Abstract
Introduction
In this study, we tested the ability of small molecule inhibitors of WNT/β-catenin signaling to block interleukin 1β (IL-1β)- and tumor necrosis factor α (TNFα)-induced cartilage degradation. Proinflammatory cytokines such as IL-1β and TNFα are potent inducers of cartilage degradation by upregulating matrix metalloproteinase (MMP) expression and activity. Because WNT/β-catenin signaling was found to be involved in IL-1β- and TNFα-induced upregulation of MMP activity, we hypothesized that inhibition of WNT/β-catenin signaling might block IL-1β- and TNFα-induced cartilage degradation. We tested the effect of small molecules that block the interaction between β-catenin and TCF/Lef transcription factors on IL-1β- and TNFα-induced cartilage degradation in mouse fetal metatarsals.
Methods
We used mouse fetal metatarsals treated with IL-1β and TNFα as an ex vivo model for cytokine-induced cartilage degradation. Metatarsals were treated with IL-1β and TNFα in combination with the small molecules PKF115-584, PKF118-310 and CGP049090 at different concentrations and then harvested them for histological and gene expression analysis.
Results
We found that IL-1β- and TNFα-induced cartilage degradation in mouse fetal metatarsals was blocked by inhibiting WNT/β-catenin signaling using small molecule PKF115-584 and partially using CGP049090 dose-dependently. In addition, we found that PKF115-584 blocked IL-1β- and TNFα-induced MMP mRNA expression, but did not reverse the inhibitory effect of IL-1β on the expression of cartilage anabolic genes.
Conclusion
In this study, we show that inhibition of WNT/β-catenin signaling by small molecules can effectively prevent IL-1β- and TNFα-induced cartilage degradation by blocking MMP expression and activity. Furthermore, we elucidate the involvement of WNT/β-catenin signaling in IL-1β- and TNFα-induced cartilage degradation.
Introduction
In degenerative cartilage diseases such as osteoarthritis (OA) and rheumatoid arthritis (RA), the balance between anabolic and catabolic processes is shifted toward breakdown of the extracellular cartilage matrix [1-3]. Cartilage destruction is thought to be the result of increased expression and activity of catabolic proteins, such as matrix metalloproteinases (MMPs) [4]. Expression of MMP1 (collagenase), MMP3 (stromelysin), MMP9 (gelatinase) and MMP13 (collagenase 3) mRNA has been found in chondrocytes in arthritic cartilage [5,6]. Increased mRNA expression of MMP1 and MMP3 was also found in the synovial tissue of OA patients [7]. In agreement with that finding, protein expression of MMP1, MMP3 and MMP9 in the synovial fluid of patients with OA in the temporomandibular joint was found to be increased compared to healthy control joints [8]. The essential role of MMPs in cartilage degradation was illustrated by experimental evidence indicating that Mmp13-deficient mice were resistant to cartilage damage in medial meniscus destabilization-induced cartilage degradation [9]. In addition, cartilage degradation induced by IL-1β and oncostatin M in human and bovine articular cartilage explants could be blocked by a specific MMP13 inhibitor [10].
Proinflammatory cytokines such as interleukin (IL)-1β and tumor necrosis factor α (TNFα) potently induce MMP expression and activity in cartilage, and these cytokines are associated with cartilage degradation in vitro and in vivo [6,11,12]. The increased expression of several MMPs in human articular cartilage explants in similar locations where IL-1β and TNFα were highly expressed is suggestive of the involvement of IL-1β and TNFα in the stimulation of MMP expression [11]. In vitro and in vivo studies have shown that proinflammatory cytokines such as IL-1β and TNFα are present in both OA and RA joint tissues and synovial fluid [1,4,13]. IL-1β is associated with cartilage degeneration, whereas TNFα was shown to be involved in driving inflammation [3]. Besides their role in cartilage degradation by stimulating MMPs, IL-1β and TNFα impair the ability of the cartilage to restore the extracellular matrix by blocking the synthesis of new extracellular matrix components [3].
Recently, the canonical WNT/β-catenin signaling pathway in the pathophysiology of cartilage degenerative disease has attracted much attention [14]. The WNT/β-catenin signaling pathway is activated upon binding of WNT to its receptor Frizzled (FZD) and coactivator low-density lipoprotein receptor-related protein 5 (LRP5)/LRP6. Subsequently, the degradation complex for β-catenin is destabilized, resulting in high cytoplasmic levels of β-catenin and translocation of β-catenin to the nucleus, where it binds to transcription factor/lymphoid enhancer-binding factor (TCF/Lef), leading to activation of target genes [15]. Several lines of evidence predominantly derived from animal models support the involvement of WNT/β-catenin signaling in the molecular mechanism underlying cartilage degradation. Conditional activation of β-catenin in articular chondrocytes in adult mice was found to result in articular cartilage destruction with accelerated terminal chondrocyte differentiation [16]. It has also been shown that knockout of FRZB, an antagonist of canonical WNT signaling makes mice more susceptible to chemically induced articular cartilage degradation [17]. Furthermore, increased expression of secreted FZD-related proteins, which prevents binding of WNTs to their receptors, was found in OA synovium, which might be indicative of a compensatory mechanism for increased WNT signaling [18].
Recently, a link between WNT/β-catenin signaling and IL-1β-induced cartilage degradation was found. Expression of WNT5a and WNT7a in articular chondrocytes was induced by IL-1β [19], and the combination of IL-1β and WNT3a induced greater loss of proteoglycans from the extracellular matrix than either one alone [12]. In addition, induction of WNT signaling by either recombinant WNT3a or glycogen synthase kinase 3β (GSK3β) inhibitor 6-bromoindirubin 3'-oxime (BIO) was shown to induce MMP mRNA expression and proteolytic activity in mouse cartilage explants. The fact that knockdown of TCF4 eliminated this effect indicates the involvement of TCF4 in WNT-induced MMP expression [20]. In addition, involvement of Lef1 was found in increased MMP13 expression upon IL-1β stimulation [21].
