Abstract
A role for phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2) in membrane fusion was originally identified for regulated dense-core vesicle exocytosis in neuroendocrine cells. Subsequent studies demonstrated essential roles for PI(4,5)P2 in regulated synaptic vesicle and constitutive vesicle exocytosis. For regulated dense-core vesicle exocytosis, PI(4,5)P2 appears to be primarily required for priming, a stage in vesicle exocytosis that follows vesicle docking and precedes Ca2+-triggered fusion. The priming step involves the organization of SNARE protein complexes for fusion. A central issue concerns the mechanisms by which PI(4,5)P2 exerts an essential role in membrane fusion events at the plasma membrane. The observed microdomains of PI(4,5)P2 in the plasma membrane of neuroendocrine cells at fusion sites has suggested possible direct effects of the phosphoinositide on membrane curvature and tension. More likely, PI(4,5)P2 functions in vesicle exocytosis as in other cellular processes to recruit and activate PI(4,5)P2-binding proteins. CAPS and Munc13 proteins, which bind PI(4,5)P2 and function in vesicle priming to organize SNARE proteins, are key candidates as effectors for the role of PI(4,5)P2 in vesicle priming. Consistent with roles prior to fusion that affect SNARE function, subunits of the exocyst tethering complex involved in constitutive vesicle exocytosis also bind PI(4,5)P2. Additional roles for PI(4,5)P2 in fusion pore dilation have been described, which may involve other PI(4,5)P2-binding proteins such as synaptotagmin. Lastly, the SNARE proteins that mediate exocytic vesicle fusion contain highly basic membrane-proximal domains that interact with acidic phospholipids that likely affect their function.
Keywords: CAPS; Munc13; PI(4, 5)P2 microdomains; SNARE proteins; Vesicle exocytosis
4.1 Introduction
The phosphoinositide PI(4,5)P2 serves many roles in cellular function. As the substrate for receptor-regulated phospholipase C (PLC)-mediated hydrolysis, its cleavage generates the signaling molecules Ins(1,4,5)P3 and DAG. The metabolism of PI(4,5)P2 also gives rise to PI(4)P or PI(3,4,5)P3 as signaling lipids. But possibly the most extensive role that PI(4,5)P2 plays is as an intact phospholipid that is characteristic of the plasma membrane. Whereas the total membrane composition of a cell consists of 1 mol% PI(4,5)P2, this lipid can achieve high local concentrations (~5 mol%) where its unique properties of high charge density and large hydrated headgroup can exert direct physical effects. Of likely greater significance for its signaling role, PI(4,5)P2 serves to recruit to or activate proteins or protein complexes in the plasma membrane. A large number of proteins have structured domains such as a PH domain or a C2 domain that interact stereoselectively with PI(4,5)P2 (Lemmon 2003, 2008). An even larger number of proteins containArg/Lys-rich+ hydrophobic regions that interact electrostatically with PI(4,5)P2 (McLaughlin et al. 2002). PI(4,5)P2 involvement in plasma membrane function extends to actin cytoskeletal regulation (Yin and Janmey 2003), channel and transporter regulation (Balla 2009; Suh and Hille 2008), virus budding (Saad et al. 2006), exocytosis (Martin 2001), phagocytosis (Grinstein 2010) and endocytosis (Martin 2001; Di Paolo and De Camilli 2006).
PI(4,5)P2 regulates vectorial membrane trafficking to and from the plasma membrane. In the anterograde direction, both constitutive and regulated vesicle exocytosis require PI(4,5)P2. Following an initial discussion of these exocytic pathways and the early discoveries that PI(4,5)P2 plays a role in membrane fusion, we will discuss mechanisms by which PI(4,5)P2 participates directly as a membrane constituent or as a cofactor for protein function in vesicle exocytosis.
4.2 Background on Membrane Fusion in Vesicle Exocytosis
PI(4,5)P2 at the plasma membrane functions in the vectorial process of exocytic vesicle fusion. All cells have an essential constitutive secretory pathway in which cargo in vesicles leaves the Golgi and transits directly or indirectly via endosomal intermediates to the plasma membrane (De Matteis and Luini 2008). In these pathways, the exocytic fusion of vesicles with the plasma membrane does not require cellular Ca2+ elevations. A second set of post-Golgi pathways found in neural, endocrine, exocrine and hematopoietic secretory cells constitute the regulated secretory pathway in which dense-core vesicles (DCVs) fuse with the plasma membrane only upon Ca2+ elevation. Additional regulated secretory pathways utilize endosome-derived vesicles such as the synaptic vesicles (SVs) in neurons that undergo Ca2+-dependent exocytosis. In regulated secretory pathways, vesicles are commonly staged at the plasma membrane prior to exocytosis in a docked configuration (Verhage and Sorensen 2008). Several lines of evidence indicate that vesicles undergo an obligatory priming step that renders them capable of engaging in Ca2+-triggered fusion (Rettig and Neher 2002). Priming is a regulated step between vesicle docking and fusion for which a number of distinct molecular constituents have been identified.
During the preceding two decades, many of the molecular constituents for vesicle exocytosis and its regulation have been identified (Jahn and Scheller 2006) (see Fig. 4.1). The core exocytic machinery consists of SNARE proteins present on vesicles and plasma membrane. The SNAREs constitute a minimal sufficient set of proteins to catalyze membrane fusion as demonstrated in liposome fusion assays (Weber et al. 1998). In cells, many other factors regulate and modulate SNARE protein function (Jahn and Scheller 2006). The pathway for membrane bilayer fusion consists of the initial merger of contacting leaflets to form a hemi-fused stalk intermediate. This is followed by the merger of non-contacting leaflets to form a fusion pore (Cohen and Melikyan 2004). The route to stalk formation and its resolution into a fusion pore involves considerable membrane bending (Chernomordik and Kozlov 2008). While membrane fusion is driven by proteins, studies over the last two decades identified phospholipids that play active roles in the membrane fusion process (van Meer and Sprong 2004; Salaun et al. 2004). This chapter will focus on PI(4,5)P2 and the role(s) it plays directly or indirectly (via proteins) in membrane fusion.
Figure 4.1.
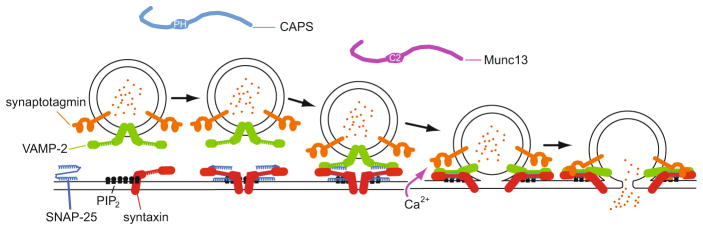
Sequential priming and fusion steps of Ca2+-triggered vesicle exocytosis are depicted with proteins that bind PI(4,5)P2. A hypothetical sequence of SNARE complex formation involving plasma membrane SNAP-25 and syntaxin and vesicle VAMP-2 is shown from left to right. Syntaxin is shown interacting with PI(4,5)P2 in PI(4,5)P2-rich microdomains with progressive segregation of PI(4,5)P2 from fusion sites. CAPS (via its PH domain) and Munc13 (via its Ca2+-dependent C2 domain) exhibit PI(4,5)P2 binding, which may mediate recruitment of these proteins to fusion sites for promoting SNARE complex formation. Synaptotagmin is shown to trigger fusion by interacting with SNAREs and membrane upon Ca2+ binding
4.3 Discovery of a Role for PI(4,5)P2 in Trafficking to the Plasma Membrane
Evidence for the involvement of PI(4,5)P2 in membrane fusion first emerged from studies of regulated DCV exocytosis in permeable neuroendocrine cells. Eberhard et al. (1990) found that that treatment of digitonin-permeabilized chromaffin cells with a bacterial PI-specific phospholipase C (PLC) decreased PI levels and inhibited Ca2+-triggered catecholamine secretion. Because inhibition was preferential for an ATP-dependent stage of DCV exocytosis, it was suggested that polyphosphoinositides may be required. Hay and Martin (1992) reported that sequential ATP-dependent and Ca2+-triggered reactions for DCV exocytosis in mechanically-permeabilized PC12 cells required distinct cytosolic protein factors. The cytosolic protein factors required for the ATP-dependent reactions were identified as phosphatidylinositol transfer protein (PITP) (Hay and Martin 1993) and phosphatidylinositol 4-monophosphate 5-kinase type Iγ (Hay et al. 1995). The identification of these factors, which mediate the ATP-dependent restoration of PI(4,5)P2 in permeable cell incubations, provided direct evidence that PI(4,5)P2 was essential for regulated DCV exocytosis. Hay et al. (1995) found that the addition of PI(4,5)P2-specific antibodies or PLCδ1 strongly inhibited regulated exocytosis. These results indicated that the intact lipid PI(4,5)P2 played a central role in a late step in the DCV exocytic pathway. Consistent with this, products derived from PI(4,5)P2 by hydrolysis (DAG, inositol phosphates, fatty acids) did not affect regulated DCV exocytosis in the Ca2+-buffered permeable PC12 cell system (Hay et al. 1995). Subsequent studies in permeable chromaffin cells by Wiedemann et al. (1996) suggested that PI 4-kinase activity on the secretory granules in chromaffin cells was also essential for regulated DCV exocytosis.
