Abstract
Introduction
The increasing use of computed tomography (CT) has led to more-frequent identification of asymptomatic lesions in the anterior mediastinum. The purpose of this study is to identify CT features that distinguish benign thymic lesions from early stage malignant thymic neoplasms.
Methods
We retrospectively reviewed preoperative CT imaging for 66 patients who had undergone thymectomy for benign thymic lesions or early stage malignant thymic neoplasms. All variables with a p-value <0.2 on univariate logistic regression analysis were evaluated by multivariate analysis. Stepwise selection was performed, and variables with a p-value <0.05 were retained in the final model.
Results
Thirty-eight malignant (58%) and 28 benign thymic lesions (42%) were included. Patients with benign thymic tumors were significantly younger (median age, 49.5 years) than patients with malignant tumors (60.0 years; p=0.007). Malignant tumors were larger in short-axis dimension (p=0.028) and more frequently in a non-midline location in the anterior mediastinum (p=0.029). Intralesional fat was seen exclusively in benign masses (p=0.002). Seven benign tumors (25%) and 1 malignant tumor (2.6%) had a triangular thymic shape (p=0.023). In multivariate analysis, lower age, smaller short-axis dimension, and lack of infiltration of the mediastinal fat were significant independent predictors of benign pathologic results.
Conclusion
Intralesional fat, midline location, and triangular thymic shape are more frequently found in benign thymic lesions. Lack of infiltration of the mediastinal fat, younger patient age, and smaller size are independent predictors of benign thymic lesions. These features may help characterize thymic masses as benign and avert potentially unnecessary invasive diagnostic procedures.
Keywords: Computed tomography, Mediastinal tumor, Thymus
Introduction
The normal thymus evolves over the course of a lifetime, with involution and gradual fatty replacement beginning around puberty. The thymus originates from 3 embryonic germ-cell layers and thus has the potential to transform along a number of neoplastic cell lines [1–3]. On computed tomography (CT), the normal thymus appears as a triangular-shaped structure in the anterior mediastinum. Variations in the morphology of the normal thymus gland, along with its association with a diverse range of pathologic processes, can make the thymus an imaging enigma and diagnostic challenge for clinicians [4, 5].
An abnormal appearance of the thymus can be attributable to either diffuse enlargement of the gland or a discrete mass. Benign thymic hyperplasia and lymphoma can both cause diffuse enlargement of the thymus. Many lesions—including thymoma, thymic carcinoma, and thymic carcinoids—as well as benign lesions—such as thymolipomas and cysts—can present with a focal thymic mass. This is often an incidental finding in an asymptomatic patient undergoing imaging for unrelated reasons. Clinical presentation can play a role in the evaluation of patients with thymic masses. One study found that more than 75% of asymptomatic patients with mediastinal masses had benign lesions, whereas almost two-thirds of symptomatic patients with mediastinal masses had malignant lesions [6]. It has been suggested that expectant management can be considered for observation of asymptomatic patients with diffuse thymic enlargement. For symptomatic patients, biopsy or, at times, resection may be appropriate [7].
At present, CT remains the imaging modality of choice for the evaluation of mediastinal masses. Magnetic resonance imaging and positron emission tomography (PET) are useful adjuncts [8]. While there is overlap in the features of many thymic lesions on imaging, some lesions have a characteristic appearance on CT. Thymolipoma, an uncommon benign thymic neoplasm, typically manifests on CT as a large anterior mediastinal mass containing fat intermingled with areas of soft-tissue attenuation, which may conform to the shape of other mediastinal structures [9, 10]. On CT, idiopathic multilocular thymic cyst, an acquired benign thymic lesion, cannot be reliably distinguished from the cystic components of malignant thymic lesions, including thymomas, Hodgkin lymphoma, and mediastinal germ-cell tumors [11]. The appearances of thymomas on imaging can vary according to their staging and histologic subtype; lobulated contours, calcifications, and heterogeneous attenuation are associated with more-advanced, rather than early stage, thymoma [12].
The increasing use of CT has led to more-frequent identification of incidental lesions in the anterior mediastinum, and with recent advances in minimally invasive surgical techniques, an increasing number of thymic lesions are referred for surgical evaluation. Consequently, some patients may undergo invasive procedures for ultimately benign disease. If the benignity of a thymic lesion could be determined on imaging with a reasonable level of confidence, then the need for invasive procedures may potentially be obviated for a number of cases [7]. The purpose of this study is to identify imaging features that help distinguish benign thymic lesions from early stage malignant thymic neoplasms.
Materials and Methods
Patient Selection
Our institutional review board granted approval and waived the informed consent requirement for this retrospective study. Patients were identified from a prospectively maintained database of surgical procedures. Patients who underwent complete thymic resections for benign lesions or early stage thymic malignant neoplasms at our institution between January 2007 and September 2010 were included. Early stage thymic malignancy was defined as stage I or II by the Masaoka-Koga classification system [13], and patients who received neoadjuvant therapy were excluded. We excluded advanced stage thymic malignancies, as these often demonstrate more-aggressive features on CT, such as vascular invasion, making them easier to distinguish from benign processes. Clinical information was abstracted retrospectively from the medical record.