Because proinflammatory cytokine-induced cartilage degradation appears to involve WNT/β-catenin signaling and increased WNT/β-catenin signaling has been implicated in the initiation and progressive deterioration of cartilage degeneration, we hypothesized that small molecule inhibitors of the interaction between β-catenin and TCF4 and Lef1 could be used to prevent cytokine-induced cartilage degradation. The aim of this study was to assess the potential effects of small molecules that inhibit the WNT/β-catenin signaling pathway on the degeneration of cartilage. We have selected the small molecules PKF115-584, PKF118-310 and CGP049090, which block the binding of β-catenin to its transcription factor TCF4. PKF115-584 and CGP049090 also block the binding between β-catenin and transcription factor Lef1 [22-24]. In addition, PKF115-584 not only blocks but also disrupts the binding between β-catenin and TCF [22-24]. To study the potential effect of these WNT inhibitors, we used explanted mouse fetal metatarsals, in which we induced cartilage degradation by adding IL-1β and TNFα.
Materials and methods
Luciferase assay
HEK-293t cells were seeded at 7,500 cells/cm2 into 96-well plates (Nalge Nunc International, Penfield, NY, USA) and cultured for 24 h in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (FBS) and 100 U of penicillin-streptomycin (Gibco/Life Technologies, Grand Island, NY, USA) prior to transfection with the TOPflash TCF/Lef luciferase reporter construct (Upstate Biotechnology/EMD Millipore, Lake Placid, NY, USA) and pRL-CMV control vector (Promega, Madison, WI, USA). Cells were stimulated with the GSK3β inhibitor BIO (Sigma-Aldrich, St Louis, MO, USA) to stimulate the WNT/β-catenin pathway in combination with the inhibitors 24 h after transfection. After 24 h of stimulation, luminescence was measured using the Dual-Glo Luciferase Assay System (Promega).
Metabolic activity
To study the effect of small molecules on metabolic activity, the preosteoblast cell line KS483-4C3 was used [25]. Twenty-four hours after seeding, KS483-4C3 cells were stimulated with different concentrations of the compounds, and, 24 h later, the metabolic activity was determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Cells were incubated with MTT for 4 h, and, after stopping the reaction by adding dimethyl sulfoxide, optical density was measured at 540 nm.
Immunofluorescence staining for nuclear accumulation of β-catenin
Nuclear accumulation of β-catenin was detected by immunofluorescence staining. KS483-4C3 cells were seeded onto glass slides (Nalgene Nunc International) and treated with LiCl, both with and without small molecule inhibitors. After 3 h, cells were washed in phosphate-buffered saline and fixed in 3.7% buffered formalin. Subsequently, cells were quenched in 50 mM NH4Cl for 10 min and incubated overnight at 4°C in NET GEL buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 5 mM ethylenediaminetetraacetic acid, 0.05% Nonidet P-40, 0.25% gelatin and 0.02% azide). The next day cells were incubated with anti-β-catenin antibody (1:500 in NET GEL buffer; BD Transduction Laboratories, San Jose, CA, USA) for 1 h at room temperature. Next, cells were incubated with anti-mouse fluorescein isothiocyanate-labeled secondary antibody (1:250 in NET GEL buffer; Sigma-Aldrich) for 1 h at room temperature and mounted using VECTASHIELD mounting medium (VECTOR Laboratories, Burlingame, CA, USA).
Mouse fetal metatarsals
Mouse fetal metatarsals were isolated from FVB mouse embryos (time-paired; Harlan Laboratories, Indianapolis, IN, USA) at day 17.5 of gestation [26,27]. After isolation, metatarsals were individually cultured in 24-well plates in 200 µl/well in Minimal Essential Medium (MEM) α supplemented with 10% FBS, 100 U of penicillin-streptomycin (Gibco/Life Technologies) and 1% GlutaMAX supplement (Invitrogen, Carlsbad, CA, USA) for 48 h. After this equilibration period, metatarsals were treated with several concentrations of the small molecules, either alone or in combination with 10 ng/ml TNFα or IL-1β (R&D Systems, Minneapolis, MN, USA) or a combination of both for 1, 4 or 7 days. Animal experiments were approved by the ethical committee of the University Medical Centre Utrecht.
Morphometric and histological analysis
Optical microscopy was performed at different time points, and the lengths of the metatarsals were measured along the sagittal axis of the bone using ImageJ image analysis software (National Institutes of Health, Bethesda, MD, USA). For histological examination, metatarsals were fixed in 10% formalin and dehydrated in ethanol series before being embedded in paraffin. Five-micrometer sections were cut using a rotary microtome (HM 355S; Microm International, Walldorf, Germany). Sections were stained for glycosaminoglycans using 0.5% Alcian Blue (Sigma-Aldrich) in H2O (pH set to 1 using HCl) for 30 min and counterstained in 1% Nuclear Fast Red solution (Sigma-Aldrich) for 5 min. For immunohistochemical staining of collagen type II, sections were preincubated in 5 µg/ml proteinase K (Sigma-Aldrich) for 10 min, followed by 1 mg/ml hyaluronidase (Sigma-Aldrich) for 30 min, both at 37°C. Rabbit polyclonal collagen type II primary antibody (Abnova, Jhongli City, Taiwan) was diluted 1:1,000 and incubated overnight at 4°C. For visualization, the EnVision+ HRP kit (Dako, Carpinteria, CA, USA) was used.
Gene expression analysis
Five metatarsals were pooled and lysed in TRIzol reagent (Ambion/Life Technologies, Austin, TX, USA) for RNA isolation, using the NucleoSpin RNA II kit (Macherey-Nagel, Düren, Germany) according to the manufacturer's protocol. Subsequently, cDNA was synthesized using the iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA, USA). Quantitative polymerase chain reaction was performed using iQ SYBR Green Supermix (Bio-Rad Laboratories) in the MyiQ 2 Two-Color Real-Time PCR Detection System (Bio-Rad Laboratories). Gene expression was normalized using glyceraldehyde 3-phosphate dehydrogenase and expressed as fold change compared to controls. Primer sequences are listed in Table 1.
Table 1.