At the time of these initial discoveries, a role for highly phosphorylated inositides in a membrane fusion process was surprising although emerging studies in yeast were revealing a requirement for a PI 3-kinase (Vps34) in protein sorting to the vacuole (Schu et al. 1993). Studies on PI(4,5)P2 from this point forward were directed at assessing its importance for regulated vesicle exocytosis in living cells and evaluating the precise steps in the regulated secretory pathway at which it functioned. Along with the advancing understanding of the role of PI(4,5)P2 in cellular function, later studies probed the mechanism(s) by which PI(4,5)P2 participates in membrane fusion mechanisms. These developments are reviewed below.
4.4 A Role for PI(4,5)P2 in the Priming Reactions of Regulated DCV Exocytosis
Several studies in intact cells confirmed an essential role for PI(4,5)P2 in regulated DCV exocytosis. PI(4,5)P2 is mainly present at the plasma membrane in cells and Holz et al. (2000) showed that the PH domain of PLCδ1 localized to the plasma membrane of chromaffin cells where it inhibited regulated DCV exocytosis. PI(4,5)P2 was not detected on DCVs although PI(4)P is present because of their origin in the Golgi. In more recent studies, 3-phosphorylated inositides have also been localized to DCVs (Wen et al. 2011). This might imply that there is trafficking between DCVs and endosomes. PI(4)P 5-kinase 1γ is one of the major 5-kinases responsible for PI(4,5)P2 synthesis at the plasma membrane (Wenk et al. 2001). Increasing PI(4)P 5-kinase activity (Aikawa and Martin 2003; Aoyagi et al. 2005) or decreasing it (Lawrence and Birnbaum 2003; Gong et al. 2005; Waselle et al. 2005) in cells with corresponding changes in cellular PI(4,5)P2 levels was associated with increased or decreased rates of DCV exocytosis, respectively. These studies extended to living cells the conclusions that PI(4)P 5-kinase and PI(4,5)P2 regulate vesicle exocytosis.
Studies in permeable PC12 cells had indicated that PI(4,5)P2 was needed for a priming step in DCV exocytosis (Hay et al. 1995; Grishanin et al. 2004). Priming of DCV exocytosis in neuroendocrine cells was observed to be ATP-dependent (Rettig and Neher 2002). To determine the site in the sequential DCV exocytic pathway at which PI(4,5)P2 was required, high resolution capacitance studies were conducted in neuroendocrine cells in which PI(4,5)P2 levels were altered. Evoked capacitance changes in response to cellular Ca2+ rises are biphasic exhibiting burst and sustained phases. The burst phase represents the exocytosis of primed DCVs from a ready release pool (RRP) whereas the sustained phase is interpreted as priming reactions that refill the RRP. Olsen et al. (2003) recorded the immediate (< 2s) capacitance increase in patch-clamped pancreatic β cells in response to depolarization-elicited Ca2+ entry as a measure of the RRP. Maintenance of and refilling of the RRP required ATP and was inhibited by phenylarsine oxide, a non-specific inhibitor of PI 4-kinase (Wiedemann et al. 1996), or by antibodies to PI(4)P or PI(4,5)P2. Remarkably the ATP requirement for priming DCV exocytosis was by-passed by direct microinjection of PI(4)P or PI(4,5)P2. This evidence indicated that the priming of DCV exocytosis in pancreatic β cells involved the synthesis of PI(4)P and PI(4,5)P2. CAPS antibody was found to block priming in response to PI(4,5)P2 injection, which suggested that CAPS was an important effector for the role of PI(4,5)P2 in DCV exocytosis (see below).
Similar studies were conducted in chromaffin cells following the perturbation of PI(4,5)P2 levels (Milosevic et al. 2005). PI(4,5)P2 levels were measured in plasma membrane sheets prepared from cells and reacted with a GFP-PH-PLCδ1 fusion protein. Overexpression of PI(4)P 5-kinase 1γ or direct microinjection of PI(4,5)P2 was used to increase cellular PI(4,5)P2 levels. Increases in PI(4,5)P2 levels correlated with the increased size of the RRP and with increased rates of DCV (re)priming. Conversely, expression of a phosphatase domain of syntaptojanin-1 was utilized to decrease plasma membrane PI(4,5)P2 levels, which strongly reduced the RRP and inhibited DCV (re)priming rates. More recent studies of chromaffin cells from a PI(4)P 5-kinase 1γ knockout mouse reached similar conclusions that a reduction in plasma membrane PI(4,5)P2 levels mainly reduced the RRP and DCV (re)priming while slightly elevating the number of docked DCVs (Gong et al. 2005). Fusion pore expansion was also somewhat delayed in the PI(4)P 5-kinase 1γ knockout chromaffin cells. Overall these studies confirmed the importance of PI(4,5)P2 for priming DCV exocytosis although the basis for the critical role for this lipid remained to be elucidated.
4.5 A Role for PI(4,5)P2 in Other Forms of Vesicle Exocytosis
Regulated synaptic vesicle (SV) exocytosis utilizes an assembly of proteins very similar to that employed for DCV exocytosis. Whereas DCVs are directly Golgi-derived, SVs are derived from recycling endosomes. SVs were reported to possess a type II PI 4-kinase (Guo et al. 2003) similar to that reported for DCVs (Barylko et al. 2001). Previous work (Wenk et al. 2001) had established that PI(4)P 5-kinase 1γ is a major PI(4,5)P2-synthesizing enzyme in synapses that could potentially utilize the PI(4)P although the actual source of PI(4)P for PI(4,5)P2 synthesis at the presynaptic plasma membrane is unclear. Early studies on whether PI(4,5)P2 was required for Ca2+-triggered SV exocytosis in synaptosome preparations produced conflicting results (Khvotchev and Sudhof 1998; Zheng et al. 2004). Because endocytosis is strongly dependent upon PI(4,5)P2 and SVs rapidly recycle, a requirement for PI(4,5)P2 in SV exocytosis has been difficult to demonstrate. Di Paolo et al. (2004) reported that evoked synaptic transmission in cortical neurons from the PI(4)P 5-kinase 1γ knockout mouse was normal but there was a reduced RRP for SVs, and the RRP underwent accelerated depletion at high frequency stimulation. A delay in the recycling/repriming time for SVs and a slowing of endocytosis in the synapses from knockout mice was also observed. These results suggested that PI(4,5)P2 may be required for the evoked exocytosis of SVs.
Vesicle exocytosis in the constitutive secretory pathway is also dependent upon plasma membrane PI(4,5)P2 although this has yet to be thoroughly examined in mammalian cells. By contrast, extensive genetic evidence in yeast indicates an essential role of PI(4,5)P2 in post-Golgi vesicle exocytosis and for cell polarity mechanisms involving the actin cytoskeleton (Yakir-Tamang and Gerst 2009b; He and Guo 2009). MSS4 corresponds to the single PI(4)P 5-kinase in yeast. At the non-permissive temperature, Mss4 cells with a temperature-sensitive PI(4)P 5-kinase exhibit defects in actin localization and in secretion (Yakir-Tamang and Gerst 2009a). Conversely, MSS4 overexpression was capable of rescuing growth defects and secretion in a number of late sec gene mutants including those that encode exocyst subunits and a plasma membrane SNARE protein Sec9p (Yakir-Tamang and Gerst 2009a; Routt et al. 2005) (see below). Reminiscent of the original findings in neuroendocrine cells, overexpression of SFH5, a phosphatidylinositol-specific PITP, was found to suppress growth defects in late sec gene mutants (Routt et al. 2005; Yakir-Tamang and Gerst 2009a). The evidence indicates that SFH5 functions in a pathway involving the Stt4 PI 4-kinase and Mss4 PI(4)P 5-kinase to synthesize plasma membrane PI(4,5)P2 and this is required for the function of the exocyst complex and SNAREs in the constitutive secretory pathway (see below). The results support a key role for PI(4,5)P2 in the constitutive exocytosis of post-Golgi vesicles.
4.6 Is PI(4,5)P2 Spatially Segregated to Sites of Exocytosis?
Several studies in neuroendocrine cells have found that plasma membrane PI(4,5)P2 is spatially inhomogeneous and distributed in microdomains (Laux et al. 2000; Caroni 2001; Milosevic et al. 2005; Aoyagi et al. 2005; James et al. 2008). This was in part demonstrated in plasma membrane lawns using a GFP-PH fusion protein from PLCδ1, which binds PI(4,5)P2 without clustering it (James et al. 2008; Milosevic et al. 2005; Aoyagi et al. 2005). In studies with PC12 cell membrane lawns, the fluorescent probe was calibrated with PI(4,5)P2-containing supported bi-layers to infer a microdomain concentration for PI(4,5)P2 corresponding to ~6 mol% (James et al. 2008). Although it had been argued that apparent sites of PI(4,5)P2 enrichment may represent membrane infoldings (van Rheenen et al. 2005), the studies in PC12 cell membranes showed that non-specific lipid staining was not increased at sites of PI(4,5)P2 enrichment (James et al. 2008; Milosevic et al. 2005). Moreover, the inferred concentrations of PI(4,5)P2 detected were proportional to ATP-dependent synthesis (James et al. 2008). In this study, many of the PI(4,5)P2-enriched microdomains corresponded to sites of DCV docking (~35%). About 50% of CAPS, which is a PI(4,5)P2-binding protein required for DCV priming (see below), co-localized at microdomains of PI(4,5)P2 that contained docked DCVs.