Imaging Analysis
All preoperative CT scans were retrospectively reviewed in a consensus fashion by 2 radiologists (M.S.G. and A.M.) with a combined 16 years of experience post–radiology fellowship training. Radiologists were blinded to the pathologic diagnoses and clinical details of the patients at the time of interpretation of the scans. All images were reviewed on a picture archiving and communication system (GE PACS; Waukesha, WI). CT imaging protocols varied, as many patients were referred from a range of institutions to our tertiary referral center following their initial imaging. Imaging was performed on a variety of multidetector CT scanners, with slice thicknesses ranging from 1.25 to 5 mm. Forty-two patients (64%) received intravenous contrast.
Imaging findings were assessed in accordance with the standard CT reporting terms for mediastinal masses suspicious for thymic malignancy, as defined by the International Thymic Malignancy Interest Group.[14]. The features on CT included size, location, contour, shape, and attenuation. We defined lesion location within the mediastinum as “anterior mediastinal” if the lesion was located posterior to the sternum and anterior to the heart, from the left brachiocephalic vein to the diaphragm, between the right and left parietal pleura. The location was further subdivided into “right,” “left,” or “midline,” according to its position relative to a line drawn between the midsternum and the trachea.
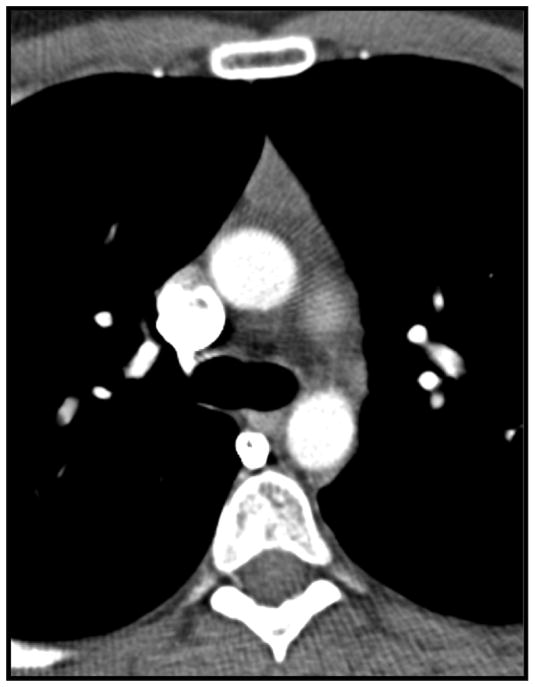
The longest lesion dimension on axial imaging and its perpendicular short-axis measurement on the same slice were obtained. The lesion contour was defined as “smooth” in the absence of spiculation, ill-defined border, or lobulation, and as “lobulated” in the presence of 1 or more lobulations, which are characterized as convex tumor contours with adjacent notches between tumor lobules. The shape was classified as “round” if the ratio between long-axis and short-axis was <1.5, as “oval” if the ratio was ≥1.5, and as “thymic shape” if the lesion had a triangular configuration (Figure 1). Attenuation was assessed by visual inspection and categorized as “homogeneous” if the lesion was of uniform attenuation and as “heterogeneous” if there were areas of low attenuation within the lesion. The presence of macroscopic intralesional fat, calcifications, and cystic components was assessed (Figures 2–5). Lesions demonstrating homogeneous water attenuation were considered “cystic.” Calcifications were described as “curvilinear,” “punctate,” or “coarse.” Clear infiltration of the mediastinal fat by the lesion was noted (Figure 6); however, the absence of a visible fat plant between abutting surfaces was not considered to indicate infiltration.
Figure 1.
Triangular thymic shaped anterior mediastinal mass on computed tomography in a 31-year-old woman. The mass was histologically confirmed to be benign thymic tissue following resection.
Figure 2.
Lymphangioma in a 35-year-old woman. Cystic well-defined left sided thymic mass on contrast enhanced CT(13 HU).
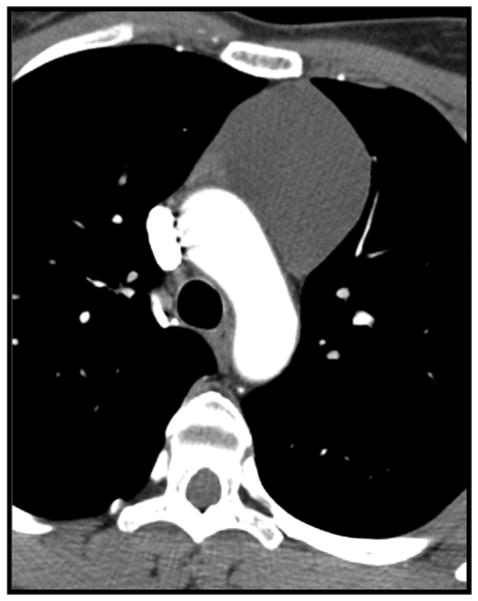
Figure 5.
37-year-old man with a heterogeneous anterior mediastinal mass containing fat (arrowheads) intermingled with areas of soft-tissue attenuation; confirmed as a thymolipoma on histological analysis following resection.
