Abstract
Endotoxemia, characterized by an excess of circulating bacterial wall lipopolysaccharide, is associated with systemic inflammation and the metabolic syndrome. Placing 8 healthy subjects on a Western-style diet for 1 month induced a 71% increase in plasma levels of endotoxin activity (endotoxemia), whereas a prudent-style diet reduced levels by 31%. The Western-style diet might, therefore, contribute to endotoxemia by causing changes in gastrointestinal barrier function or the composition of the microbiota. Endotoxemia might also develop in individuals with gastrointestinal barrier impairment. Therapeutic reagents that reduce endotoxemia might reduce systemic inflammation in patients with gastrointestinal diseases or metabolic syndrome.
Keywords: Gastrointestinal Barrier Function, Immune Response, Circulating Cytokines, LPS
Endotoxin or lipopolysaccharide (LPS) is a major glycolipid component of the cell wall of gram-negative bacteria and, if absorbed into the circulation, activates the release of host-derived inflammatory mediators to induce a systemic inflammatory response. Endotoxemia occurs in obesity, is accompanied by several metabolic disorders, and is associated with low-grade systemic inflammation.1 The source of and the mechanism for endotoxemia in obesity is controversial. Endotoxemia is derived from the gut and must reflect enhanced intestinal permeability or major changes in gut bacterial species.
Lifestyle changes, including a Western-style diet, cause obesity, and previous studies have shown that an enriched-fat diet induces endotoxemia compared with a control diet.2 Infusion of LPS leads to weight gain and insulin resistance.3 Others have reported acute rises in endotoxin levels with high energy intake.4 No direct demonstration of changes in endotoxemia with a Western-style diet compared with a more prudent diet has been reported. The present study describes these effects in subjects studied in metabolically controlled conditions.
A detailed description of volunteer subjects and methods is available in Supplementary Patients and Methods. Briefly, 8 healthy subjects were fed a Western-style and a “prudent-style” diet for 1 month as inpatients hospitalized in a metabolic ward in a crossover study separated by a 1-month washout period. Blood endotoxin levels (endotoxemia) were measured by a neutrophil priming method that detects LPS from gram-negative organisms and is the only method approved by the Food and Drug Administration for this purpose. Serum cytokines were determined before and after each dietary study period.
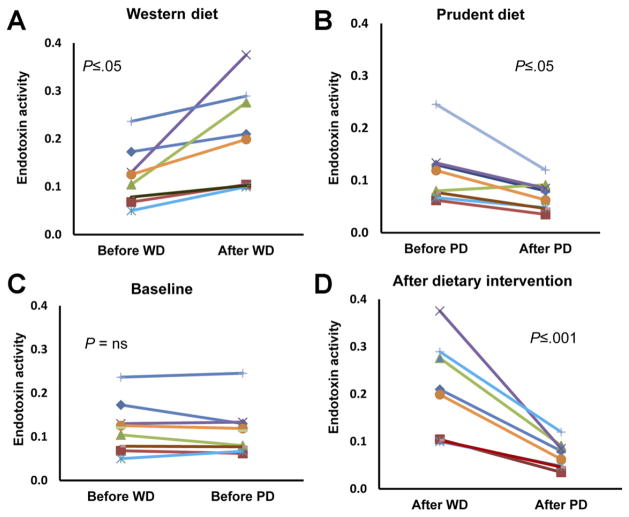
Our study shows that 4 weeks of feeding a Western-style diet induced highly significant increases (by 71%) in plasma endotoxin activity levels, in contrast to a major decrease (by 38%) after the prudent-style diet (Figure 1) (P < .001). Baseline endotoxin activity levels were about identical, and energy density and physical activity measurements were very similar. Simultaneously, the feeding of a prudent-style diet resulted in a significant decrease in serum tumor necrosis factor α with a trend for reduced monocyte chemotactic protein 1 and interleukin-8 levels (Table 1), whereas these levels were unchanged after the Western-style diet (Table 1). These changes were small but in line with previous data showing increased circulating cytokines accompanying endotoxemia.5
Figure 1.
Plasma endotoxin activity levels. Data from 8 individual subjects shown as endotoxin activity units for the Western-style diet (WD) and the prudent-style diet (PD). Paired t tests were used to compare effects of the diets on endotoxin activity levels. The Mann–Whitney sum test was used to compare effects of the 2 diets on endotoxin activity levels. (A) Effect of WD administration. (B) Effect of PD administration. (C) Differences between baseline measures before administration of the 2 diets. (D) Differences between measurements after administration of the 2 diets.
Table 1.
