Abstract
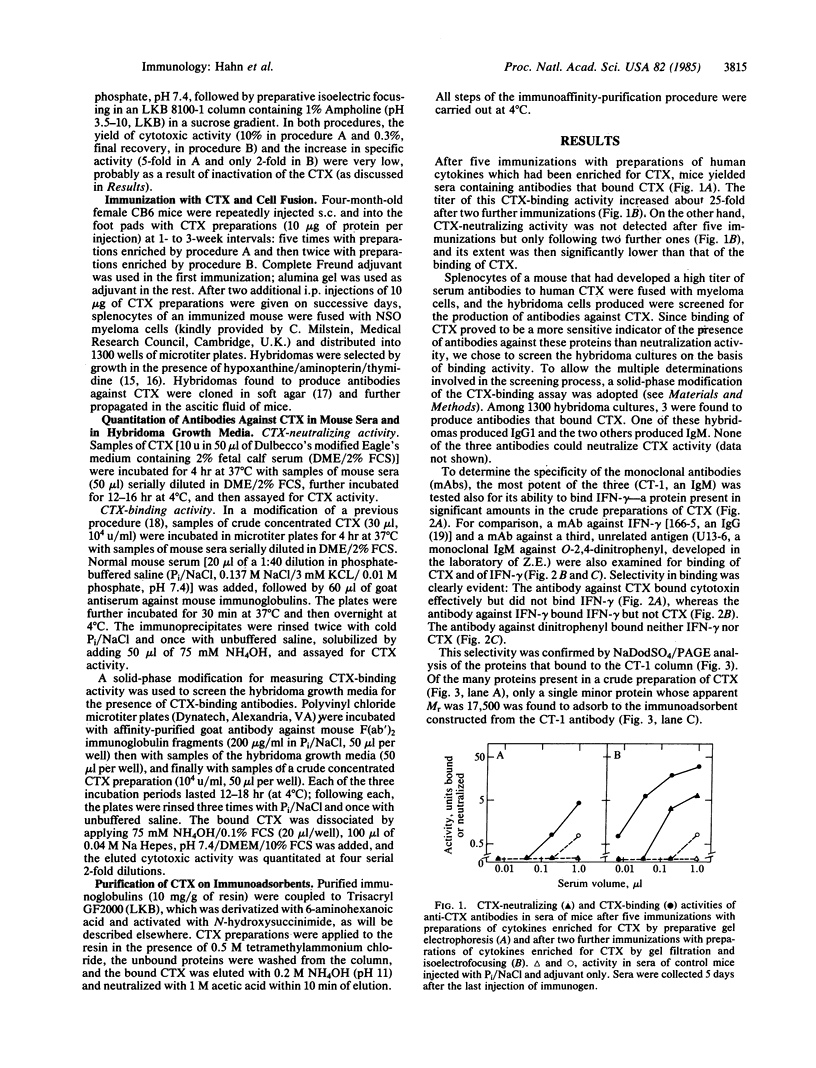
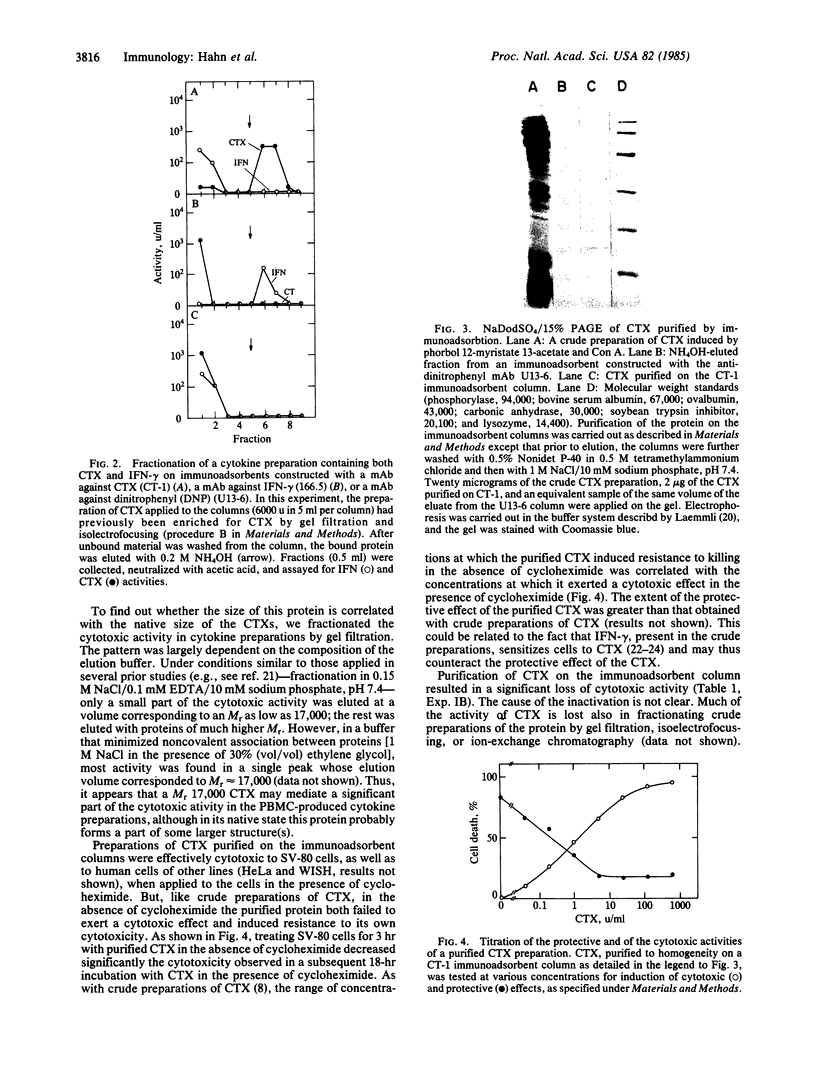
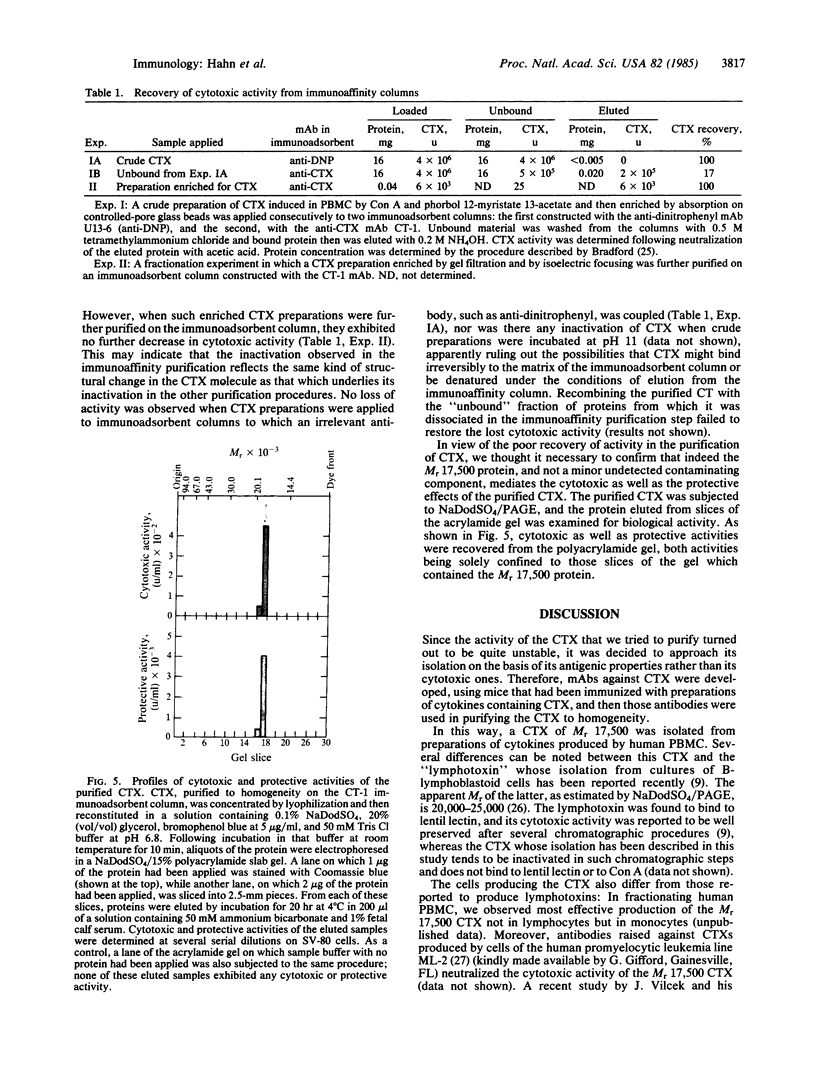
Crude preparations of cytotoxins (CTXs) produced by human peripheral blood mononuclear cells exert a marked cytotoxic effect when applied to cells in the presence of cycloheximide but in its absence can induce resistance to cytotoxicity. To examine the relationship between these cytotoxic and protective activities, we attempted to fully dissociate the CTX from the other proteins secreted by mononuclear cells. Mice injected with preparations of the cytokines secreted by peripheral blood mononuclear cells developed significant titers of serum antibodies to CTX(s). Splenocytes of such immunized mice were fused with NSO myeloma cells; a few among the resulting hybridoma cells secreted CTX-binding antibodies. Immunoadsorbents constructed with a monoclonal antibody produced by one of these hybridomas were used to purify to homogeneity a CTX (Mr approximately 17,500) from crude preparations of cytokines, by a single adsorption and elution cycle. Purified CTX was cytotoxic in the presence of cycloheximide but in its absence induced resistance to cytotoxicity; this resistance was manifested by decreased vulnerability to CTX in a subsequent incubation in the presence of cycloheximide. We conclude that CTX itself can induce certain changes in cells, which are reflected in resistance to its own cytotoxic effect.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggarwal B. B., Moffat B., Harkins R. N. Human lymphotoxin. Production by a lymphoblastoid cell line, purification, and initial characterization. J Biol Chem. 1984 Jan 10;259(1):686–691. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Eardley D. D., Shen F. W., Gershon R. K., Ruddle N. H. Lymphotoxin production by subsets of T cells. J Immunol. 1980 Mar;124(3):1199–1202. [PubMed] [Google Scholar]