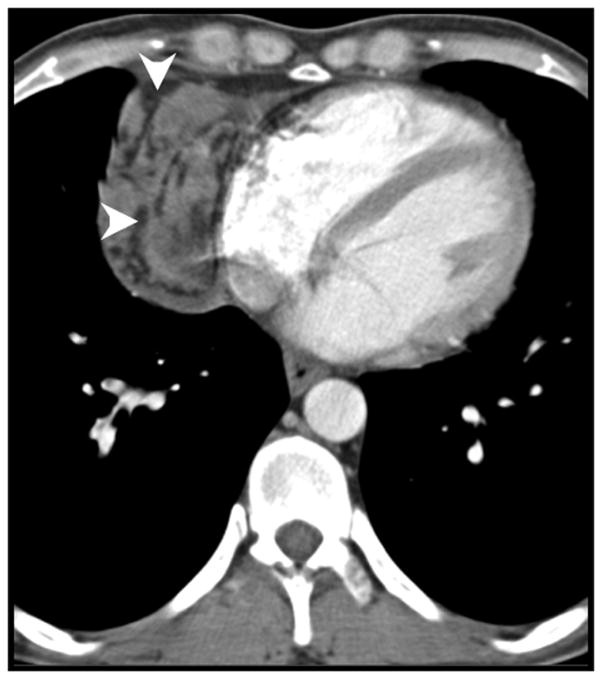
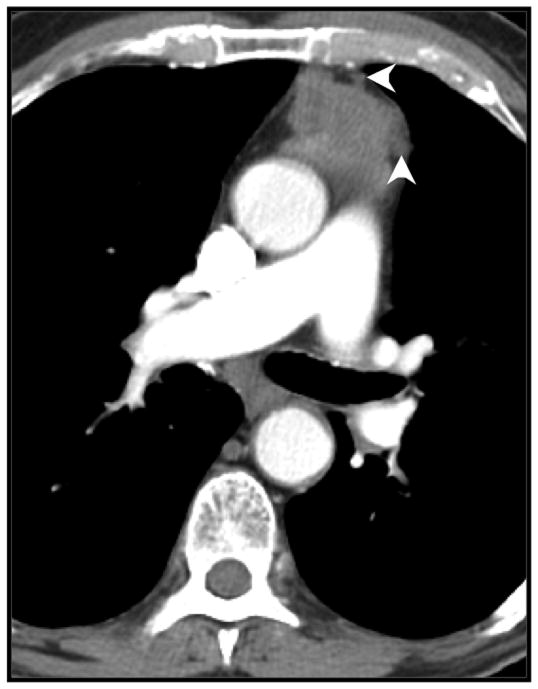
Figure 6.
Mediastinal fat invasion (arrowheads), confirmed by pathologic analysis, in an 87-year-old man with thymoma.
Statistical Analysis
Logistic regression and Fisher’s exact test were used for univariate analysis. Variables with a p value <0.2 on univariate analysis were evaluated by multivariate analysis. A multivariate logistic regression model was constructed using stepwise selection in which only variables with an overall p value <0.05 were retained in the final model. p < 0.05 was considered to indicate statistical significance. All analyses were conducted using SAS version 9.2 (SAS Institute, Cary, NC).
Results
The study group consisted of 66 patients (32 men and 34 women; age range, 22 to 88 years; median age, 57 years). Twenty-one patients (32%) had a history of nonthymic malignancy. Thirty-one patients (47%) reported chest-related symptoms (pain, shortness of breath, cough, and symptoms warranting cardiac evaluation), which prompted their initial imaging assessment. Two patients underwent CT imaging for evaluation of symptomatic myasthenia gravis; both had thymomas on pathologic analysis. For the remaining 33 patients (50%), anterior mediastinal masses were incidentally detected on imaging performed for a variety of clinical indications (Table 1).
Table 1.
Indication or Symptoms Precipitating Initial Imaging
| Indication/Symptom(s) | Overall (N = 66) | Benign (N = 28) | Malignant (N = 38) |
|---|---|---|---|
| Respiratory symptoms | 15 (22.7%) | 5 | 10 |
| Chest pain | 13 (19.7%) | 8 | 5 |
| Staging of nonthymic cancer | 9 (13.6%) | 5 | 4 |
| Nonrespiratory indicationsa | 9 (13.6%) | 3 | 6 |
| Routine imagingb | 9 (13.6%) | 2 | 7 |
| Cardiovascular imaging | 4 (6.1%) | 1 | 3 |
| Unknown reason | 3 (4.5%) | 2 | 1 |
| Evaluation of pleural effusions/retrosternal goiter | 2 (3.0%) | 2 | 0 |
| Myasthenia gravis | 2 (3.0%) | 0 | 2 |
Including trauma, positive tuberculin skin test, anemia, renal colic, hematuria, back pain, and high white cell count.
For example, preoperative chest radiograph.