Changes in Serum Cytokine Concentrations (N = 8)
| Cytokines | Before diet, mean ± SD (pg/mL) | After diet, mean ± SD (pg/mL) | Change (%) |
|---|---|---|---|
| Prudent diet | |||
| TNF-α | 10 ± 2.5 | 8.4 ± 1.5 | −17a |
| MCP-1 | 195 ± 27 | 178 ± 32 | −9 (NS) |
| IL-6 | 1.6 ± 0.5 | 1.4 ± 0.3 | −11 (NS) |
| IL-8 | 19 ± 12 | 14 ± 1.6 | −28 (NS) |
| Western diet | |||
| TNF-α | 9 ± 1.6 | 9 ± 1.7 | −1 (NS) |
| MCP-1 | 198 ± 37 | 180 ± 50 | −9 (NS) |
| IL-6 | 1.8 ± 0.7 | 1.8 ± 0.8 | −2 (NS) |
| IL-8 | 17 ± 8 | 15 ± 3.8 | −13 (NS) |
NOTE. Data before the 2 diets did not differ. Statistical analysis by paired t tests.
IL, interleukin; MCP-1, monocyte chemotactic protein 1; NS, not significant; TNF, tumor necrosis factor.
P ≤ .05.
Chronic endotoxemia commonly occurs in obesity and is an important factor inducing systemic inflammation leading to the metabolic syndrome.6 Moderate increases in bacterial LPS have been described after fat-enriched meals in mice6 and in volunteer subjects7 as well as with a high energy intake.4 In the mouse, a high-fat diet induces endotoxemia, inflammation in visceral adipose tissue, and glucose imbalance ameliorated by antibiotics.8
Cell wall products of luminal commensal microbiota that cross the gastrointestinal barrier can induce endotoxemia. Thus, endotoxemia must result either from changes in microbiota or/and increased gastrointestinal permeability. Intestinal microbiota are implicated in causing obesity,9 and the diet including fiber intake can induce changes in gut microbiota.10 Furthermore, increased gut permeability has been described with high-fat feeding in study subjects,11 which can be modulated by increased proglucagon-derived peptide in the mouse.12
The present study directly shows diet-modified endotoxemia in humans. The study was performed in only 8 subjects, but it had a crossover design in the same individuals at a metabolic inpatient facility under strict nutritional and activity controls13 whose endotoxin activity levels before diet administration were almost identical.
The present study raises several questions. Does diet-induced endotoxemia in humans reflect changes in permeability or in microbiota? Is diet-induced endotoxemia an epiphenomenon or pathogenic in the absence of gastrointestinal disease? In gastrointestinal diseases that have been shown to be accompanied by increased intestinal permeability such as celiac disease or inflammatory bowel disease,14 does diet-induced endotoxemia increase the risk of systemic inflammation? May feeding prebiotics or probiotics reduce endotoxemia and its concomitant complications?
Supplementary Material
Acknowledgments
Funding
Supported in part by CTSC grant no. 5UL1 RR024143-5.
Abbreviations used in this paper
- LPS
lipopolysaccharide
Footnotes
Conflicts of Interest
The authors disclose no conflicts.
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at doi: 10.1053/j.gastro.2012.01.034.
References
- 1.Wellen KE, et al. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghanim H, et al. Diabetes Care. 2009;32:2281–2287. doi: 10.2337/dc09-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehta NN, et al. Diabetes. 2010;59:172–181. doi: 10.2337/db09-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amar J, et al. Am J Clin Nutr. 2008;87:1219–1223. doi: 10.1093/ajcn/87.5.1219. [DOI] [PubMed] [Google Scholar]
- 5.Michie HR, et al. N Engl J Med. 1988;318:1481–1486. doi: 10.1056/NEJM198806093182301. [DOI] [PubMed] [Google Scholar]
- 6.Cani PD, et al. Diabetologia. 2007;50:2374–2383. doi: 10.1007/s00125-007-0791-0. [DOI] [PubMed] [Google Scholar]
- 7.Erridge C, et al. Am J Clin Nutr. 2007;86:1286–1292. doi: 10.1093/ajcn/86.5.1286. [DOI] [PubMed] [Google Scholar]
- 8.Cani PD, et al. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 9.Backhed F, et al. Proc Natl Acad Sci U S A. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turnbaugh PJ, et al. Cell Host Microbe. 2008;3:213–223. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brun P, et al. Am J Physiol Gastrointest Liver Physiol. 2007;292:G518–G525. doi: 10.1152/ajpgi.00024.2006. [DOI] [PubMed] [Google Scholar]
- 12.Cani PD, et al. Gut. 2009;58:1091–1103. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pendyala S, et al. Am J Clin Nutr. 2011;93:234–242. doi: 10.3945/ajcn.110.002683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gardiner KR, et al. Gut. 1995;36:897–901. doi: 10.1136/gut.36.6.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.