Earlier studies by Aoyagi et al. (2005) had found that ~13% of the docked DCVs in PC12 cells resided at membrane sites that were enriched for both syntaxin-1 and PI(4,5)P2. Brief depolarization to elicit DCV exocytosis reduced this co-localization to 3%. The extent of co-localization of DCVs with syntaxin-1/PI(4,5)P2 clusters increased with cellular overexpression of PI(4)P 5-kinase, which also increased Ca2+-triggered DCV exocytosis (Aoyagi et al. 2005). Overall these studies (Aoyagi et al. 2005; James et al. 2008) suggested that plasma membrane sites for DCV docking, priming and fusion may be enriched for PI(4,5)P2. This work on isolated plasma membrane lawns has not yet been extended to living cells. Bodipy TMR-PI(4,5)P2 microinjected into cells was shown to exhibit ~3-fold reduced diffusion compared to the diffusion of other lipids leading the authors (Golebiewska et al. 2008) to conclude that ~2/3 of the PI(4,5)P2 was reversibly bound. However, it will be important to directly image PI(4,5)P2 in cells at sites of exocytosis to determine if membrane fusion occurs in PI(4,5)P2-rich membrane microdomains. The tools available currently to detect PI(4,5)P2 in living cells (e.g., PH-GFP) simultaneously inhibit Ca2+-triggered DCV exocytosis (Holz et al. 2000) so additional methods to detect and quantify PI(4,5)P2 in living cells will be needed.
While there is considerable evidence for independent pools of PI(4,5)P2 in the plasma membrane (Janmey and Lindberg 2004), the basis for PI(4,5)P2 microdomains in the plasma membrane is unknown. Even at concentrated sites of synthesis, diffusion is expected to rapidly dissipate concentration gradients of the lipid. PI(4,5)P2 would need to be “captured” at such sites. This might be achieved by interactions with proteins that have specific PI(4,5)P2-binding domains such as dynamin with its PH domain that in turn could oligomerize and cluster PI(4,5)P2 (Bethoney et al. 2009). Alternatively, the electrostatic clustering of PI(4,5)P2 by proteins that contain basic/hydrophobic regions could alter the diffusion of PI(4,5)P2 away from localized sites of synthesis (McLaughlin and Murray 2005). Proteins such as GAP-43, MARCKS, CAP-23, and NAP-22 contain “basic effector domains” capable of electrostatically sequestering PI(4,5)P2. The 13 basic residues in the MARCKS effector domain sequesters three PI(4,5)P2 molecules (McLaughlin and Murray 2005). Indeed overexpression of MARCKS in PC12 cells was found to increase PI(4,5)P2 clusters in the plasma membrane whereas overexpression of a dominant interfering mutant was found to decrease PI(4,5)P2 clusters (Laux et al. 2000). Many transmembrane proteins have Lys/Arg-rich segments on their cytoplasmic membrane-proximal domains, which would enable formation of a diversity of distinct PI(4,5)P2 microdomains containing different protein clusters. SNARE proteins such as syntaxin-1 that undergo cholesterol-dependent clustering at sites of DCV exocytosis have basic juxtamembrane regions that might sequester PI(4,5)P2 into associated microdomains (see below).
PI(4,5)P2 microdomains on the cytoplasmic leaflet have been suggested to align with extracellular leaflet liquid-ordered lipid rafts enriched in sphingolipids and cholesterol. This was based on biochemical methods isolating detergent-resistant membranes (Hope and Pike 1996). The unsaturated sn-2 acyl chain of PI(4,5)P2 renders this unlikely given the tight packing of saturated acyl chains in the classical lipid raft. However, recent work has indicated that proteins with highly basic domains that sequester PI(4,5)P2 may also partition into raft domains because of their myristoylation or palmitoylation. Studies of the HIV Gag protein suggested that the binding of PI(4,5)P2 by the Gag protein displaces a myristate buried in a hydrophobic pocket of the protein that inserts into a raft domain (Saad et al. 2006). In vitro studies of a palmitoylated GAP-43 peptide showed that it partitioned PI(4,5)P2 into liquid-ordered domains on giant unilamellar liposomes (Tong et al. 2008). Additional studies will be needed to determine the relationship, if any, between cytoplasmic leaflet PI(4,5)P2 microdomains and the lipid raft domains in the extracellular leaflet.
4.7 Mechanisms for PI(4,5)P2 Function in Membrane Fusion
A central question concerns the mechanism(s) by which PI(4,5)P2 affects membrane fusion. PI(4,5)P2 plays a strong positive role in regulated DCV exocytosis (Hay et al. 1995) where it regulates a priming step. As discussed below, there may be additional roles for PI(4,5)P2 at later steps in DCV exocytosis. Below we consider a number of suggested mechanisms for both positive and negative effects of PI(4,5)P2 on membrane fusion. Firstly, if PI(4,5)P2 is localized at membrane fusion sites at the high concentrations (~6 mol%) detected (James et al. 2008), it would contribute bulk properties to the local membrane environment including curvature and charge density. High local concentrations and domain segregation may affect membrane tension in fusion mechanisms. Secondly, PI(4,5)P2 is a substrate for enzymatic conversion as well as an activator of enzymes that generate lipid products (DAG, PA) that affect membrane curvature, fluidity and fusion. Thirdly, and the most generally established mechanism for PI(4,5)P2 in cellular processes, is that the lipid recruits cytosolic proteins to specific locations on a membrane surface (Martin 1998; Lemmon 2003; 2008; Kutateladze 2010). Regulation of integral membrane protein function is also well-characterized (Balla 2009; Suh and Hille 2008). The functional diversity of PI(4,5)P2-binding proteins is enormous and could contribute to membrane fusion by a variety of mechanisms. We discuss mechanisms that operate at vesicle priming as well as later steps in vesicle exocytosis.
4.8 Direct Effects of Membrane PI(4,5)P2
Membrane preparations from PC12 cells exhibit spatially-restricted microdomains of PI(4,5)P2 near docked DCVs (James et al. 2008; Aoyagi et al. 2005; Milosevic et al. 2005). PI(4,5)P2 concentrations in microdomains may exceed 5 mol% in contrast to interdomain regions at ~2 mol% (James et al. 2008). PI(4,5)P2 is considered to be an inverted cone-shaped lipid that would exert positive curvature in a localized region (Chernomordik and Kozlov 2008). PI(4,5)P2 at 5 mol% in either v-SNARE donor or t-SNARE acceptor liposomes was found to inhibit SNARE-dependent liposome fusion. Vicogne and co-workers also found that PI(4,5)P2 was inhibitory when included in t-SNARE liposomes (Vicogne et al. 2006). Inhibition by PI(4,5)P2 was comparable to that by another inverted cone-shaped lipid, lysophosphatidylcholine, at 5 mol% and was attributed to the positive curvature-promoting properties of PI(4,5)P2 that would counter formation of a stalk intermediate (James et al. 2008). This inhibitory mechanism observed in liposomes was partially counteracted by the sequestration of PI(4,5)P2 by a basic charge-rich linker domain in syntaxin-1. There may be other mechanisms in cells for which PI(4,5)P2 exerts stimulatory effects on fusion.
Classical (Chandler and Heuser 1980) and more recent studies (Anantharam et al. 2010) indicate that the plasma membrane invaginates toward DCVs during membrane fusion. The induction of local curvature in the plasma membrane by PI(4,5)P2 at fusion sites could play a role in promoting bilayer apposition as well as creating tension in the plasma membrane to facilitate fusion (Kozlov et al. 2010). PI(4,5)P2 microdomains in the plasma membrane may exhibit positive curvature but in addition many PI(4,5)P2-binding proteins undergo hydrophobic insertion, which would further amplify positive curvature. Many types of PI(4,5)P2-binding proteins exhibit bilayer insertion including PH domain-containing proteins such as CAPS and dynamin (Ramachandran et al. 2009), tandem C2 domain-containing proteins such as synaptotagmin (Martens et al. 2007), and ENTH domain containing proteins such as epsin (Ford et al. 2002). There is evidence that Ca2+-triggered membrane insertion of synaptotagmin into the plasma membrane during fusion increases membrane curvature and tension to promote fusion pore dilation (Martens et al. 2007; Lynch et al. 2008; Hui et al. 2009). Thus, the overall local membrane curvature imparted by PI(4,5)P2 within plasma membrane microdomains and enhanced by protein insertion could play a significant positive role in promoting membrane transitions during fusion.
4.9 Role of PI(4,5)P2-Derived or Activated Metabolites
Under mild Ca2+ stimulation conditions, DCV exocytosis requires PI(4,5)P2 as the intact phospholipid (Eberhard et al. 1990; Hay et al. 1995). However, under strong stimulation conditions, PI(4,5)P2 can be metabolized by phospholipase C (PLC) (Micheva et al. 2001). One of the metabolites of PI(4,5)P2, DAG, has been strongly linked to activation mechanisms for regulated vesicle exocytosis. Protein kinase C and brain isoforms of Munc13 have DAG-binding C1 domains that mediate activation of these proteins (Brose et al. 2004). It has also been suggested that the transformation of PI(4,5)P2 to DAG could exert dramatic effects on the shape of membranes to trigger fusion (Janmey and Kinnunen 2006) but an essential role for PLCs in fusion per se remains to be demonstrated. Whether DAG is generated at exocytic fusion sites and whether DAG, as a cone-shaped lipid, has additional positive roles in affecting membrane curvature remain to be explored.