Of the 66 patients in the study group, 38 (58%) had malignant thymic lesions, and 28 (42%) had benign lesions. The malignant lesions included thymoma, thymic carcinoma, thymic carcinoid, and thymic lymphoma, whereas the benign group was more heterogeneous and included a wide spectrum of pathologies (Table 2). Patients with malignant thymic tumors were significantly older (p = 0.007), with a median age of 60 years (range, 34 to 88 years), compared with 49.5 years (range, 22 to 77 years) in the benign group. All lesions were completely resected (Table 3). An open surgical approach was used in 40 patients (61%), which included 13 patients with benign lesions (46% of the benign study group) and 27 patients with malignant lesions (71% of the malignant study group). Of the remaining 26 patients (39%), 12 (43%) of those with benign lesions and 8 (21%) of those with malignant lesions underwent robotically assisted minimally invasive resection. The remaining 6 patients (9%) underwent standard thoracoscopic resection. The most common reason for surgery was to rule out malignancy (97%); the remaining 2 patients (3%) underwent surgery for symptomatic myasthenia gravis.
Table 2.
Pathologic Diagnosis
| Pathologic Diagnosis | No. (%) |
|---|---|
| Benign | |
| Thymic cyst | 10 (15.2) |
| Thymic hyperplasia | 6 (9.1) |
| Benign thymus | 3 (4.5) |
| Thymolipoma | 3 (4.5) |
| Benign nodular thyroid hyperplasia | 1 (1.5) |
| Bronchogenic cyst | 1 (1.5) |
| Cystic lymphoid thymic hyperplasia | 1 (1.5) |
| Fibrous tumor | 1 (1.5) |
| Lymphangioma | 1 (1.5) |
| Thymic fibrosis | 1 (1.5) |
| Malignant | |
| Thymoma | 32 (48.5) |
| Thymic carcinoma | 3 (4.5) |
| Thymic carcinoid | 2 (3) |
| Thymic lymphoma | 1 (1.5) |
Table 3.
Surgical Approach
| Surgical Approach | Overall (N = 66) | Benign (N = 28) | Malignant (N = 38) |
|---|---|---|---|
| VATS | 26 (39.4) | 15 (53.6) | 11 (28.9) |
| Standard | 6 (9.1) | 3 (10.7) | 3 (7.9) |
| Robotically assisted | 20 (30.3) | 12 (42.8) | 8 (21.0) |
| Open | 40 (60.6) | 13 (46.4) | 27 (71.1) |
Data are no. (%). VATS = video assisted thoracoscopic surgery
The results of the univariate analysis of CT findings, for all thymic lesions, are summarized in Table 4. Malignant tumors were larger, with a statistically significant difference in short-axis dimension (p = 0.028): for malignant and benign thymic tumors, median short-axis dimensions were 31 mm (range, 12 to 89 mm) and 19.5 mm (range, 10 to 56 mm), respectively. Anterior mediastinal location differed significantly (p = 0.029): benign lesions were more frequently located in the midline and less frequently located to the left or the right of the midline, compared with malignant lesions (Figures 7 and 8).
Table 4.
Univariate Analysis
| Variable | Overall (N = 66) | Benign (N = 28) | Malignant (N = 38) | pa |
|---|---|---|---|---|
| Age (years)b | 57 (22–88) | 49.5 (22–77) | 60 (34–88) | 0.007 |
| Sex | 0.433 | |||
| Male | 32 (48.5) | 12 (42.9) | 20 (52.6) | |
| Female | 34 (51.5) | 16 (57.1) | 18 (47.4) | |
| Cancer history | 0.961 | |||
| Yes | 21 (31.8) | 9 (32.1) | 12 (31.6) | |
| No | 45 (68.2) | 19 (67.9) | 26 (68.4) | |
| Size (mm)b | ||||
| Long-axis | 38 (12–144) | 33.5 (12–74) | 40.5 (15–144) | 0.226 |
| Short-axis | 25 (10–89) | 19.5 (10–56) | 31.0 (12–89) | 0.028 |
| Location | 0.029 | |||
| Left | 33 (50.0) | 12 (42.9) | 21 (55.3) | |
| Right | 18 (27.3) | 5 (17.9) | 13 (34.2) | |
| Midline | 15 (22.7) | 11 (39.3) | 4 (10.5) | |
| Contour | 0.083 | |||
| Smooth | 44 (66.7) | 22 (78.6) | 22 (57.9) | |
| Lobulated | 22 (33.3) | 6 (21.4) | 16 (42.1) | |
| Shape | 0.023 | |||
| Round or ovoid | 58 (87.9) | 21 (75.0) | 37 (97.4) | |
| Thymic shape | 8 (12.1) | 7 (25.0) | 1 (2.6) | |
| Attenuation | 0.892 | |||
| Homogeneous | 36 (54.5) | 15 (53.6) | 21 (55.3) | |
| Heterogeneous | 30 (45.5) | 13 (46.4) | 17 (44.7) | |
| Intralesional fat | 0.002c | |||
| Yes | 7 (10.6) | 7 (25.0) | 0 (0.0) | |
| No | 59 (89.4) | 21 (75.0) | 38 (100.0) | |
| Calcification | 0.417 | |||
| Yes | 5 (7.6) | 3 (10.7) | 2 (5.3) | |
| No | 61 (92.4) | 25 (89.3) | 36 (94.7) | |
| Mediastinal fat infiltration | 0.051 | |||
| Yes | 10 (15.2) | 1 (3.6) | 9 (23.7) | |
| No | 56 (84.8) | 27 (96.4) | 29 (76.3) | |
Data are no. (%), unless otherwise noted.