Primer sequences for quantitative polymerase chain reaction
| Gene name | Primer sequence | Product size | Annealing temperature |
|---|---|---|---|
| ACAN | Forward: 5'-AGGCAGCGTGATCCTTACC-3' Reverse: 5'-GGCCTCTCCAGTCTCATTCTC-3' |
136 bp | 60°C |
| COL2A1 | Forward: 5'-CGTCCAGATGACCTTCCTACG-3' Reverse: 5'-TGAGCAGGGCCTTCTTGAG-3' |
122 bp | 60°C |
| SOX9 | Forward: 5'-TGGGCAAGCTCTGGAGACTTC-3' Reverse: 5'-ATCCGGGTGGTCCTTCTTGTG-3' |
98 bp | 60°C |
| MMP3 | Forward: 5'-TGGCATTCAGTCCCTCTATGG-3' Reverse: 5'-AGGACAAAGCAGGATCACAGTT-3' |
116 bp | 60°C |
| MMP9 | Forward: 5'-GGTGATTGACGACGCCTTTGC-3' Reverse: 5' CGCGACACCAAACTGGATGAC 3' |
115 bp | 60°C |
| MMP13 | Forward: 5'-AAGGAGCATGGCGACTTCT-3' Reverse: 5'-TGGCCCAGGAGGAAAAGC-3' |
72 bp | 60°C |
| GAPDH | Forward: 5'-CGCTCTCTGCTCCTCCTGTT-3' Reverse: 5'-CCATGGTGTCTGAGCGATGT-3' |
82 bp | 60°C |
| B2M | Forward: 5'-GACTTGTCTTTCAGCAAGGA-3' Reverse: 5'-ACAAAGTCACATGGTTCACA-3' |
106 bp | 60°C |
Statistical analysis
The results are expressed as mean values with 95% confidence intervals (CIs), and statistical significance was tested using analysis of variance with PASW Statistics 18 software (SPSS, Inc, Chicago, IL, USA).
Results
Effect of small molecules on TCF/Lef reporter activity, nuclear translocation of β-catenin and metabolic activity
We first tested the efficacy and specificity of the small molecule compounds to inhibit canonical WNT/β-catenin signaling in HEK-293t cells transiently transfected with the TOPflash TCF/Lef luciferase reporter construct. The compounds were tested in the presence or absence of BIO, a potent activator of WNT/β-catenin signaling by blocking GSK3β [28]. We found a dose-dependent decrease in reporter activity when the cells were treated with BIO and the small molecule inhibitors. PKF115-584 treatment resulted in a sixfold decrease in reporter activity at a concentration of 1.0 µM. At a concentration of 3.0 µM, luciferase reporter activity increased again, most likely due to notable cell death upon visual inspection of the cultures. PKF118-310 and CGP049090 were slightly less effective. Maximal inhibition was found at 3.0 µM, which decreased reporter activity by fourfold and sixfold, respectively (Figure 1A).
Figure 1.
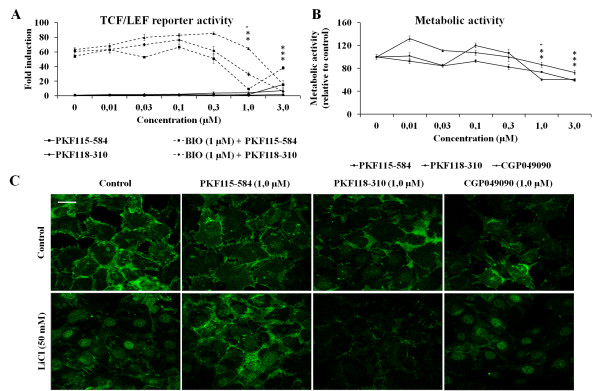
Small molecule inhibitors of WNT/β-catenin signaling effectively block TCF/Lef-mediated activity of β-catenin. (A) Small molecules dose-dependently inhibit transcription factor/lymphoid enhancer-binding factor (TCF/LEF) reporter activity in HEK-293t cells, induced by the glycogen synthase kinase 3β (GSK3β) inhibitor 6-bromoindirubin 3'-oxime (BIO) (1.0 µM). Data represent the means of three independent experiments with 95% confidence intervals (CIs). (B) Metabolic activity, measured using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay in KS483-4C3 cells, was not affected by small molecules at lower concentrations; however, at 1.0 µM (except for CGP049090) and 3.0 µM, metabolic activity was significantly decreased. Data represent the means of three independent experiments with 95% CI. (C) Treatment with 50 mM LiCl induced nuclear translocation of β-catenin. Small molecules by themselves had no effect on cellular localization of β-catenin, whereas PKF118-310 and PKF115-584 blocked LiCl-induced translocation of β-catenin to the nucleus. CGP049090 did not affect nuclear accumulation of β-catenin after LiCl treatment. A representative example of three independent experiments is shown. Scale bar represents 10 µm *P < 0.05 placed in the order relative to the order of the data points below..
The effect of small molecules on the metabolic activity of cells was tested in KS483-4C3 cells using an MTT assay [25]. No significant effects on metabolic activity were found when cells were treated with lower concentrations of the compound; however, 1.0 µM or 3.0 µM PKF115-584 and PKF118-310 and 3.0 µM CGP049090 did cause a significant decrease in metabolic activity (Figure 1B).
Because β-catenin can effectively activate the WNT/β-catenin pathway only after nuclear translocation, the cellular localization of β-catenin was determined by immunofluorescence staining. Figure 1C shows that stimulation of the WNT/β-catenin pathway by LiCl, which also inhibits GSK3β, resulted in translocation and accumulation of β-catenin in the nucleus. At 1.0 µM, CGP049090 reduced the intensity of β-catenin staining but did not inhibit nuclear translocation induced by LiCl, whereas PKF118-310 reduced the intensity of β-catenin staining and also inhibited LiCl-induced nuclear translocation. In contrast, PKF115-584 did not affect the intensity of the β-catenin staining under basal conditions. In the presence of LiCl, β-catenin membrane staining was increased, but nuclear translocation was markedly inhibited by PKF115-584.
