Abstract
Oncolytic vaccinia virus (VV) therapy has shown promise in preclinical models and in clinical studies. However, complete responses have rarely been observed. This lack of efficacy is most likely due to suboptimal virus spread through the tumor resulting in limited tumor cell destruction. We reasoned that redirecting T cells to the tumor has the potential to improve the antitumor activity of oncolytic VVs. We, therefore, constructed a VV encoding a secretory bispecific T-cell engager consisting of two single- chain variable fragments specific for CD3 and the tumor cell surface antigen EphA2 (EphA2-T-cell engager-armed VV (EphA2-TEA-VV)). In vitro, EphA2-TEA-VV's ability to replicate and induce oncolysis was similar to that of unmodified virus. However, only tumor cells infected with EphA2-TEA-VV induced T-cell activation as judged by the secretion of interferon-γ and interleukin-2. In coculture assays, EphA2-TEA-VV not only killed infected tumor cells, but in the presence of T cells, it also induced bystander killing of noninfected tumor cells. In vivo, EphA2-TEA-VV plus T cells had potent antitumor activity in comparison with control VV plus T cells in a lung cancer xenograft model. Thus, arming oncolytic VVs with T-cell engagers may represent a promising approach to improve oncolytic virus therapy.
Introduction
Oncolytic vaccinia viruses (VVs) are promising anticancer agents owing to their ability to infect, replicate in, and lyse tumor cells and spread to other tumor cells in successive rounds of replication.1,2 Oncolytic VVs' major mode of action is the destruction of tumor cells, which may then induce antigen-specific T-cell responses against the tumor thereby targeting metastatic disease even after local injection of virus. Oncolytic VV therapy has shown promise in preclinical models and in clinical studies. Complete clinical responses have, however, been rarely observed.3,4,5,6,7,8,9,10 This is most likely due to the limited virus spread through the tumor and limited activation of antitumor T-cell responses by oncolytic VVs.1,2,3,4,5,6,7,8,9,10
T cells play a critical role in controlling tumor growth. There is increasing evidence that T cells are able to control tumor growth and survival in cancer patients, both in early and late stages of the disease. For example, adoptive transfer of T cells has been demonstrated to effectively treat disseminated tumors, including Hodgkin's lymphoma, nasopharyngeal carcinoma, neuroblastoma, and melanoma.11,12,13,14,15 However, tumor-specific T-cell responses are difficult to induce and sustain in cancer patients and are limited by numerous immune escape mechanisms of tumor cells selected during immunoediting.16,17 T-cell engager (CD3-scFv, single- chain variable fragment) provides an alternative approach to engage T cells for cancer therapy. CD3-scFv has been used in bispecific T-cell engagers (BiTEs) that consist of a CD3-scFv and a scFv specific for a tumor cell surface antigen.18,19,20,21,22,23,24,25,26 Clinical studies have shown that infusion of CD19-specific BiTEs results in highly effective killing of tumor cells in patients with non-Hodgkin's lymphomas and B-cell precursor acute lymphoblastic leukemia.24,25,26
In this article, we report the development of a T-cell engager-armed oncolytic VV (TEA-VV) encoding secretory bispecific T-cell engagers (TEs) that bind both to human CD3 and a tumor cell surface antigen EphA2 (EphA2-TEA-VV). Upon infection of EphA2-positive tumor cells, EphA2-TEA-VVs activated T cells and induced T-cell killing of viral infected and noninfected tumor cells, indicative of “bystander killing”. In vivo, EphA2-TEA-VV plus T cells had greater antitumor activity than that of unarmed VV plus T cells in a lung cancer xenograft model.
Results
TEs induce killing of tumor cells
To investigate the ability of TEs to induce bystander killing of tumor cells, we first generated recombinant lentiviral vectors expressing a secretory bispecific EphA2-specific TEs (EphA2-TE; Lv-EphA2-TE), a membrane-bound CD3-specific TE (Lv-mTE), or GFP (Lv-GFP) (Supplementary Figure S1A). The EphA2-positive lung cancer cell line A549 was transduced with Lv-EphA2-TE, Lv-mTE, or Lv-GFP. Virus-infected A549 (GFP+) cells were mixed with noninfected A549 (GFP−) cells and cocultured with nonstimulated peripheral blood mononuclear cells (PBMCs). After 48 hours, the presence of tumor cells was determined using immunofluorescence and fluorescence-activated cell sorting (FACS) analysis (Supplementary Figure S1B–D). Flow analysis demonstrated that Lv-EphA2-TE induced killing of both virus-infected A549 (GFP+) and noninfected A549 (GFP−) cells, whereas Lv-mTE only induced killing of virus-infected A549 (GFP+) cells. These results indicate that the lentivirus encoding the secretable EphA2-TE induced bystander killing of noninfected tumor cells.