From univariate logistic regression analysis evaluating the association between each patient and lesion feature and lesion status as benign versus malignant, unless otherwise noted.
Median (range).
Fisher’s exact test.
Figure 7.
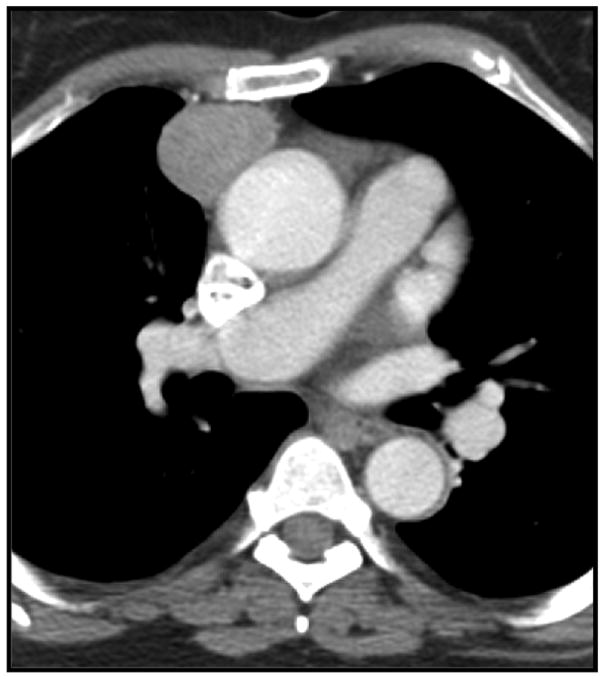
Thymoma in a 63-year-old woman. Contrast-enhanced CT shows a round, right-sided enhancing anterior mediastinal mass.
Figure 8.
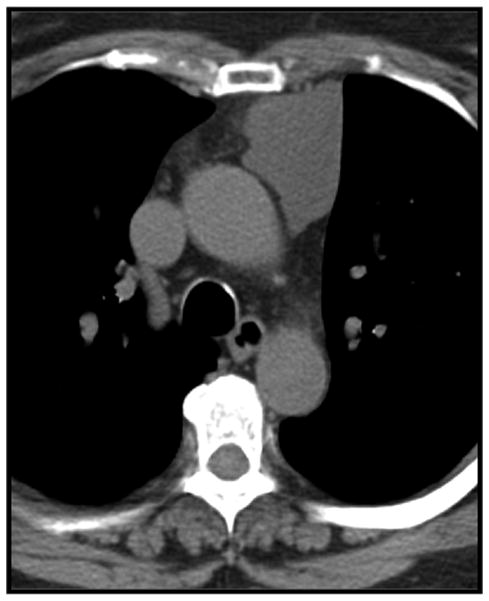
Thymic carcinoma in a 45-year-old man. Contrast enhanced CT shows an ovoid heterogeneous left-sided anterior mediastinal mass.
There were significant differences in tumor shape (p = 0.023): 7 benign thymic tumors (25%) had a thymic shape, compared with 1 malignant tumor (3%), which was shown to be a thymoma on final histologic analysis. Macroscopic intralesional fat was seen in 7 benign tumors (25%) and in 0 malignant tumors (p = 0.002). The majority of tumors did not have calcifications (p = 0.417) and more often had a smooth (n = 44; 67%) rather than a lobulated (n = 22; 33%) contour (p = 0.083). Lesion attenuation (p = 0.892) was not significantly different between benign and malignant lesions. Four benign lesions (14%) and 1 malignant lesion (3%) were cystic. Infiltration of the mediastinal fat was visualized on CT in 10 patients: 1 (4%) had a benign tumor with pathologic results demonstrating thymic hyperplasia, and 9 (24%) had malignant tumors, 4 of which had mediastinal fat invasion on histologic analysis (p = 0.051).
In multivariate analysis, patient age, short-axis dimension, and lack of infiltration of the mediastinal fat remained significantly associated with benign pathologic results (Table 5). As none of the malignant tumors had macroscopic intralesional fat on CT, this feature could not be analyzed in the multivariate model. For each year of increase in age, the odds of having a benign lesion decreased by 7% (odds ratio [OR], 0.93; 95% confidence interval [CI], 0.89 to 0.98) (p = 0.003). With regard to size, for each millimeter increase in short-axis dimension, the odds of the lesion being benign decreased by 5% (OR, 0.95; 95% CI, 0.91 to 1.00) (p = 0.040). When mediastinal fat infiltration was present, lesions were 92% less likely to be benign, compared with when mediastinal fat infiltration was absent (OR, 0.08; 95% CI, 0.001 to 0.85) (p = 0.036).
Table 5.