Tumor necrosis factor α and interleukin 1β induce cartilage degradation in mouse fetal metatarsals
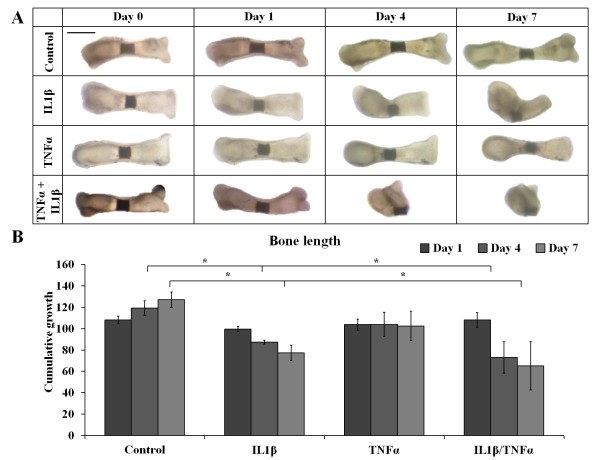
Metatarsals were cultured in medium containing IL-1β or TNFα or a combination of both at concentrations of 10 ng/ml. TNFα tended to blunt longitudinal bone growth, although this did not reach significance. In contrast, IL-1β alone induced bone and cartilage resorption, resulting in a significant reduction in bone length after 4 and 7 days of treatment. Cotreatment of IL-1β with TNFα caused even more abundant bone and cartilage resorption, resulting in a significant reduction in bone length (Figures 2A and 2B). Because IL-1β and TNFα together were more effective than either IL-1β or TNFα alone, we used the combination of IL-1β and TNFα to induce cartilage degradation in further experiments.
Figure 2.
Combined treatment with interleukin 1β and tumor necrosis factor α caused cartilage degradation in mouse fetal metatarsals. (A) Mouse fetal metatarsals treated with a combination of interleukin 1β (IL-1β) and tumor necrosis factor α (TNFα) exhibit abundant cartilage resorption, whereas treatment with IL-1β alone had minor effects and TNFα tended to blunt longitudinal growth only. A representative picture of six independent experiments is shown. Scale bar represents 500 µm. (B) Treatment with IL-1β or a combination of IL-1β and TNFα significantly decreased bone length after 4 days and 7 days of treatment. Data represent the means of six independent experiments with 95% confidence intervals. *P < 0.05).
Inhibitory effect of small molecules on cartilage degradation in mouse fetal metatarsals
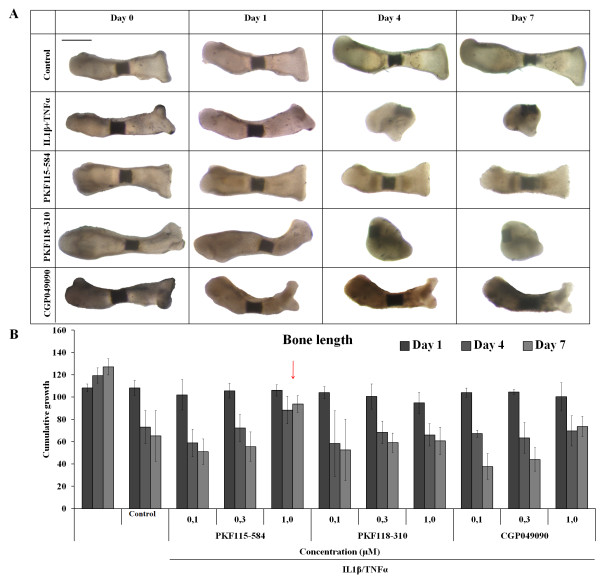
Because visual inspection of cell cultures treated with concentrations higher than 1.0 µM revealed increased cell death, most likely due to toxic side effects, we chose to test the effect of the three WNT inhibitors at a dose range from 0.1 µM to 1.0 µM. When metatarsals were treated with the small molecules only, no effect on the in vitro growth of the metatarsals was observed at any of the concentrations that were tested (Additional file 1: Figure S1). The decrease in growth and resorption of the metatarsals when treated with TNFα and IL-1β was counteracted when metatarsals were treated with small molecules. At a concentration of 1.0 µM, PKF115-584 blocked bone and cartilage resorption most effectively. Also, CGP049090 counteracted the detrimental effects of TNFα and IL-1β on explant resorption, albeit less effectively than PKF115-584, whereas PKF118-310 had no significant effect (Figure 3A).
Figure 3.
Cartilage degradation induced by interleukin 1β and tumor necrosis factor α in mouse fetal metatarsals can be blocked by small molecule WNT inhibitors. (A) Morphological changes of metatarsals caused by interleukin 1β (IL-1β) and tumor necrosis factor α (TNFα) (10 ng/ml each) can be blocked by cotreatment with PKF115-584 at a concentration of 1.0 µM. CGP049090 partially blocks resorption of the metatarsals, whereas PKF118-310 did not have an effect. A representative picture of three independent experiments is shown. Scale bar represents 500 µm. (B) PKF115-584 dose-dependently blocked a decrease in bone length caused by IL-1β/TNFα treatment (10 ng/ml each) over time (indicated by red arrow). Other compounds and other concentrations did not counteract detrimental effects of IL-1β/TNFα treatment. Data represent the means of three independent experiments with 95% confidence intervals.
Alcian Blue staining for glycosaminoglycans demonstrated that the glycosaminoglycan content of the cartilaginous matrix of IL-1β/TNFα-treated metatarsals was decreased. In line with the effect of IL-1β and TNFα on glycosaminoglycans in the extracellular matrix, Alcian Blue staining was partially preserved by cotreatment with PKF115-584 and PKF118-310, but not with CGP049090 (Figures 4A and 4B). Also, collagen II staining of the extracellular matrix was completely lacking after treatment with IL-1β and TNFα, whereas cotreatment with PKF115-584, but not with PKF118-310 or CGP049090, could prevent loss of collagen II from the extracellular matrix (Figure 4C).
Figure 4.
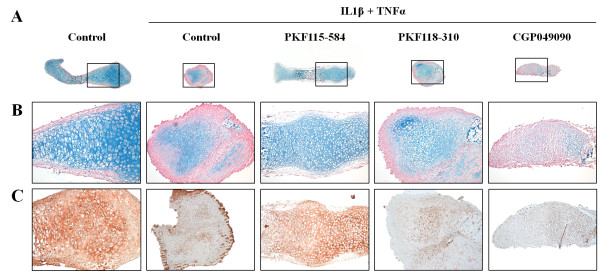
Interleukin 1β/tumor necrosis factor α-induced loss of glycosaminoglycans and collagen II was blocked by cotreatment with PKF115-584. (A) Metatarsals were treated with interleukin 1β (IL-1β) and tumor necrosis factor α (TNFα) (10 ng/ml each) in combination with small molecule WNT/β-catenin inhibitors (1.0 µM). PKF115-584 preserved morphology and glycosaminoglycan staining. A representative picture of two independent experiments is shown. Scale bar represents 500 µm. Boxed areas represent areas represented in B. (B) Magnification of the boxed region in (A). Scale bar represents 100 µm. (C) After 7 days of treatment, PKF115-584 (1.0 µM) prevented IL-1β and TNFα (10 ng/ml each)-induced loss of collagen II staining. A representative picture of three independent experiments is shown. Scale bar represents 100 µm.