- Eshhar Z., Ofarim M., Waks T. Generation of hybridomas secreting murine reaginic antibodies of anti-DNP specificity. J Immunol. 1980 Feb;124(2):775–780. [PubMed] [Google Scholar]
- Fair D. S., Jeffes E. W., 3rd, Granger G. A. Release of LT molecules with restricted physical heterogeneity by a continuous human lymphoid cell line in vitro. Mol Immunol. 1979 Mar;16(3):185–192. doi: 10.1016/0161-5890(79)90144-5. [DOI] [PubMed] [Google Scholar]
- Gifford G. E., Flick D. A., AbdAllah N. A., Fisch H. Production of a cytotoxin from phorbol myristate acetate-treated human promyelocytes. J Natl Cancer Inst. 1984 Jul;73(1):69–73. [PubMed] [Google Scholar]
- Granger G. A., Kolb W. P. Lymphocyte in vitro cytotoxicity: mechanisms of immune and non-immune small lymphocyte mediated target L cell destruction. J Immunol. 1968 Jul;101(1):111–120. [PubMed] [Google Scholar]
- Gray P. W., Aggarwal B. B., Benton C. V., Bringman T. S., Henzel W. J., Jarrett J. A., Leung D. W., Moffat B., Ng P., Svedersky L. P. Cloning and expression of cDNA for human lymphotoxin, a lymphokine with tumour necrosis activity. Nature. 1984 Dec 20;312(5996):721–724. doi: 10.1038/312721a0. [DOI] [PubMed] [Google Scholar]
- HAYFLICK L. The establishment of a line (WISH) of human amnion cells in continuous cultivation. Exp Cell Res. 1961 Feb;23:14–20. doi: 10.1016/0014-4827(61)90059-3. [DOI] [PubMed] [Google Scholar]
- Hiserodt J. C., Prieur A. M., Granger G. A. In vitro lymphocyte cytotoxicity. I. Evidence of multiple cytotoxic molecules secreted by mitogen activated human lymphoid cells in vitro. Cell Immunol. 1976 Jun 15;24(2):277–288. doi: 10.1016/0008-8749(76)90212-4. [DOI] [PubMed] [Google Scholar]