Multivariate Logistic Regression Model for Association with Benign Thymic Lesions
| Variable | OR (95% CI) | p |
|---|---|---|
| Age | 0.93 (0.89–0.98) | 0.003 |
| Short-axis dimension | 0.95 (0.91–1.00) | 0.040 |
| Infiltration of mediastinal fat | 0.08 (0.01–0.85) | 0.036 |
CI = confidence interval; OR = odds ratio
Discussion
Thymic tumors are relatively uncommon, and a diverse spectrum of pathologic processes can affect the thymus, as reflected in our study. Because of its location, lesions in the thymus are not always readily accessible by percutaneous biopsy. Many patients may undergo invasive procedures for ultimately benign disease. Twenty-eight patients in our study underwent resection and 13 underwent sternotomy for ultimately benign disease. Our aim was to identify features on CT imaging that would assist in distinguishing benign thymic masses from early stage thymic malignances.
Our findings concur with those of previous studies, which found that malignant thymic tumors occur in an older patient population. In a European study, the median age at diagnosis was 59 years; in the United States, the mean age was 56 years [1, 15, 16]. From a demographic standpoint, we found that indeterminate thymic lesions in younger patients (<43 years) tended more often to be benign.
Symptomatology has been described as a useful predictor of malignancy in patients with mediastinal masses. In one study, up to two-thirds of symptomatic patients had a malignancy [6]. Singla et al. found that the presence of thoracic symptoms, such as chest discomfort or shortness of breath, was a useful discriminator in patients with a diffusely enlarged thymus gland on CT. Of 36 patients with thymic hyperplasia, no asymptomatic patient had malignant findings on resection, leading the authors to conclude that follow-up with serial imaging, rather than biopsy, may be appropriate [7]. With the exception of myasthenia, there are no specific symptoms that can be definitively attributed to the thymus gland. In our study, 50% of patients had either symptoms anatomically attributable to the chest or myasthenia; of these, 58% (19 patients) had malignant lesions, compared with 42% (14 patients) who had benign lesions. Therefore, in our experience, clinical presentation would not help in the diagnostic algorithm.
Although most of the thymic lesions in our study were located to the right or left of the midline, 39% of benign masses, compared with only 10% of malignant tumors, were midline, although this association did not retain statistical significance in multivariate analysis. This finding is somewhat comparable to those of studies of thymic epithelial tumors, which found that <25% of lesions were in the midline [17, 18]. We found that malignant tumors were significantly larger in short-axis dimension than benign tumors. In a small study by Travaini et al., all benign lesions (8 of 8 masses) were <5 cm in long-axis dimension, compared with only 3 of 12 malignant lesions [19]. This pattern is also seen in the spectrum of thymic epithelial tumors. Thymic carcinomas, which exhibit more-aggressive behavior and a poorer outcome, compared with thymomas, have been shown to be significantly larger [17].
Identification of thymic mass morphology may be helpful. In our study group, in univariate analysis, lesions that retained a triangular thymic shape were significantly more likely to be benign, compared with those with a round or oval shape. Jeong et al. found that thymic epithelial tumors with an oval shape had a worse prognosis [18]. In our series, the presence of macroscopic intralesional fat was seen exclusively in benign tumors; however, we had no cases of teratoma or malignant germ-cell tumor, which can have macroscopic fat visible on CT [20], and we were not able to examine this feature in multivariate analysis. In 97% of our patients, malignancy could not be ruled out by CT alone, prompting additional diagnostic procedures. This finding indicates that our ability to discern a benign lesion from a malignant one leaves room for improvement.
Evidence of infiltration of the mediastinal fat on CT is associated with more-aggressive malignant tumors of the thymus. Jeong et al. observed mediastinal fat invasion more commonly in thymic carcinomas (5 of 15) than in low-grade thymomas (1 of 31) (p = 0.033) [18]. Jung et al. had similar findings [17]. In our study, we identified infiltration of the mediastinal fat on CT in 1 benign lesion (which was determined to be thymic hyperplasia on histologic analysis) and in 9 malignant lesions, 4 of which had confirmed mediastinal fat invasion on histologic analysis, which concurs with published results. Infiltration of the mediastinal fat remained a strong independent predictor in multivariate analysis.
The small number of patients in our cohort is a limitation of our study, and additional confirmation of our findings by use of a larger study group would certainly be valuable. However, thymic tumors are uncommon. In one population study, the incidence of thymomas was reported as 3.2 per 1 million people [15]. In addition, CT imaging did not follow a standardized protocol, which was inevitable, since many patients were referred to our tertiary referral oncology center following initial evaluation at other institutions. However, to our knowledge, our study is the largest to compare the imaging features of benign and malignant thymic lesions. Travaini et al. evaluated the role of F-18-fluorodeoxyglucose PET CT to differentiate benign and malignant thymic masses but was limited by a small sample size of 20 patients [19]. In a study of 109 thymic masses, Singla et al. recommend that it is reasonable to observe asymptomatic patients with diffusely enlarged thymic glands by use of serial CT imaging; however, they did not address the imaging characterization of discrete thymic masses seen on CT [7].
In conclusion, there are features that can be helpful in determining a benign etiology for a thymic mass. Intralesional fat, midline location, and triangular thymic shape are more frequently found in benign thymic lesions. Infiltration of the mediastinal fat, particularly in the setting of older age or a large mass, may increase the likelihood of a malignant lesion.