Small molecule WNT/β-catenin inhibitors decrease expression of matrix catabolic genes
Because the catabolic effect of cytokines on cartilage consists of both induction of MMP expression and downregulated expression of cartilage matrix genes, we tested the effect of small molecule inhibitors on mRNA expression of these genes. In line with the findings reported in previous studies [6,12], IL-1β and TNFα significantly induced expression of Mmp3, Mmp9 and Mmp13. Small molecule PKF115-584 significantly downregulated IL-1β/TNFα-induced expression of Mmp9 and Mmp13 after 4 days of treatment, whereas CGP049090 only blocked the IL-1β/TNFα-induced upregulation of Mmp9 after 4 days (Figures 5A and 5B). The expression of cartilage matrix genes Acan and Col2a1 was significantly downregulated, and the expression of Sox9 tended to decrease, upon treatment with IL-1β and TNFα after 1 and 4 days of treatment. PKF115-584 and CGP049090, and to a lesser extent PKF118-310, also decreased the mRNA expression of Acan and Col2a1 from day 1 forward. Neither compound was able to counteract IL-1β/TNFα-induced reduction in gene expression neither at day 1 nor at day 4. The three inhibitors did not affect Sox9 or counteract the effect of IL-1β/TNFα on Sox9 expression after 1 day of treatment. Prolonged treatment with small molecules, with the exception of PKF118-310, decreased Sox9 expression.
Figure 5.
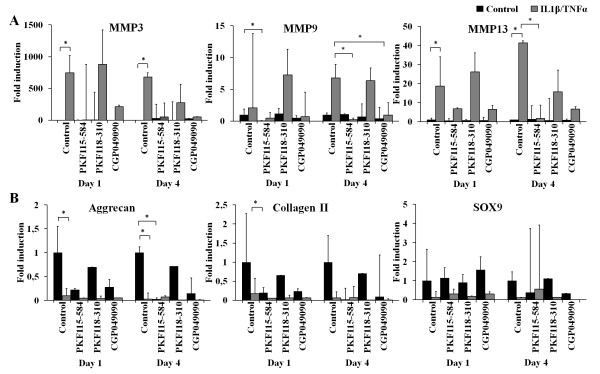
Small molecule inhibitors block interleukin 1β (IL-1β)/tumor necrosis factor α (TNFα)-induced expression of matrix metalloproteinases without affecting the IL-1β/TNFα-induced decrease in mRNA expression of cartilage markers. (A) Significant upregulation of Mmp3 expression was found when metatarsals were treated with IL-1β and TNFα. Both PKF115-584 and CGP049090 decreased this upregulation after 4 days of cotreatment, whereas PKF118-310 did not have an effect. Mmp9 expression was significantly downregulated by PKF115-584 after 1 day and IL-1β/TNFα-induced upregulation was prevented by both PKF115-584 and CGP049090, but not by PKF118-310, after 4 days of culture. Expression of Mmp13 was significantly upregulated by IL-1β and TNFα, whereas this effect was blocked by cotreatment with PKF115-584 or CGP049090, but not with PKF118-310. MMP, matrix metalloproteinase. (B) Acan expression is significantly decreased by IL-1β and TNFα at both day 1 and day 4. Also, small molecules PKF115-584 and CGP049090 downregulated expression of Acan by themselves. Expression of Col2a1 is downregulated by IL-1β and TNFα, and this effect could not be counteracted by small molecules. No significant effects on Sox9 expression were found. Data represent the means of two independent experiments with 95% confidence intervals. *P < 0.05.
Discussion
We hypothesized that inhibition of WNT/β-catenin signaling in cartilage might be an effective therapeutic strategy for the treatment of cytokine induced cartilage degradation. Therefore, in this study we have tested this hypothesis by assessing the effect of recently identified small molecule inhibitors of WNT/β-catenin signaling on cartilage degradation in the absence or presence of the pro-inflammatory cytokines IL-1β and TNFα, which are known to potently stimulate cartilage degradation by upregulating the expression of MMPs and aggrecanases [1,11].
Using the TOPflash TCF/Lef luciferase reporter construct experiments, we have shown that the small molecule inhibitors effectively block WNT/β-catenin signaling while having only a minor unfavorable effect on the metabolic activity of KS483-4C3 cells at higher concentrations as measured by MTT assay. Immunofluorescence staining for β-catenin revealed that PKF115-584 blocked nuclear translocation of β-catenin upon LiCl stimulation, without altering total β-catenin expression in basal conditions or after stimulation. PKF118-310 and CGP049090 slightly decreased the expression of β-catenin under basal conditions as well as upon LiCl stimulation. PKF118-310 blocked β-catenin translocation after LiCl stimulation, whereas CGP049090 did not affect nuclear translocation of β-catenin. Taking these findings together, we conclude that the small molecule inhibitors we selected can be used in further experiments to assess the effect on in vitro cartilage degradation. The discrepancy in the effect of the different small molecule inhibitors on nuclear translocation of β-catenin might indicate different mechanisms of action between these compounds.
To study the effects of the compounds on IL-1β- and TNFα-induced cartilage degradation, we used an ex vivo model consisting of mouse fetal metatarsals [29,30]. In degenerative cartilage disease, not only chondrocytes but also osteoblasts in the underlying bone are involved. The organ culture system that we used includes the primary center of ossification and the developing bone collar, as well as the cartilage template, providing chondrocytes as well as osteoblasts. Previously, it has also been shown that, in this model, system immune cells, including macrophages and osteoclasts, reside in the perichondrium [31]. This allows for communication between different cell types implemented in degenerative joint diseases that cannot be mimicked in other in vitro models, such as cartilage explants. Furthermore, in this system, chondrocytes and osteoblasts are in their natural environment, allowing the different cell types to interact with each other and with the extracellular matrix as they would in vivo. In line with the findings reported in previous studies, we observed that IL-1β and TNFα are potent inducers of cartilage and bone degradation in mouse fetal metatarsals [32-34]. Therefore, we considered this model to be suitable for studying the effect of small molecule inhibitors of the WNT/β-catenin signaling pathway on cartilage degradation induced by proinflammatory cytokines. In line with data reported in the literature, IL-1β alone demonstrated a mild effect on explant degradation. TNFα did not have an effect, but acted synergistically with IL-1β [29]. We therefore have chosen the combination of these cytokines to induce explant degradation and to evaluate the potential effect of the WNT/β-catenin inhibitors. Cartilage degradation is mainly due to increased expression and activity of MMPs, which can be induced by, among others, IL-1β and TNFα [1,11]. Indications of the involvement of WNT/β-catenin in IL-1β/TNFα-induced upregulation of MMPs have been found previously [12]. On the basis of morphometric and histological examination, we have shown that inhibition of WNT/β-catenin signaling by PKF115-584 can prevent the catabolic effects of IL-1β and TNFα on cartilage. CGP049090 prevented degradation of the extracellular matrix as well, albeit less effectively, whereas PKF118-310 did not have an anticatabolic effect.