- Kull F. C., Jr, Cuatrecasas P. Necrosin: purification and properties of a cytotoxin derived from a murine macrophage-like cell line. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7932–7936. doi: 10.1073/pnas.81.24.7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewis J. E., Carmack C. E., Yamamoto R., Granger G. A. Antibodies against human lymphokines: I. Methods for induction of antibodies capable of neutralizing stable (alpha) and unstable (beta) lymphotoxins released in vitro by activated human lymphocytes. J Immunol Methods. 1977;14(2):163–176. doi: 10.1016/0022-1759(77)90006-0. [DOI] [PubMed] [Google Scholar]
- Novick D., Eshhar Z., Fischer D. G., Friedlander J., Rubinstein M. Monoclonal antibodies to human interferon-gamma: production, affinity purification and radioimmunoassay. EMBO J. 1983;2(9):1527–1530. doi: 10.1002/j.1460-2075.1983.tb01618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick D., Eshhar Z., Rubinstein M. Monoclonal antibodies to human alpha-interferon and their use for affinity chromatography. J Immunol. 1982 Nov;129(5):2244–2247. [PubMed] [Google Scholar]
- Pennica D., Nedwin G. E., Hayflick J. S., Seeburg P. H., Derynck R., Palladino M. A., Kohr W. J., Aggarwal B. B., Goeddel D. V. Human tumour necrosis factor: precursor structure, expression and homology to lymphotoxin. Nature. 1984 Dec 20;312(5996):724–729. doi: 10.1038/312724a0. [DOI] [PubMed] [Google Scholar]
- Pluznik D. H., Sachs L. The cloning of normal "mast" cells in tissue culture. J Cell Physiol. 1965 Dec;66(3):319–324. doi: 10.1002/jcp.1030660309. [DOI] [PubMed] [Google Scholar]
- Rosenau W., Goldberg M. L., Burke G. C. Early biochemical alterations induced by lymphotoxin in target cells. J Immunol. 1973 Oct;111(4):1128–1135. [PubMed] [Google Scholar]
- Rosenau W., Stites D., Jemtrud S. Elaboration of lymphotoxin by freshly isolated human T lymphocytes and continuous lymphoid-cell lines. Cell Immunol. 1979 Mar 15;43(2):235–244. doi: 10.1016/0008-8749(79)90169-2. [DOI] [PubMed] [Google Scholar]