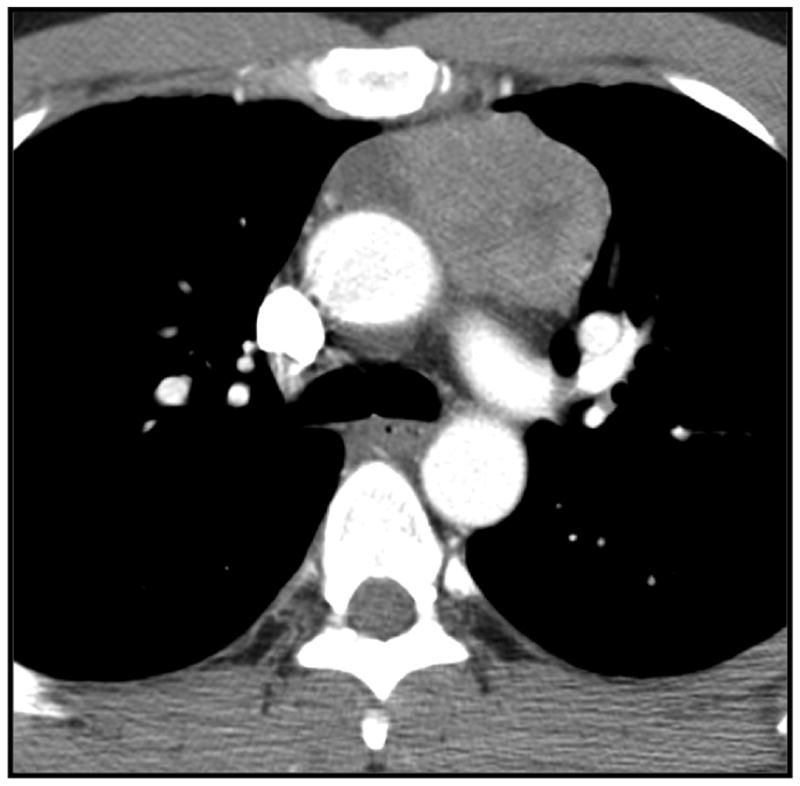
Figure 3.
Simple thymic cyst in a 62-year-old woman. Non contrast CT image shows a low attenuation (10 HU) cystic left sided anterior mediastinal mass.
Figure 4.
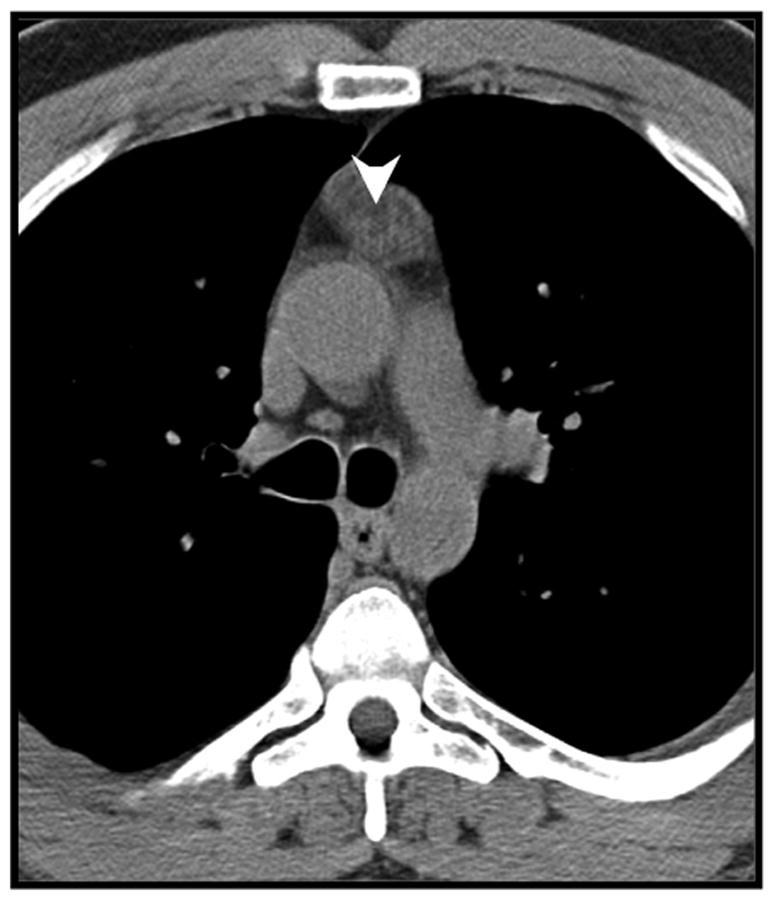
Example of macroscopic intralesional fat (arrowheads) in pathologically confirmed true thymic hyperplasia in a 48-year-old man.