Gene expression analysis revealed that the compounds, particularly PKF115-584 and CGP049090, inhibit IL-1β/TNFα-induced expression of catabolic genes Mmp3, Mmp9 and Mmp13. This indicates that inhibition of WNT/β-catenin signaling has an anticatabolic effect by blocking the induction of MMPs by IL-1β and TNFα. In line with the findings reported in previous studies [12], this observation further indicates that WNT/β-catenin signaling is involved in IL-1β/TNFα-induced MMP expression. As mentioned above, the catabolic effect of inflammatory cytokines consists, on the one hand, of the increased expression and activity of matrix-degrading proteins and, on the other hand, of decreased expression of cartilage anabolic genes. For the WNT/β-catenin inhibitors to block cartilage degradation effectively, they should interfere with both components of cartilage destruction. We found that small molecule inhibitors do block the catabolic process induced by IL-1β and TNFα. However, we did not find an effect of WNT/β-catenin inhibition on recovery of basal gene expression levels of extracellular matrix components Acan and Col2a1 after the use of IL-1β/TNFα, indicating that the synthesis of new extracellular matrix is not stimulated by small molecule inhibition. In addition, small molecules seem to have a repressive effect on bone growth, indicating a combined inhibitory effect on differentiation. These findings implicate the involvement of WNT/β-catenin signaling in the IL-1β/TNFα-induced effect on catabolic genes, but not in the effect on cartilage anabolic genes. In skeletal development, low levels of β-catenin are thought to promote chondroprogenitor differentiation, whereas, in later stages, high levels of β-catenin promote chondrocyte hypertrophic differentiation and subsequent endochondral ossification [35-37]. Based on these findings, inhibition of WNT/β-catenin signaling could be expected to induce cartilage matrix formation. However, low levels of WNT/β-catenin signaling seem not to have a stimulating effect on extracellular matrix formation after IL-1β- and TNFα-induced cartilage degradation. Other pathways, such as the MAPK/ERK pathway (mitogen-activated protein kinase and extracellular signal-regulated kinase) [38] and the nuclear factor κB pathway [39], were suggested to regulate the IL-1β-induced inhibition of gene expression of ACAN and COL2A1. Furthermore, immunofluorescence staining of β-catenin indicated that PKF115-584 might stabilize β-catenin in the cytosol, allowing for β-catenin to exert alternative effects such as direct binding to SOX9 and sequestering of SOX9 in the cytoplasm, thereby inhibiting expression of matrix genes.
Both PKF115-584 and PKF118-310 inhibit WNT/β-catenin signaling by blocking the binding of β-catenin to the transcription factor TCF4 [23]. We found differential effects of PKF115-584 and PKF118-310 on IL-1β/TNFα-induced cartilage degradation, which might be due to the fact that PKF115-584 inhibits translocation of β-catenin to the nucleus upon LiCl stimulation without affecting the basal amount of β-catenin, whereas PKF118-310 reduced both. In addition, CGP049090, which blocks the binding of β-catenin to TCF4 and Lef1 [24], was not as effective as PKF115-584. This might be due to the fact that PKF115-584 not only blocks binding of β-catenin to TCF4 but also disrupts binding of TCF4 to DNA [24].
Conclusion
This study provides evidence for the involvement of WNT/β-catenin signaling in MMP-mediated cartilage degradation induced by IL-1β and TNFα. Furthermore, we show that WNT/β-catenin signaling is not involved in the repressive effects of IL-1β and TNFα on cartilage matrix proteins such as ACAN and COL2A1. Instead, we provide evidence that WNT/β-catenin signaling may be directly involved in the regulation of the expression of these extracellular matrix proteins via an as yet unknown mechanism.
Abbreviations
ACAN: Aggrecan; cDNA: Complementary deoxyribonucleic acid; Col2a1: Collagen type 2a1; FZD: Frizzled; GAPDH: Glyceraldehyde 3-phosphate dehydrogenase; GSK3β: Glycogen synthase kinase 3β; IL-1β: Interleukin 1β; Lef1: Lymphoid enhancer-binding factor 1; LiCl: Lithium chloride; LRP: low-density lipoprotein receptor-related protein; MMP: Matrix metalloproteinase; mRNA: Messenger ribonucleic acid; OA: Osteoarthritis; RA: Rheumatoid arthritis; TCF4: Transcription factor 4; TNFα: Tumor necrosis factor α
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
EL performed luciferase and MTT assays, analyzed data and drafted the manuscript. RM performed immunofluorescent staining for β-catenin. Both EL and RM performed ex vivo experiments. MK and CB contributed extensively to the discussion of experimental design and data interpretation. All authors read and approved the final manuscript.
Supplementary Material
Figure S1 Small molecule inhibitors do not affect mouse fetal metatarsals. (A) No morphological changes were found in metatarsals treated with small molecules PKF115-584, PKF118-310 or CGP049090 at a concentration of 1.0 µM. A representative picture of two independent experiments is shown. Scale bar represents 500 µm. (B) No significant differences were found in the bone length when metatarsals were treated with small molecules. Data are means with 95% confidence intervals of three independent experiments.
Contributor Information
Ellie BM Landman, Email: ellielandman@gmail.com.
Razvan L Miclea, Email: r.l.miclea@lumc.nl.
Clemens A van Blitterswijk, Email: c.a.vanblitterswijk@utwente.nl.
Marcel Karperien, Email: h.b.j.karperien@utwente.nl.