- Ruddle N. H., Waksman B. H. Cytotoxicity mediated by soluble antigen and lymphocytes in delayed hypersensitivity. 3. Analysis of mechanism. J Exp Med. 1968 Dec 1;128(6):1267–1279. doi: 10.1084/jem.128.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone-Wolff D. S., Yip Y. K., Kelker H. C., Le J., Henriksen-Destefano D., Rubin B. Y., Rinderknecht E., Aggarwal B. B., Vilcek J. Interrelationships of human interferon-gamma with lymphotoxin and monocyte cytotoxin. J Exp Med. 1984 Mar 1;159(3):828–843. doi: 10.1084/jem.159.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G. J., Green H., Swift M. R. Susceptibility of human diploid fibroblast strains to transformation by SV40 virus. Science. 1966 Sep 9;153(3741):1252–1254. doi: 10.1126/science.153.3741.1252. [DOI] [PubMed] [Google Scholar]
- Walker S. M., Lucas Z. J. Cytotoxic activity of lymphocytes. IV. Preparation of heterologous antibody to human lymphotoxin. J Immunol. 1974 Sep;113(3):813–823. [PubMed] [Google Scholar]
- Wallach D., Hahn T., Budilovsky S. Translation of mRNA for human lymphotoxin in microinjected Xenopus oocytes. FEBS Lett. 1984 Dec 10;178(2):257–263. doi: 10.1016/0014-5793(84)80612-2. [DOI] [PubMed] [Google Scholar]
- Wallach D. Preparations of lymphotoxin induce resistance to their own cytotoxic effect. J Immunol. 1984 May;132(5):2464–2469. [PubMed] [Google Scholar]
- Williams T. W., Bellanti J. A. In vitro synergism between interferons and human lymphotoxin: enhancement of lymphotoxin-induced target cell killing. J Immunol. 1983 Feb;130(2):518–520. [PubMed] [Google Scholar]
- Williams T. W., Granger G. A. Lymphocyte in vitro cytotoxicity: mechanism of human lymphotoxin-induced target cell destruction. Cell Immunol. 1973 Feb;6(2):171–185. doi: 10.1016/0008-8749(73)90020-8. [DOI] [PubMed] [Google Scholar]
- Wright S. C., Bonavida B. Studies on the mechanism of natural killer (NK) cell-mediated cytotoxicity (CMC). I. Release of cytotoxic factors specific for NK-sensitive target cells (NKCF) during co-culture of NK effector cells with NK target cells. J Immunol. 1982 Jul;129(1):433–439. [PubMed] [Google Scholar]