pancreas. 2007;35:16–21. doi: 10.1097/MPA.0b013e3180547709. [DOI] [PubMed] [Google Scholar]
- 232.Krechler T, Zeman M, Vecka M, et al. Leptin and adiponectin in pancreatic cancer: connection with diabetes mellitus. Neoplasma. 2011;58:58–64. doi: 10.4149/neo_2011_01_58. [DOI] [PubMed] [Google Scholar]
- 233.Huxley R, Ansary-Moghaddam A, Berrington de Gonzalez A, et al. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer. 2005;92:2076–83. doi: 10.1038/sj.bjc.6602619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Tang H, Dong X, Hassan M, et al. Body mass index and obesity- and diabetes-associated genotypes and risk for pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:779–92. doi: 10.1158/1055-9965.EPI-10-0845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Krishnan A, Nair SA, Pillai MR. Biology of PPAR gamma in cancer: a critical review on existing lacunae. Curr Mol Med. 2007;7:532–40. doi: 10.2174/156652407781695765. [DOI] [PubMed] [Google Scholar]