References
- Fernandes JC, Martel-Pelletier J, Pelletier JP. The role of cytokines in osteoarthritis pathophysiology. Biorheology. 2002;15:237–246. [PubMed] [Google Scholar]
- Hedbom E, Häuselmann HJ. Molecular aspects of pathogenesis in osteoarthritis: the role of inflammation. Cell Mol Life Sci. 2002;15:45–53. doi: 10.1007/s00018-002-8404-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;15:33–42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- Pelletier JP, Martel-Pelletier J, Abramson SB. Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis Rheum. 2001;15:1237–1247. doi: 10.1002/1529-0131(200106)44:6<1237::AID-ART214>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Freemont AJ, Hampson V, Tilman R, Goupille P, Taiwo Y, Hoyland JA. Gene expression of matrix metalloproteinases 1, 3, and 9 by chondrocytes in osteoarthritic human knee articular cartilage is zone and grade specific. Ann Rheum Dis. 1997;15:542–549. doi: 10.1136/ard.56.9.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reboul P, Pelletier JP, Tardif G, Cloutier JM, Martel-Pelletier J. The new collagenase, collagenase-3, is expressed and synthesized by human chondrocytes but not by synoviocytes: a role in osteoarthritis. J Clin Invest. 1996;15:2011–2019. doi: 10.1172/JCI118636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyszer GM, Heer AH, Gay S. Cytokines and oncogenes in cellular interactions of rheumatoid arthritis. Stem Cells. 1994;15:75–86. doi: 10.1002/stem.5530120114. [DOI] [PubMed] [Google Scholar]
- Kanyama M, Kuboki T, Kojima S, Fujisawa T, Hattori T, Takigawa M, Yamashita A. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in synovial fluids of patients with temporomandibular joint osteoarthritis. J Orofac Pain. 2000;15:20–30. [PubMed] [Google Scholar]
- Little CB, Barai A, Burkhardt D, Smith SM, Fosang AJ, Werb Z, Shah M, Thompson EW. Matrix metalloproteinase 13-deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis Rheum. 2009;15:3723–3733. doi: 10.1002/art.25002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piecha D, Weik J, Kheil H, Becher G, Timmermann A, Jaworski A, Burger M, Hofmann MW. Novel selective MMP-13 inhibitors reduce collagen degradation in bovine articular and human osteoarthritis cartilage explants. Inflamm Res. 2010;15:379–389. doi: 10.1007/s00011-009-0112-9. [DOI] [PubMed] [Google Scholar]
- Tetlow LC, Adlam DJ, Woolley DE. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: associations with degenerative changes. Arthritis Rheum. 2001;15:585–594. doi: 10.1002/1529-0131(200103)44:3<585::AID-ANR107>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Yuasa T, Otani T, Koike T, Iwamoto M, Enomoto-Iwamoto M. Wnt/β-catenin signaling stimulates matrix catabolic genes and activity in articular chondrocytes: its possible role in joint degeneration. Lab Invest. 2008;15:264–274. doi: 10.1038/labinvest.3700747. [DOI] [PubMed] [Google Scholar]
- Caron JP, Fernandes JC, Martel-Pelletier J, Tardif G, Mineau F, Geng C, Pelletier JP. Chondroprotective effect of intraarticular injections of interleukin-1 receptor antagonist in experimental osteoarthritis: suppression of collagenase-1 expression. Arthritis Rheum. 1996;15:1535–1544. doi: 10.1002/art.1780390914. [DOI] [PubMed] [Google Scholar]
- Wu Q, Zhu M, Rosier RN, Zuscik MJ, O'Keefe RJ, Chen D. β-catenin, cartilage, and osteoarthritis. Ann N Y Acad Sci. 2010;15:344–350. doi: 10.1111/j.1749-6632.2009.05212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H. Wnt/β-catenin signaling in development and disease. Cell. 2006;15:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Zhu M, Tang D, Wu Q, Hao S, Chen M, Xie C, Rosier RN, O'Keefe RJ, Zuscik M, Chen D. Activation of β-catenin signaling in articular chondrocytes leads to osteoarthritis-like phenotype in adult β-catenin conditional activation mice. J Bone Miner Res. 2009;15:12–21. doi: 10.1359/jbmr.080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lories RJU, Peeters J, Bakker A, Tylzanowski P, Derese I, Schrooten J, Thomas JT, Luyten FP. Articular cartilage and biomechanical properties of the long bones in Frzb-knockout mice. Arthritis Rheum. 2007;15:4095–4103. doi: 10.1002/art.23137. [DOI] [PubMed] [Google Scholar]
- Imai K, Morikawa M, D'Armiento J, Matsumoto H, Komiya K, Okada Y. Differential expression of WNTs and FRPs in the synovium of rheumatoid arthritis and osteoarthritis. Biochem Biophys Res Commun. 2006;15:1615–1620. doi: 10.1016/j.bbrc.2006.05.075. [DOI] [PubMed] [Google Scholar]
- Hwang SG, Ryu JH, Kim IC, Jho EH, Jung HC, Kim K, Kim SJ, Chun JS. Wnt-7a causes loss of differentiated phenotype and inhibits apoptosis of articular chondrocytes via different mechanisms. J Biol Chem. 2004;15:26597–26604. doi: 10.1074/jbc.M401401200. [DOI] [PubMed] [Google Scholar]
- Ma B, van Blitterswijk CA, Karperien M. A Wnt/β-catenin negative feedback loop inhibits interleukin-1-induced matrix metalloproteinase expression in human articular chondrocytes. Arthritis Rheum. 2012;15:2589–2600. doi: 10.1002/art.34425. [DOI] [PubMed] [Google Scholar]
- Yun K, Im SH. Transcriptional regulation of MMP13 by Lef1 in chondrocytes. Biochem Biophys Res Commun. 2007;15:1009–1014. doi: 10.1016/j.bbrc.2007.10.121. [DOI] [PubMed] [Google Scholar]
- Lepourcelet M, Chen YN, France DS, Wang H, Crews P, Petersen F, Bruseo C, Wood AW, Shivdasani RA. Small-molecule antagonists of the oncogenic Tcf/β-catenin protein complex. Cancer Cell. 2004;15:91–102. doi: 10.1016/S1535-6108(03)00334-9. [DOI] [PubMed] [Google Scholar]