Phospholipase D (PLD), which is a PH domain-containing, PI(4,5)P2-activated enzyme that hydrolyzes PC to PA, has been strongly implicated both in regulated DCV exocytosis and in constitutive vesicle exocytosis (Bader and Vitale 2009). PA is a cone-shaped phospholipid so its presence in the cytoplasmic leaflet could enhance the transition of merged membranes into a stalk intermediate to promote fusion. In PC12 cells in which Ca2+ entry was stimulated by depolarization, an accumulation of PA at the plasma membrane was detected using a PA-binding protein-GFP fusion protein (Zeniou-Meyer et al. 2007) although this was delayed compared to evoked DCV exocytosis. Nonetheless, the down regulation of PLD1 by siRNA was found to block PA accumulation as well as evoked DCV exocytosis. Capacitance recordings in chromaffin cells indicated that PLD1 siRNA reduced the RRP size as well as DCV priming. Application of lysophosphatidylcholine, an inverted cone-shaped lipid, to the extracellular leaflet reversed the inhibitory effect of PA depletion on DCV exocytosis in PC12 cells. The authors (Zeniou-Meyer et al. 2007) suggested that PLD1 activation resulted in membrane bending through the generation of PA. This might be expected to function in DCV fusion rather than in DCV priming. These studies suggested that PLD1 is an important effector for the role of PI(4,5)P2 in DCV exocytosis. Other studies have suggested that SCAMP2, a membrane tetraspanin protein that binds PI(4,5)P2 and PLD1, may regulate a late step in DCV exocytosis involving fusion pore formation (Liao et al. 2007).
4.10 Protein Recruitment and Activation by PI(4,5)P2
At present, the best established mechanisms for the function of PI(4,5)P2 in actin polymerization (Janmey and Lindberg 2004) and endocytosis (Di Paolo and De Camilli 2006) involve protein recruitment. In each of these cases, proteins interact with PI(4,5)P2 either through specific binding domains such as PH domains or through electrostatic interactions with domains that are rich in basic and hydrophobic residues. PI(4,5)P2-binding proteins with PH domains, C2 domains, or Lys/Arg-rich regions play a major role in various steps of vesicle exocytosis including priming.
4.11 SNARE Protein Interactions with Acidic Phospholipids
SNARE proteins, the core constituents of the fusion machinery, are directly regulated by the acidic phospholipids in the cytoplasmic leaflet of membranes. Syntaxin-1/SNAP-25 t-SNARE heterodimers were reported to exhibit reduced mobility in supported bilayers that contained PI(4,5)P2 (Wagner and Tamm 2001). PI(4,5)P2 itself exhibits reduced mobility in supported bilayers (Baumann et al. 2010), which suggests that direct interactions with PI(4,5)P2 may reduce the mobility of t-SNAREs to organize them at sites in the membrane. PI(4,5)P2 may also activate syntaxin-1 for assembly with SNAP-25 as recent studies (Murray and Tamm 2009) indicated that the cholesterol-dependent self-clustering of syntaxin-1 in liposomes was decreased by the inclusion of PI(4,5)P2 at 1–5 mol%. Direct binding of the cytoplasmic domain of syntaxin-1 to acidic phospholipids has been demonstrated (Lam et al. 2008).
A conserved binding site for PI(4,5)P2 (or PA) among exocytic syntaxins consists of K252KAVKYQSKARRKK265 (for syntaxin-1) in the membrane-proximal linker domain that is C-terminal to the SNARE motif. Mutations of K residues in this juxtamembrane segment results in a loss of evoked DCV exocytosis in cells and in decreased SNARE-dependent fusion on PI(4,5)P2-containing liposomes in vitro (Lam et al. 2008; James et al. 2008). Both of these results indicate that syntaxin interactions with PI(4,5)P2 (James et al. 2008) or PA (Lam et al. 2008) play a positive role in membrane fusion. As noted previously, interactions with syntaxin were proposed to segregate PI(4,5)P2 in the membrane to prevent the steric inhibition of fusion (James et al. 2008) (see Fig. 4.1). Alternatively, for the cellular studies, it was suggested that syntaxin interacted with PA to concentrate this negative curvature-preferring lipid at the periphery of contacting leaflets to reduce the energy requirement for stalk formation (Lam et al. 2008).
Interactions of the juxtamembrane segment with acidic phospholipids could also drive conformational changes in syntaxin. Soluble versions of syntaxin adopt a closed configuration that blocks the interaction of syntaxin with other SNARE proteins (Chen et al. 2008). The conformation of syntaxin in the membrane could be affected by juxtamembrane segment interactions with acidic phospholipids. Alternatively, PI(4,5)P2 interactions with syntaxin could play a role in localizing the protein on the membrane or in promoting SNAP-25 interactions (Aoyagi et al. 2005; Murray and Tamm 2009). Either of these effects might explain a positive role for PI(4,5)P2 in priming DCV exocytosis. While these studies indicate an important role for the highly basic linker domain of syntaxin in interactions with acidic phospholipids, many roles for this interaction seem possible and need further evaluation. Based on the effects of PI(4.5)P2 in SNARE-dependent liposome fusion (James et al. 2008), the mechanisms discussed here are unlikely to provide a complete explanation for the strong role for PI(4,5)P2 in priming DCV exocytosis (see below).
The vesicle SNARE VAMP-2 also interacts with acidic phospholipids through membrane-proximal linker segments containing K83LKRKYWWKNLK94 (for VAMP-2) (Williams et al. 2009; Kweon et al. 2003; De Haro et al. 2003). Seagar and co-workers (De Haro et al. 2003; Quetglas et al. 2002) reported that a region of VAMP-2 overlapping this one binds Ca2+/calmodulin and acidic phospholipids in a mutually exclusive manner. They provided evidence that Ca2+/calmodulin binding to VAMP-2 switched its cis interactions with vesicle membrane lipids to trans interactions with the plasma membrane. These interactions might be expected to promote fusion but recent liposome fusion assay studies showed that Ca2+/calmodulin inhibited SNARE-dependent fusion (Di Giovanni et al. 2010). Williams et al. (2009) reported that the overexpression of a VAMP-2 K85E/R86D mutant inhibited evoked DCV exocytosis and they suggested that the basic juxtamembrane region of wild-type VAMP-2 acts in trans to counteract charge repulsion between the bilayers at approaches of < 1 nm. The principle electrostatic interaction forVAMP-2 in trans would be with PI(4,5)P2 in the plasma membrane. It was also proposed (Williams et al. 2009) that the basic juxtamembrane regions on both VAMP-2 and syntaxin-1 may function symmetrically through nonspecific electrostatic interactions in trans to promote close membrane apposition and trans SNARE complex assembly. These studies indicate an important role for basic charge-containing residues in the membrane-proximal region of VAMP-2 but the role these play remain uncertain.
Interactions with acidic phospholipids for exocytic SNARE proteins are quite general. For example, the yeast syntaxin Sso1p binds acidic phospholipids via membrane-proximal basic residues. About half of the stimulation of SNARE-dependent liposome fusion by PA was attributed to this interaction (Liu et al. 2007b). Additional studies are needed to determine whether there are common mechanisms at work in SNARE protein-lipid interactions and what function they play.
4.12 CAPS and Munc13 as Lipid-Binding Proteins for Priming Vesicle Exocytosis
As indicated previously, the major role for PI(4,5)P2 in the regulated secretory pathway relates to a function in priming DCV exocytosis. Two of the major priming proteins for regulated vesicle exocytosis, CAPS and Munc13, are regulated by PI(4,5)P2 and by PI(4,5)P2 or DAG, respectively. CAPS was discovered as a protein in rat brain cytosol that reconstitutes Ca2+-triggered DCV exocytosis in mechanically-permeabilized PC12 cells (Walent et al. 1992). The activity of CAPS in permeable cells is only evident after ATP-dependent reactions involving PITP and PI(4)P 5-kinase that restore PI(4,5)P2 have gone to completion (Grishanin et al. 2004). CAPS binds PI(4,5)P2 in part through its central PH domain, which is required for CAPS activity in evoked DCV exocytosis (Grishanin et al. 2002, 2004; Loyet et al. 1998). Recent studies reconstituted part of the function of CAPS in a SNARE protein-dependent liposome fusion assay (James et al. 2008, 2009). CAPS activity in the liposome fusion assay requires that PI(4,5)P2 is present in the acceptor liposomes that contain the plasma membrane t-SNAREs syntaxin-1 and SNAP-25. By contrast, PI(4,5)P2 in the donor VAMP-2-containing liposomes failed to support CAPS function. As anticipated for essential interactions with PI(4,5)P2, the PH domain of CAPS was required for its activity in liposome fusion (James et al. 2008). CAPS functions in vesicle priming where it likely promotes the assembly of SNARE protein complexes in advance of triggered fusion (James et al. 2009). On liposomes, heterotrimeric SNARE complex formation is accelerated by CAPS but only when PI(4,5)P2 is present on the t-SNARE liposomes. This contrasts with the lack of a requirement for PI(4,5)P2 for CAPS binding to SNARE proteins (Daily et al. 2010). Thus, the current evidence indicates that CAPS may function in vesicle priming through dual interactions with PI(4,5)P2 via its PH domain and with SNARE proteins via a C-terminal domain in CAPS. Anchorage in the membrane through PI(4,5)P2 interactions may allow CAPS to exert force on the SNARE proteins to mediate rearrangements. This model can account for a positive role for PI(4,5)P2 in priming Ca2+-triggered DCV exocytosis (see Fig. 4.1).