Acknowledgments
Funding: None
Footnotes
Disclosures: None
References
- 1.Strollo DC, Rosado-de-Christenson ML. Tumors of the thymus. J Thorac Imaging. 1999;14:152–171. doi: 10.1097/00005382-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Suster S, Rosai J. Histology of the normal thymus. Am J Surg Pathol. 1990;14:284–303. doi: 10.1097/00000478-199003000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Suster S, Moran CA. Thymic carcinoma: spectrum of differentiation and histologic types. Pathology. 1998;30:111–122. doi: 10.1080/00313029800169056. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs MT, Frush DP, Donnelly LF. The right place at the wrong time: historical perspective of the relation of the thymus gland and pediatric radiology. Radiology. 1999;210:11–16. doi: 10.1148/radiology.210.1.r99ja4511. [DOI] [PubMed] [Google Scholar]
- 5.Nicolaou S, Muller NL, Li DK, et al. Thymus in myasthenia gravis: comparison of CT and pathologic findings and clinical outcome after thymectomy. Radiology. 1996;201:471–474. doi: 10.1148/radiology.201.2.8888243. [DOI] [PubMed] [Google Scholar]
- 6.Davis RD, Jr, Oldham HN, Jr, Sabiston DC., Jr Primary cysts and neoplasms of the mediastinum: recent changes in clinical presentation, methods of diagnosis, management, and results. Ann Thorac Surg. 1987;44:229–237. doi: 10.1016/s0003-4975(10)62059-0. [DOI] [PubMed] [Google Scholar]
- 7.Singla S, Litzky LA, Kaiser LR, et al. Should asymptomatic enlarged thymus glands be resected? J Thorac Cardiovasc Surg. 2010;140:977–983. doi: 10.1016/j.jtcvs.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Marom EM. Imaging thymoma. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2010;5:S296–303. doi: 10.1097/JTO.0b013e3181f209ca. [DOI] [PubMed] [Google Scholar]
- 9.Rosado-de-Christenson ML, Pugatch RD, Moran CA, et al. Thymolipoma: analysis of 27 cases. Radiology. 1994;193:121–126. doi: 10.1148/radiology.193.1.8090879. [DOI] [PubMed] [Google Scholar]
- 10.Gamanagatti S, Sharma R, Hatimota P, et al. Giant thymolipoma. AJR Am J Roentgenol. 2005;185:283–284. doi: 10.2214/ajr.185.1.01850283a. [DOI] [PubMed] [Google Scholar]
- 11.Choi YW, McAdams HP, Jeon SC, et al. Idiopathic multilocular thymic cyst: CT features with clinical and histopathologic correlation. AJR Am J Roentgenol. 2001;177:881–885. doi: 10.2214/ajr.177.4.1770881. [DOI] [PubMed] [Google Scholar]
- 12.Tomiyama N, Muller NL, Ellis SJ, et al. Invasive and noninvasive thymoma: distinctive CT features. J Comput Assist Tomogr. 2001;25:388–393. doi: 10.1097/00004728-200105000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Detterbeck FC, Nicholson AG, Kondo K, et al. The Masaoka-Koga stage classification for thymic malignancies: clarification and definition of terms. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2011;6:S1710–1716. doi: 10.1097/JTO.0b013e31821e8cff. [DOI] [PubMed] [Google Scholar]
- 14.Marom EM, Rosado-de-Christenson ML, Bruzzi JF, et al. Standard report terms for chest computed tomography reports of anterior mediastinal masses suspicious for thymoma. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2011;6:S1717–1723. doi: 10.1097/JTO.0b013e31821e8cd6. [DOI] [PubMed] [Google Scholar]
- 15.de Jong WK, Blaauwgeers JL, Schaapveld M, et al. Thymic epithelial tumours: a population-based study of the incidence, diagnostic procedures and therapy. Eur J Cancer. 2008;44:123–130. doi: 10.1016/j.ejca.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Engels EA, Pfeiffer RM. Malignant thymoma in the United States: demographic patterns in incidence and associations with subsequent malignancies. Int J Cancer. 2003;105:546–551. doi: 10.1002/ijc.11099. [DOI] [PubMed] [Google Scholar]
- 17.Jung KJ, Lee KS, Han J, et al. Malignant thymic epithelial tumors: CT-pathologic correlation. AJR Am J Roentgenol. 2001;176:433–439. doi: 10.2214/ajr.176.2.1760433. [DOI] [PubMed] [Google Scholar]
- 18.Jeong YJ, Lee KS, Kim J, et al. Does CT of thymic epithelial tumors enable us to differentiate histologic subtypes and predict prognosis? AJR Am J Roentgenol. 2004;183:283–289. doi: 10.2214/ajr.183.2.1830283. [DOI] [PubMed] [Google Scholar]
- 19.Travaini LL, Petralia G, Trifiro G, et al. [18F]FDG positron emission tomography/computed tomography and multidetector computed tomography roles in thymic lesion treatment planning. Lung Cancer. 2008;61:362–368. doi: 10.1016/j.lungcan.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 20.Rosado-de-Christenson ML, Templeton PA, Moran CA. From the archives of the AFIP. Mediastinal germ cell tumors: radiologic and pathologic correlation. Radiographics. 1992;12:1013–1030. doi: 10.1148/radiographics.12.5.1326777. [DOI] [PubMed] [Google Scholar]