- Wei W, Chua MS, Grepper S, So S. Small molecule antagonists of Tcf4/β-catenin complex inhibit the growth of HCC cells in vitro and in vivo. Int J Cancer. 2010;15:2426–2436. doi: 10.1002/ijc.24810. [DOI] [PubMed] [Google Scholar]
- Gandhirajan RK, Staib PA, Minke K, Gehrke I, Plickert G, Schlösser A, Schmitt EK, Hallek M, Kreuzer KA. Small molecule inhibitors of Wnt/β-catenin/Lef-1 signaling induces apoptosis in chronic lymphocytic leukemia cells in vitro and in vivo. Neoplasia. 2010;15:326–335. doi: 10.1593/neo.91972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Horst G, van der Werf SM, Farih-Sips H, van Bezooijen RL, Löwik CWGM, Karperien M. Downregulation of Wnt signaling by increased expression of Dickkopf-1 and -2 is a prerequisite for late-stage osteoblast differentiation of KS483 cells. J Bone Miner Res. 2005;15:1867–1877. doi: 10.1359/JBMR.050614. [DOI] [PubMed] [Google Scholar]
- van Beek E, Oostendorp-van de Ruit M, van der Wee-Pals L, Bloys H, van de Bent C, Papapoulos S, Löwik C. Effects of experimental conditions on the release of 45calcium from prelabeled fetal mouse long bones. Bone. 1995;15:63–69. doi: 10.1016/8756-3282(95)00135-Z. [DOI] [PubMed] [Google Scholar]
- Haaijman A, Karperien M, Lanske B, Hendriks J, Löwik CWGM, Bronckers AL, Burger EH. Inhibition of terminal chondrocyte differentiation by bone morphogenetic protein 7 (OP-1) in vitro depends on the periarticular region but is independent of parathyroid hormone-related peptide. Bone. 1999;15:397–404. doi: 10.1016/S8756-3282(99)00189-1. [DOI] [PubMed] [Google Scholar]
- Yasuhara R, Yuasa T, Williams JA, Byers SW, Shah S, Pacifici M, Iwamoto M, Enomoto-Iwamoto M. Wnt/β-catenin and retinoic acid receptor signaling pathways interact to regulate chondrocyte function and matrix turnover. J Biol Chem. 2010;15:317–327. doi: 10.1074/jbc.M109.053926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bezooijen RL, van Der Wee-Pals L, Papapoulos SE, Löwik CWGM. Interleukin 17 synergises with tumour necrosis factor α to induce cartilage destruction in vitro. Ann Rheum Dis. 2002;15:870–876. doi: 10.1136/ard.61.10.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miclea RL, Siebelt M, Finos L, Goeman JJ, Löwik CWGM, Oostdijk W, Weinans H, Wit JM, Robanus-Maandag EC, Karperien M. Inhibition of Gsk3β in cartilage induces osteoarthritic features through activation of the canonical Wnt signaling pathway. Osteoarthritis Cartilage. 2011;15:1363–1372. doi: 10.1016/j.joca.2011.07.014. [DOI] [PubMed] [Google Scholar]
- van der Pluijm G, Deckers M, Sijmons B, de Groot H, Bird J, Wills R, Papapoulos S, Baxter A, Löwik CWGM. In vitro and in vivo endochondral bone formation models allow identification of anti-angiogenic compounds. Am J Pathol. 2003;15:157–163. doi: 10.1016/S0002-9440(10)63639-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre V, Peeters-Joris C, Vaes G. Modulation by interleukin 1 and tumor necrosis factor α of production of collagenase, tissue inhibitor of metalloproteinases and collagen types in differentiated and dedifferentiated articular chondrocytes. Biochim Biophys Acta. 1990;15:366–378. doi: 10.1016/0167-4889(90)90145-4. [DOI] [PubMed] [Google Scholar]
- Mårtensson K, Chrysis D, Sävendahl L. Interleukin-1β and TNF-α act in synergy to inhibit longitudinal growth in fetal rat metatarsal bones. J Bone Miner Res. 2004;15:1805–1812. doi: 10.1359/JBMR.040805. [DOI] [PubMed] [Google Scholar]
- MacRae VE, Farquharson C, Ahmed SF. The restricted potential for recovery of growth plate chondrogenesis and longitudinal bone growth following exposure to pro-inflammatory cytokines. J Endocrinol. 2006;15:319–328. doi: 10.1677/joe.1.06609. [DOI] [PubMed] [Google Scholar]
- Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/β-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005;15:739–750. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Tamamura Y, Otani T, Kanatani N, Koyama E, Kitagaki J, Komori T, Yamada Y, Costantini F, Wakisaka S, Pacifici M, Iwamoto M, Enomoto-Iwamoto M. Developmental regulation of Wnt/β-catenin signals is required for growth plate assembly, cartilage integrity, and endochondral ossification. J Biol Chem. 2005;15:19185–19195. doi: 10.1074/jbc.M414275200. [DOI] [PubMed] [Google Scholar]
- Hill TP, Später D, Taketo MM, Birchmeier W, Hartmann C. Canonical Wnt/β-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell. 2005;15:727–738. doi: 10.1016/j.devcel.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Wang X, Li F, Fan C, Wang C, Ruan H. Effects and relationship of ERK1 and ERK2 in interleukin-1β-induced alterations in MMP3, MMP13, type II collagen and aggrecan expression in human chondrocytes. Int J Mol Med. 2011;15:583–589. doi: 10.3892/ijmm.2011.611. [DOI] [PubMed] [Google Scholar]
- Yuasa T, Kondo N, Yasuhara R, Shimono K, Mackem S, Pacifici M, Iwamoto M, Enomoto-Iwamoto M. Transient activation of Wnt/β-catenin signaling induces abnormal growth plate closure and articular cartilage thickening in postnatal mice. Am J Pathol. 2009;15:1993–2003. doi: 10.2353/ajpath.2009.081173. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Small molecule inhibitors do not affect mouse fetal metatarsals. (A) No morphological changes were found in metatarsals treated with small molecules PKF115-584, PKF118-310 or CGP049090 at a concentration of 1.0 µM. A representative picture of two independent experiments is shown. Scale bar represents 500 µm. (B) No significant differences were found in the bone length when metatarsals were treated with small molecules. Data are means with 95% confidence intervals of three independent experiments.