Studies in PC12 cells, chromaffin cells and neurons indicate that C2 domain-containing proteins cofunction with CAPS in vesicle priming reactions (Liu et al. 2010; Jockusch et al. 2007). Munc13 proteins exhibit sequence homology to CAPS in C-terminal regions that mediate SNARE interactions (Koch et al. 2000). Genetic disruption of Munc13 isoforms in mice strongly inhibits neurotransmitter release at the stage of priming SVs (Varoqueaux et al. 2002). Brain-specific isoforms of Munc13 lack a PH domain but contain three C2 domains and a C1 domain. The second C2 domain of Munc13-1 binds Ca2+ and exhibits Ca2+-dependent PI(4,5)P2 binding (Shin et al. 2010). A gain of function C2B domain mutant of Munc13 exhibited increased neurotransmitter release evoked by single action potentials whereas a C2B mutant abrogated for Ca2+ binding showed decreased release with trains of action potentials (Shin et al. 2010). As noted previously, brain Munc13 isoforms also contain a C1 domain that binds DAG. Munc13 with a C1 domain mutation is dysfunctional in potentiating SV or DCV exocytosis (Bauer et al. 2007; Rhee et al. 2002; Rosenmund et al. 2002). Munc13 as a priming factor may be recruited to sites of exocytosis, either to PI(4,5)P2 during Ca2+ rises, or to DAG arising from Ca2+ activation of PLC (Rosenmund et al. 2002; Rhee et al. 2002) (see Fig. 4.1). Thus, for some forms of regulated vesicle exocytosis, PI(4,5)P2 hydrolysis may be required for function to generate DAG (Hammond et al. 2006). Overall, both major priming proteins that function in regulated vesicle exocytosis in neural and endocrine cells, CAPS and Munc13, utilize PI(4,5)P2 or its metabolite DAG for activation. In future studies, it will be important to determine the plasma membrane sites for PI(4,5)P2 and DAG synthesis relative to vesicle exocytosis and establish whether CAPS and Munc13 proteins are recruited to these sites.
4.13 Roles for Other PI(4,5)P2-Binding Proteins in Regulated Vesicle Exocytosis
Additional steps in vesicle exocytosis beyond priming may require PI(4,5)P2 and PI(4,5)P2-binding proteins. In capacitance recordings of DCV exocytosis in chromaffin cells (Milosevic et al. 2005), modulation of PI(4,5)P2 levels affected the RRP and rates of [re]priming. However, rates of evoked exocytosis were not affected, which implies that proteins required for fusion per se or its Ca2+ triggering were not strongly dependent upon PI(4,5)P2. Similar findings emerged in the capacitance studies of chromaffin cells from PI(4)P 5-kinase 1γ knockout mice (Di Paolo et al. 2004). Synaptotagmins, the major Ca2+ sensors for regulated vesicle exocytosis, exhibit Ca2+-dependent binding to PI(4,5)P2 in vitro (Bai et al. 2004). Loss of synaptotagmin function is associated with decreased rates of triggered exocytosis (Voets et al. 2001). Possibly residual levels of PI(4,5)P2 in cells from the PI(4)P 5-kinase 1γ knockout mice are sufficient to maintain synaptotagmin function. Additional studies in the knockout mice indicated that the number of DCVs docked at the plasma membrane were unaltered, which implies that proteins involved in DCV docking are not greatly affected by PI(4,5)P2. However, in amperometric measurements of catecholamine secretion, subtle differences were observed in the amperometric spikes from chromaffin cells of control and knockout mice (Di Paolo et al. 2004). In the latter, a longer duration pre-spike foot was observed, which may indicate altered fusion pore dynamics in cells with decreased PI(4,5)P2. Proteins that bind PI(4,5)P2 and regulate fusion pore dynamics might include dynamin, a PH domain-containing protein (Tsuboi et al. 2004), synaptotagmin, a tandem C2 domain-containing protein (Wang et al. 2001; Lynch et al. 2008), and SCAMP2, a PI(4,5)P2-binding tetraspanin protein (Liao et al. 2007). In addition, a decrease of PI(4,5)P2 would result in decreased F actin polymerization, which would alter fusion pore dilation (Berberian et al. 2009).
4.14 Tethering Complexes Bind PI(4,5)P2 in Constitutive Vesicle Exocytosis
Considerable genetic evidence indicates that plasma membrane PI(4,5)P2 is an important component for establishing the polarity of the actin cytoskeleton and the selection of exocytic fusion sites for post-Golgi vesicles (reviewed in (Yakir-Tamang and Gerst 2009b; He and Guo 2009)). Studies of the constitutive exocytic pathway reinforce the view that PI(4,5)P2 plays an important role in recruiting proteins to the target membrane. In the budding yeast, post-Golgi vesicles are delivered on a polarized F actin cytoskeleton to bud sites on the plasma membrane. As noted previously, mutants in MSS4, the sole PI(4)P 5-kinase in yeast, exhibit defects in actin localization and defects in secretion (Yakir-Tamang and Gerst 2009a). The former results in part from failure to recruit the PH domain-containing Rho GEF Rom2 to the plasma membrane. The latter results in part due to mislocalization of a vesicle tethering complex called the exocyst complex. The exocyst complex consists of 8 subunits encoded by late Sec genes (Sec3, Sec5, Sec6, Sec8, Sec10, Sec15, Exo70, Exo84) (He and Guo 2009). Sec3 and Exo70 localize to the plasma membrane largely independent of actin whereas other exocyst subunits are vesicle-associated. Actin-dependent delivery of vesicles to the plasma membrane completes formation of the exocyst complex, which activates SNARE complexes for fusion. Sec3 was found to bind PI(4,5)P2 via N-terminal polybasic sequences (Zhang et al. 2008) and Exo70 to bind PI(4,5)P2 via a C-terminal domain (He et al. 2007). These subunits also interact with GTPases required for their localization and function (He and Guo 2009; Yakir-Tamang and Gerst 2009b). Recent studies demonstrated that the mammalian exocyst Exo70 also interacts with PI(4,5)P2, which was essential for the docking and fusion of post-Golgi secretory vesicles in the constitutive secretory pathway (Liu et al. 2007a). These studies indicate that plasma membrane PI(4,5)P2 plays an important role in recruiting subunits that enable the assembly of an essential tethering complex that activates SNARE-dependent vesicle fusion.
At each stage of vesicle trafficking in the secretory pathway, a diverse set of tethering factors or tethering complexes mediate contact between an incoming vesicle and a target membrane (Sztul and Lupashin 2006). It was recently suggested that CAPS and Munc13 exhibit significant homology to other tethering factor subunits such as exocyst Sec6 suggesting a common ancestral origin (Pei et al. 2009). Many other tethering factors bind to the phosphoinositides that are characteristic of the target membrane. For example, EEA1 in endosome tethering binds PI(3)P (Gaullier et al. 1999). The HOPS complex in vacuole tethering binds PI(3)P and other phosphoinositides (Stroupe et al. 2006). A general prediction for vesicle exocytosis is that proteins involved in vesicle tethering and priming at the plasma membrane will bind PI(4,5)P2.
4.15 Conclusions
As a specific constituent characteristic of the plasma membrane in resting cells, PI(4,5)P2 likely participates in all vectorial processes involving the plasma membrane. As an abundant highly-charged constituent in the cytoplasmic leaflet, PI(4,5)P2 affects many plasma membrane processes through electrostatic interactions with commonly-occurring Arg/Lys/hydrophobic sequences in proteins or through specific PH or C2 domains. The major role for PI(4,5)P2 in vesicle exocytosis involves protein recruitment and activation. The possibility that this abundant lipid may be concentrated in enriched microdomains where it could exert direct effects on membrane curvature and tension needs to be further assessed. PI(4,5)P2-binding proteins such as CAPS and Munc13 play a major role in vesicle priming reactions where the principal role of PI(4,5)P2 is exerted. Other proteins such as PLD1, synaptotagmin and SNAREs may mediate the regulation by PI(4,5)P2 at other stages of vesicle exocytosis.
References
- Aikawa Y, Martin TF. ARF6 regulates a plasma membrane pool of phosphatidylinositol(4,5)bisphosphate required for regulated exocytosis. J Cell Biol. 2003;162:647–659. doi: 10.1083/jcb.200212142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anantharam A, Axelrod D, Holz RW. Polarized TIRFM reveals changes in plasma membrane topology before and during granule fusion. Cell Mol Neurobiol. 2010;30:1343–1349. doi: 10.1007/s10571-010-9590-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyagi K, Sugaya T, Umeda M, Yamamoto S, Terakawa S, Takahashi M. The activation of exocytotic sites by the formation of phosphatidylinositol 4,5-bisphosphate microdomains at syntaxin clusters. J Biol Chem. 2005;280:17346–17352. doi: 10.1074/jbc.M413307200. [DOI] [PubMed] [Google Scholar]
- Bader MF, Vitale N. Phospholipase D in calcium-regulated exocytosis: lessons from chromaffin cells. Biochim Biophys Acta. 2009;1791:936–941. doi: 10.1016/j.bbalip.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Bai J, Tucker WC, Chapman ER. PIP2 increases the speed of response of synaptotagmin and steers its membrane-penetration activity toward the plasma membrane. Nat Struct Mol Biol. 2004;11:36–44. doi: 10.1038/nsmb709. [DOI] [PubMed] [Google Scholar]
- Balla T. Regulation of Ca2+ entry by inositol lipids in mammalian cells by multiple mechanisms. Cell Calcium. 2009;45:527–534. doi: 10.1016/j.ceca.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barylko B, Gerber SH, Binns DD, Grichine N, Khvotchev M, Sudhof TC, Albanesi JP. A novel family of phosphatidylinositol 4-kinases conserved from yeast to humans. J Biol Chem. 2001;276:7705–7708. doi: 10.1074/jbc.C000861200. [DOI] [PubMed] [Google Scholar]
- Bauer CS, Woolley RJ, Teschemacher AG, Seward EP. Potentiation of exocytosis by phospholipase C-coupled G-protein-coupled receptors requires the priming protein Munc13-1. J Neurosci. 2007;27:212–219. doi: 10.1523/JNEUROSCI.4201-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MK, Amstad E, Mashaghi A, Textor M, Reimhult E. Characterization of supported lipid bilayers incorporating the phosphoinositides phosphatidylinositol 4,5-biphosphate and phosphoinositol-3,4,5-triphosphate by complementary techniques. Biointerphases. 2010;5:114–119. doi: 10.1116/1.3516485. [DOI] [PubMed] [Google Scholar]
- Berberian K, Torres AJ, Fang Q, Kisler K, Lindau M. F-actin and myosin II accelerate catecholamine release from chromaffin granules. J Neurosci. 2009;29:863–870. doi: 10.1523/JNEUROSCI.2818-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethoney KA, King MC, Hinshaw JE, Ostap EM, Lemmon MA. A possible effector role for the pleckstrin homology (PH) domain of dynamin. Proc Natl Acad Sci U S A. 2009;106:13359–13364. doi: 10.1073/pnas.0906945106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brose N, Betz A, Wegmeyer H. Divergent and convergent signaling by the diacylglycerol second messenger pathway in mammals. Curr Opin Neurobiol. 2004;14:328–340. doi: 10.1016/j.conb.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Caroni P. New EMBO members’ review: actin cytoskeleton regulation through modulation of PI(4,5)P(2) rafts. EMBO J. 2001;20:4332–4336. doi: 10.1093/emboj/20.16.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler DE, Heuser JE. Arrest of membrane fusion events in mast cells by quick-freezing. J Cell Biol. 1980;86:666–674. doi: 10.1083/jcb.86.2.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Lu J, Dulubova I, Rizo J. NMR analysis of the closed conformation of syntaxin-1. J Biomol NMR. 2008;41:43–54. doi: 10.1007/s10858-008-9239-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernomordik LV, Kozlov MM. Mechanics of membrane fusion. Nat Struct Mol Biol. 2008;15:675–683. doi: 10.1038/nsmb.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen FS, Melikyan GB. The energetics of membrane fusion from binding, through hemifusion, pore formation, and pore enlargement. J Membr Biol. 2004;199:1–14. doi: 10.1007/s00232-004-0669-8. [DOI] [PubMed] [Google Scholar]
- Daily NJ, Boswell KL, James DJ, Martin TF. Novel interactions of CAPS (Ca2+-dependent activator protein for secretion) with the three neuronal SNARE proteins required for vesicle fusion. J Biol Chem. 2010;285:35320–35329. doi: 10.1074/jbc.M110.145169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Haro L, Quetglas S, Iborra C, Leveque C, Seagar M. Calmodulin-dependent regulation of a lipid binding domain in the v-SNARE synaptobrevin and its role in vesicular fusion. Biol Cell. 2003;95:459–464. doi: 10.1016/s0248-4900(03)00076-5. [DOI] [PubMed] [Google Scholar]
- De Matteis MA, Luini A. Exiting the Golgi complex. Nat Rev Mol Cell Biol. 2008;9:273–284. doi: 10.1038/nrm2378. [DOI] [PubMed] [Google Scholar]
- Di Giovanni J, Iborra C, Maulet Y, Leveque C, El Far O, Seagar M. Calcium-dependent regulation of SNARE-mediated membrane fusion by calmodulin. J Biol Chem. 2010;285:23665–23675. doi: 10.1074/jbc.M109.096073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- Di Paolo G, Moskowitz HS, Gipson K, Wenk MR, Voronov S, Obayashi M, Flavell R, Fitzsimonds RM, Ryan TA, De Camilli P. Impaired PtdIns(4,5)P2 synthesis in nerve terminals produces defects in synaptic vesicle trafficking. Nature. 2004;431:415–422. doi: 10.1038/nature02896. [DOI] [PubMed] [Google Scholar]
- Eberhard DA, Cooper CL, Low MG, Holz RW. Evidence that the inositol phospholipids are necessary for exocytosis. Loss of inositol phospholipids and inhibition of secretion in permeabilized cells caused by a bacterial phospholipase C and removal of ATP. Biochem J. 1990;268:15–25. doi: 10.1042/bj2680015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MG, Mills IG, Peter BJ, Vallis Y, Praefcke GJ, Evans PR, McMahon HT. Curvature of clathrin-coated pits driven by epsin. Nature. 2002;419:361–366. doi: 10.1038/nature01020. [DOI] [PubMed] [Google Scholar]
- Gaullier JM, Simonsen A, D’arrigo A, Bremnes B, Stenmark H. FYVE finger proteins as effectors of phosphatidylinositol 3-phosphate. Chem Phys Lipids. 1999;98:87–94. doi: 10.1016/s0009-3084(99)00021-3. [DOI] [PubMed] [Google Scholar]
- Golebiewska U, Nyako M, Woturski W, Zaitseva I, McLaughlin S. Diffusion coefficient of fluorescent phosphatidylinositol 4,5-bisphosphate in the plasma membrane of cells. Mol Biol Cell. 2008;19:1663–1669. doi: 10.1091/mbc.E07-12-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong LW, Di Paolo G, Diaz E, Cestra G, Diaz ME, Lindau M, De Camilli P, Toomre D. Phosphatidylinositol phosphate kinase type I gamma regulates dynamics of large dense-core vesicle fusion. Proc Natl Acad Sci U S A. 2005;102:5204–5209. doi: 10.1073/pnas.0501412102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinstein S. Imaging signal transduction during phagocytosis: phospholipids, surface charge, and electrostatic interactions. Am J Physiol Cell Physiol. 2010;299:C876–C881. doi: 10.1152/ajpcell.00342.2010. [DOI] [PubMed] [Google Scholar]
- Grishanin RN, Klenchin VA, Loyet KM, Kowalchyk JA, Ann K, Martin TF. Membrane association domains in Ca2+-dependent activator protein for secretion mediate plasma membrane and dense-core vesicle binding required for Ca2+-dependent exocytosis. J Biol Chem. 2002;277:22025–22034. doi: 10.1074/jbc.M201614200. [DOI] [PubMed] [Google Scholar]
- Grishanin RN, Kowalchyk JA, Klenchin VA, Ann K, Earles CA, Chapman ER, Gerona RR, Martin TF. CAPS acts at a prefusion step in dense-core vesicle exocytosis as a PIP2 binding protein. Neuron. 2004;43:551–562. doi: 10.1016/j.neuron.2004.07.028. [DOI] [PubMed] [Google Scholar]
- Guo J, Wenk MR, Pellegrini L, Onofri F, Benfenati F, De Camilli P. Phosphatidylinositol 4-kinase type IIalpha is responsible for the phosphatidylinositol 4-kinase activity associated with synaptic vesicles. Proc Natl Acad Sci U S A. 2003;100:3995–4000. doi: 10.1073/pnas.0230488100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond GR, Dove SK, Nicol A, Pinxteren JA, Zicha D, Schiavo G. Elimination of plasma membrane phosphatidylinositol (4,5)-bisphosphate is required for exocytosis from mast cells. J Cell Sci. 2006;119:2084–2094. doi: 10.1242/jcs.02912. [DOI] [PubMed] [Google Scholar]
- Hay JC, Martin TF. Resolution of regulated secretion into sequential MgATP-dependent and calcium-dependent stages mediated by distinct cytosolic proteins. J Cell Biol. 1992;119:139–151. doi: 10.1083/jcb.119.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay JC, Martin TF. Phosphatidylinositol transfer protein required forATP-dependent priming of Ca(2+)-activated secretion. Nature. 1993;366:572–575. doi: 10.1038/366572a0. [DOI] [PubMed] [Google Scholar]
- Hay JC, Fisette PL, Jenkins GH, Fukami K, Takenawa T, Anderson RA, Martin TF. ATP-dependent inositide phosphorylation required for Ca(2+)-activated secretion. Nature. 1995;374:173–177. doi: 10.1038/374173a0. [DOI] [PubMed] [Google Scholar]
- He B, Guo W. The exocyst complex in polarized exocytosis. Curr Opin Cell Biol. 2009;21:537–542. doi: 10.1016/j.ceb.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Xi F, Zhang X, Zhang J, Guo W. Exo70 interacts with phospholipids and mediates the targeting of the exocyst to the plasma membrane. EMBO J. 2007;26:4053–4065. doi: 10.1038/sj.emboj.7601834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz RW, Hlubek MD, Sorensen SD, Fisher SK, Balla T, Ozaki S, Prestwich GD, Stuenkel EL, Bittner MA. A pleckstrin homology domain specific for phosphatidylinositol 4, 5-bisphosphate (PtdIns-4,5-P2) and fused to green fluorescent protein identifies plasma membrane PtdIns-4,5-P2 as being important in exocytosis. J Biol Chem. 2000;275:17878–17885. doi: 10.1074/jbc.M000925200. [DOI] [PubMed] [Google Scholar]
- Hope HR, Pike LJ. Phosphoinositides and phosphoinositide-utilizing enzymes in detergent-insoluble lipid domains. Mol Biol Cell. 1996;7:843–851. doi: 10.1091/mbc.7.6.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui E, Johnson CP, Yao J, Dunning FM, Chapman ER. Synaptotagmin-mediated bending of the target membrane is a critical step in Ca(2+)-regulated fusion. Cell. 2009;138:709–721. doi: 10.1016/j.cell.2009.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R, Scheller RH. SNAREs—engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- James DJ, Khodthong C, Kowalchyk JA, Martin TF. Phosphatidylinositol 4,5-bisphosphate regulates SNARE-dependent membrane fusion. J Cell Biol. 2008;182:355–366. doi: 10.1083/jcb.200801056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James DJ, Kowalchyk J, Daily N, Petrie M, Martin TF. CAPS drives trans-SNARE complex formation and membrane fusion through syntaxin interactions. Proc Natl Acad Sci U S A. 2009;106:17308–17313. doi: 10.1073/pnas.0900755106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janmey PA, Kinnunen PK. Biophysical properties of lipids and dynamic membranes. Trends Cell Biol. 2006;16:538–546. doi: 10.1016/j.tcb.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Janmey PA, Lindberg U. Cytoskeletal regulation: rich in lipids. Nat Rev Mol Cell Biol. 2004;5:658–666. doi: 10.1038/nrm1434. [DOI] [PubMed] [Google Scholar]
- Jockusch WJ, Speidel D, Sigler A, Sorensen JB, Varoqueaux F, Rhee JS, Brose N. CAPS-1 and CAPS-2 are essential synaptic vesicle priming proteins. Cell. 2007;131:796–808. doi: 10.1016/j.cell.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Khvotchev M, Sudhof TC. Newly synthesized phosphatidylinositol phosphates are required for synaptic norepinephrine but not glutamate or gamma-aminobutyric acid (GABA) release. J Biol Chem. 1998;273:21451–21454. doi: 10.1074/jbc.273.34.21451. [DOI] [PubMed] [Google Scholar]
- Koch H, Hofmann K, Brose N. Definition of Munc13-homology-domains and characterization of a novel ubiquitously expressed Munc13 isoform. Biochem J. 2000;349:247–253. doi: 10.1042/0264-6021:3490247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlov MM, McMahon HT, Chernomordik LV. Protein-driven membrane stresses in fusion and fission. Trends Biochem Sci. 2010;35:699–706. doi: 10.1016/j.tibs.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutateladze TG. Translation of the phosphoinositide code by PI effectors. Nat Chem Biol. 2010;6:507–513. doi: 10.1038/nchembio.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kweon DH, Kim CS, Shin YK. Regulation of neuronal SNARE assembly by the membrane. Nat Struct Biol. 2003;10:440–447. doi: 10.1038/nsb928. [DOI] [PubMed] [Google Scholar]
- Lam AD, Tryoen-Toth P, Tsai B, Vitale N, Stuenkel EL. SNARE-catalyzed fusion events are regulated by Syntaxin 1A-lipid interactions. Mol Biol Cell. 2008;19:485–497. doi: 10.1091/mbc.E07-02-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laux T, Fukami K, Thelen M, Golub T, Frey D, Caroni P. GAP43, MARCKS, and CAP23 modulate PI(4,5)P(2) at plasmalemmal rafts, and regulate cell cortex actin dynamics through a common mechanism. J Cell Biol. 2000;149:1455–1472. doi: 10.1083/jcb.149.7.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence JT, Birnbaum MJ. ADP-ribosylation factor 6 regulates insulin secretion through plasma membrane phosphatidylinositol 4,5-bisphosphate. Proc NatlAcad Sci U SA. 2003;100:13320–13325. doi: 10.1073/pnas.2232129100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon MA. Phosphoinositide recognition domains. Traffic. 2003;4:201–213. doi: 10.1034/j.1600-0854.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- Lemmon MA. Membrane recognition by phospholipid-binding domains. Nat Rev Mol Cell Biol. 2008;9:99–111. doi: 10.1038/nrm2328. [DOI] [PubMed] [Google Scholar]
- Liao H, Ellena J, Liu L, Szabo G, Cafiso D, Castle D. Secretory carrier membrane protein SCAMP2 and phosphatidylinositol 4,5-bisphosphate interactions in the regulation of dense core vesicle exocytosis. Biochemistry. 2007;46:10909–10920. doi: 10.1021/bi701121j. [DOI] [PubMed] [Google Scholar]
- Liu J, Zuo X, Yue P, Guo W. Phosphatidylinositol 4,5-bisphosphate mediates the targeting of the exocyst to the plasma membrane for exocytosis in mammalian cells. Mol Biol Cell. 2007a;18:4483–4492. doi: 10.1091/mbc.E07-05-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Wilson KA, Rice-Stitt T, Neiman AM, McNew JA. In vitro fusion catalyzed by the sporulation-specific t-SNARE light-chain Spo20p is stimulated by phosphatidic acid. Traffic. 2007b;8:1630–1643. doi: 10.1111/j.1600-0854.2007.00628.x. [DOI] [PubMed] [Google Scholar]
- Liu Y, Schirra C, Edelmann L, Matti U, Rhee J, Hof D, Bruns D, Brose N, Rieger H, Stevens DR, Rettig J. Two distinct secretory vesicle-priming steps in adrenal chromaffin cells. J Cell Biol. 2010;190:1067–1077. doi: 10.1083/jcb.201001164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyet KM, Kowalchyk JA, Chaudhary A, Chen J, Prestwich GD, Martin TF. Specific binding of phosphatidylinositol 4,5-bisphosphate to calcium-dependent activator protein for secretion (CAPS), a potential phosphoinositide effector protein for regulated exocytosis. J Biol Chem. 1998;273:8337–8343. doi: 10.1074/jbc.273.14.8337. [DOI] [PubMed] [Google Scholar]
- Lynch KL, Gerona RR, Kielar DM, Martens S, McMahon HT, Martin TF. Synaptotagmin-1 utilizes membrane bending and SNARE binding to drive fusion pore expansion. Mol Biol Cell. 2008;19:5093–5103. doi: 10.1091/mbc.E08-03-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens S, Kozlov MM, McMahon HT. How synaptotagmin promotes membrane fusion. Science. 2007;316:1205–1208. doi: 10.1126/science.1142614. [DOI] [PubMed] [Google Scholar]
- Martin TF. Phosphoinositide lipids as signaling molecules: common themes for signal transduction, cytoskeletal regulation, and membrane trafficking. Annu Rev Cell Dev Biol. 1998;14:231–264. doi: 10.1146/annurev.cellbio.14.1.231. [DOI] [PubMed] [Google Scholar]
- Martin TF. PI(4,5)P(2) regulation of surface membrane traffic. Curr Opin Cell Biol. 2001;13:493–499. doi: 10.1016/s0955-0674(00)00241-6. [DOI] [PubMed] [Google Scholar]
- McLaughlin S, Murray D. Plasma membrane phosphoinositide organization by protein electrostatics. Nature. 2005;438:605–611. doi: 10.1038/nature04398. [DOI] [PubMed] [Google Scholar]
- McLaughlin S, Wang J, Gambhir A, Murray D. PIP(2) and proteins: interactions, organization, and information flow. Annu Rev Biophys Biomol Struct. 2002;31:151–175. doi: 10.1146/annurev.biophys.31.082901.134259. [DOI] [PubMed] [Google Scholar]
- Micheva KD, Holz RW, Smith SJ. Regulation of presynaptic phosphatidylinositol 4,5-biphosphate by neuronal activity. J Cell Biol. 2001;154:355–368. doi: 10.1083/jcb.200102098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milosevic I, Sorensen JB, Lang T, Krauss M, Nagy G, Haucke V, Jahn R, Neher E. Plasmalemmal phosphatidylinositol-4,5-bisphosphate level regulates the releasable vesicle pool size in chromaffin cells. J Neurosci. 2005;25:2557–2565. doi: 10.1523/JNEUROSCI.3761-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray DH, Tamm LK. Clustering of syntaxin-1A in model membranes is modulated by phosphatidylinositol 4,5-bisphosphate and cholesterol. Biochemistry. 2009;48:4617–4625. doi: 10.1021/bi9003217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen HL, Hoy M, Zhang W, Bertorello AM, Bokvist K, Capito K, Efanov AM, Meister B, Thams P, Yang SN, Rorsman P, Berggren PO, Gromada J. Phosphatidylinositol 4-kinase serves as a metabolic sensor and regulates priming of secretory granules in pancreatic beta cells. Proc Natl Acad Sci U S A. 2003;100:5187–5192. doi: 10.1073/pnas.0931282100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei J, Ma C, Rizo J, Grishin NV. Remote homology between Munc13 MUN domain and vesicle tethering complexes. J Mol Biol. 2009;391:509–517. doi: 10.1016/j.jmb.2009.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quetglas S, Iborra C, Sasakawa N, De Haro L, Kumakura K, Sato K, Leveque C, Seagar M. Calmodulin and lipid binding to synaptobrevin regulates calcium-dependent exocytosis. EMBO J. 2002;21:3970–3979. doi: 10.1093/emboj/cdf404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R, Pucadyil TJ, Liu YW, Acharya S, Leonard M, Lukiyanchuk V, Schmid SL. Membrane insertion of the pleckstrin homology domain variable loop 1 is critical for dynamin-catalyzed vesicle scission. Mol Biol Cell. 2009;20:4630–4639. doi: 10.1091/mbc.E09-08-0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettig J, Neher E. Emerging roles of presynaptic proteins in Ca + +-triggered exocytosis. Science. 2002;298:781–785. doi: 10.1126/science.1075375. [DOI] [PubMed] [Google Scholar]
- Rhee JS, Betz A, Pyott S, Reim K, Varoqueaux F, Augustin I, Hesse D, Sudhof TC, Takahashi M, Rosenmund C, Brose N. Beta phorbol ester- and diacylglycerol-induced augmentation of transmitter release is mediated by Munc13s and not by PKCs. Cell. 2002;108:121–133. doi: 10.1016/s0092-8674(01)00635-3. [DOI] [PubMed] [Google Scholar]
- Rosenmund C, Sigler A, Augustin I, Reim K, Brose N, Rhee JS. Differential control of vesicle priming and short-term plasticity by Munc13 isoforms. Neuron. 2002;33:411–424. doi: 10.1016/s0896-6273(02)00568-8. [DOI] [PubMed] [Google Scholar]
- Routt SM, Ryan MM, Tyeryar K, Rizzieri KE, Mousley C, Roumanie O, Brennwald PJ, Bankaitis VA. Nonclassical PITPs activate PLD via the Stt4p PtdIns-4-kinase and modulate function of late stages of exocytosis in vegetative yeast. Traffic. 2005;6:1157–1172. doi: 10.1111/j.1600-0854.2005.00350.x. [DOI] [PubMed] [Google Scholar]
- Saad JS, Miller J, Tai J, Kim A, Ghanam RH, Summers MF. Structural basis for targeting HIV-1 Gag proteins to the plasma membrane for virus assembly. Proc Natl Acad Sci U S A. 2006;103:11364–11369. doi: 10.1073/pnas.0602818103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salaun C, James DJ, Chamberlain LH. Lipid rafts and the regulation of exocytosis. Traffic. 2004;5:255–264. doi: 10.1111/j.1600-0854.2004.0162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schu PV, Takegawa K, Fry MJ, Stack JH, Waterfield MD, Emr SD. Phosphatidylinositol 3-kinase encoded by yeast VPS34 gene essential for protein sorting. Science. 1993;260:88–91. doi: 10.1126/science.8385367. [DOI] [PubMed] [Google Scholar]
- Shin OH, Lu J, Rhee JS, Tomchick DR, Pang ZP, Wojcik SM, Camacho-Perez M, Brose N, Machius M, Rizo J, Rosenmund C, Sudhof TC. Munc13 C2B domain is an activity-dependent Ca2+ regulator of synaptic exocytosis. Nat Struct Mol Biol. 2010;17:280–288. doi: 10.1038/nsmb.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroupe C, Collins KM, Fratti RA, Wickner W. Purification of active HOPS complex reveals its affinities for phosphoinositides and the SNARE Vam7p. EMBO J. 2006;25:1579–1589. doi: 10.1038/sj.emboj.7601051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh BC, Hille B. PIP2 is a necessary cofactor for ion channel function: how and why? Annu Rev Biophys. 2008;37:175–195. doi: 10.1146/annurev.biophys.37.032807.125859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztul E, Lupashin V. Role of tethering factors in secretory membrane traffic. Am J Physiol Cell Physiol. 2006;290:C11–C26. doi: 10.1152/ajpcell.00293.2005. [DOI] [PubMed] [Google Scholar]
- Tong J, Nguyen L, Vidal A, Simon SA, Skene JH, McIntosh TJ. Role of GAP-43 in sequestering phosphatidylinositol 4,5-bisphosphate to Raft bilayers. Biophys J. 2008;94:125–133. doi: 10.1529/biophysj.107.110536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi T, McMahon HT, Rutter GA. Mechanisms of dense core vesicle recapture following “kiss and run” (“cavicapture”) exocytosis in insulin-secreting cells. J Biol Chem. 2004;279:47115–47124. doi: 10.1074/jbc.M408179200. [DOI] [PubMed] [Google Scholar]
- Van Meer G, Sprong H. Membrane lipids and vesicular traffic. Curr Opin Cell Biol. 2004;16:373–378. doi: 10.1016/j.ceb.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Van Rheenen J, Achame EM, Janssen H, Calafat J, Jalink K. PIP2 signaling in lipid domains: a critical re-evaluation. EMBO J. 2005;24:1664–1673. doi: 10.1038/sj.emboj.7600655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varoqueaux F, Sigler A, Rhee JS, Brose N, Enk C, Reim K, Rosenmund C. Total arrest of spontaneous and evoked synaptic transmission but normal synaptogenesis in the absence of Munc13-mediated vesicle priming. Proc Natl Acad Sci U S A. 2002;99:9037–9042. doi: 10.1073/pnas.122623799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhage M, Sorensen JB. Vesicle docking in regulated exocytosis. Traffic. 2008;9:1414–1424. doi: 10.1111/j.1600-0854.2008.00759.x. [DOI] [PubMed] [Google Scholar]
- Vicogne J, Vollenweider D, Smith JR, Huang P, Frohman MA, Pessin JE. Asymmetric phospholipid distribution drives in vitro reconstituted SNARE-dependent membrane fusion. Proc Natl Acad Sci U S A. 2006;103:14761–14766. doi: 10.1073/pnas.0606881103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voets T, Moser T, Lund PE, Chow RH, Geppert M, Sudhof TC, Neher E. Intracellular calcium dependence of large dense-core vesicle exocytosis in the absence of synaptotagmin I. Proc Natl Acad Sci U S A. 2001;98:11680–11685. doi: 10.1073/pnas.201398798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner ML, Tamm LK. Reconstituted syntaxin1a/SNAP25 interacts with negatively charged lipids as measured by lateral diffusion in planar supported bilayers. Biophys J. 2001;81:266–275. doi: 10.1016/S0006-3495(01)75697-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walent JH, Porter BW, Martin TF. A novel 145 kd brain cytosolic protein reconstitutes Ca(2+)-regulated secretion in permeable neuroendocrine cells. Cell. 1992;70:765–775. doi: 10.1016/0092-8674(92)90310-9. [DOI] [PubMed] [Google Scholar]
- Wang CT, Grishanin R, Earles CA, Chang PY, Martin TF, Chapman ER, Jackson MB. Synaptotagmin modulation of fusion pore kinetics in regulated exocytosis of dense-core vesicles. Science. 2001;294:1111–1115. doi: 10.1126/science.1064002. [DOI] [PubMed] [Google Scholar]
- Waselle L, Gerona RR, Vitale N, Martin TF, Bader MF, Regazzi R. Role of phosphoinositide signaling in the control of insulin exocytosis. Mol Endocrinol. 2005;19:3097–3106. doi: 10.1210/me.2004-0530. [DOI] [PubMed] [Google Scholar]
- Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Sollner TH, Rothman JE. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- Wen PJ, Osborne SL, Meunier FA. Dynamic control of neuroexocytosis by phosphoinositides in health and disease. Prog Lipid Res. 2011;50:52–61. doi: 10.1016/j.plipres.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Wenk MR, Pellegrini L, Klenchin VA, Di Paolo G, Chang S, Daniell L, Arioka M, Martin TF, De Camilli P. PIP kinase Igamma is the major PI(4,5)P(2) synthesizing enzyme at the synapse. Neuron. 2001;32:79–88. doi: 10.1016/s0896-6273(01)00456-1. [DOI] [PubMed] [Google Scholar]
- Wiedemann C, Schafer T, Burger MM. Chromaffin granule-associated phosphatidylinositol 4-kinase activity is required for stimulated secretion. EMBO J. 1996;15:2094–2101. [PMC free article] [PubMed] [Google Scholar]
- Williams D, Vicogne J, Zaitseva I, McLaughlin S, Pessin JE. Evidence that electrostatic interactions between vesicle-associated membrane protein 2 and acidic phospholipids may modulate the fusion of transport vesicles with the plasma membrane. Mol Biol Cell. 2009;20:4910–4919. doi: 10.1091/mbc.E09-04-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakir-Tamang L, Gerst JE. A phosphatidylinositol-transfer protein and phosphatidylinositol-4-phosphate 5-kinase control Cdc42 to regulate the actin cytoskeleton and secretory pathway in yeast. Mol Biol Cell. 2009a;20:3583–3597. doi: 10.1091/mbc.E08-10-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakir-Tamang L, Gerst JE. Phosphoinositides, exocytosis and polarity in yeast: all about actin? Trends Cell Biol. 2009b;19:677–684. doi: 10.1016/j.tcb.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Yin HL, Janmey PA. Phosphoinositide regulation of the actin cytoskeleton. Annu Rev Physiol. 2003;65:761–789. doi: 10.1146/annurev.physiol.65.092101.142517. [DOI] [PubMed] [Google Scholar]
- Zeniou-Meyer M, Zabari N, Ashery U, Chasserot-Golaz S, Haeberle AM, Demais V, Bailly Y, Gottfried I, Nakanishi H, Neiman AM, Du G, Frohman MA, Bader MF, Vitale N. Phospholipase D1 production of phosphatidic acid at the plasma membrane promotes exocytosis of large dense-core granules at a late stage. J Biol Chem. 2007;282:21746–21757. doi: 10.1074/jbc.M702968200. [DOI] [PubMed] [Google Scholar]
- Zhang X, Orlando K, He B, Xi F, Zhang J, Zajac A, Guo W. Membrane association and functional regulation of Sec3 by phospholipids and Cdc42. J Cell Biol. 2008;180:145–158. doi: 10.1083/jcb.200704128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Q, McFadden SC, Bobich JA. Phosphatidylinositol 4,5-bisphosphate promotes both [3H]-noradrenaline and [14C]-glutamate exocytosis from nerve endings. Neurochem Int. 2004;44:243–250. doi: 10.1016/s0197-0186(03)00149-9. [DOI] [PubMed] [Google Scholar]