Generation of EphA2-TEA-VV and GFP-VV
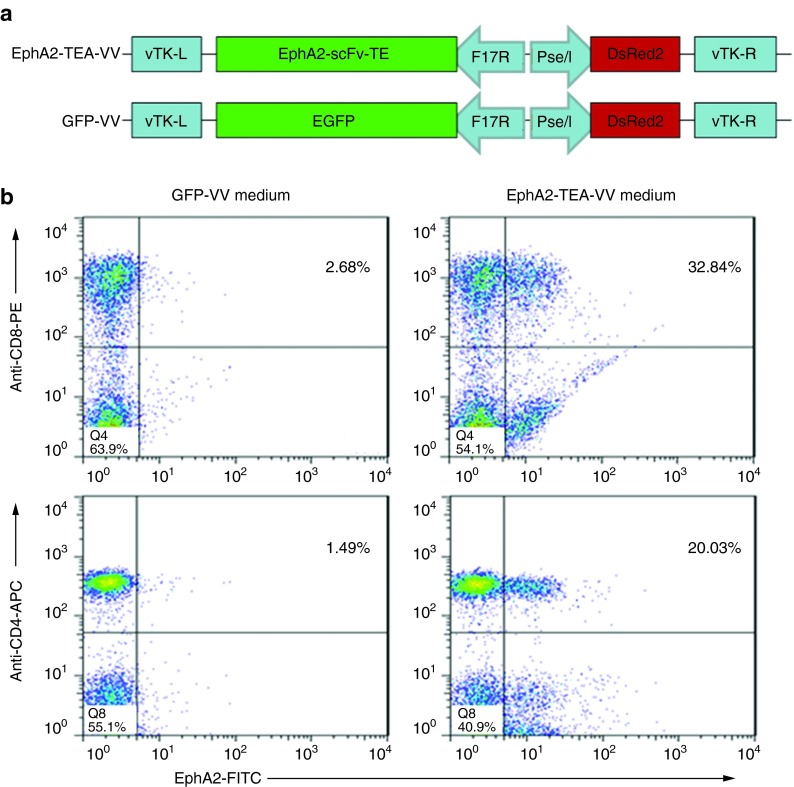
We generated double-deleted VVs (vvDD, Western Reserve strain) expressing EphA2-scFv-CD3-scFv (EphA2-TEA-VV) or GFP (GFP-VV) (Figure 1a). To investigate whether EphA2-TEA-VV–infected tumor cells express secretory bispecific EphA2-TE, A549 cells were transduced with EphA2-TEA-VV or GFP-VV at a multiplicity of infection (MOI) of 5. After 24 hours, media were collected and mixed with unstimulated human PBMCs followed by FACS analysis using EphA2-FITC (EphA2 protein labeled with fluorescein isothiocyanate) as a probe to detect CD3-bound EphA2-TE. CD4- and CD8-positive T cells were positive for EphA2-FITC indicating that EphA2-TEs are secreted from infected A549 cells and are able to bind CD3 and EphA2 (Figure 1b).
Figure 1.
The EphA2-TEA-VV express functional EphA2-T-cell engagers. (a) Scheme of expression cassettes encoding EphA2-scFv-CD3-scFv (EphA2-TEA-VV) or GFP (GFP-VV). (b) EphA2-TEA-VV–infected cells secrete bispecific EphA2-scFv-CD3-scFv with the ability of binding to T cells and EphA2. A549 tumor cells (1 × 106/2 ml/well in 6-well plate) were infected with EphA2-TEA-VV or GFP-VV at a multiplicity of infection of 5. The culture media was collected after 24 hours of incubation and added to 1 × 106 unstimulated peripheral blood mononuclear cells (PBMCs), followed by staining with EphA2-FITC, CD8-PE, and CD4-APC. EphA2-FITC, EphA2 protein labeled with fluorescein isothiocyanate; EphA2-TEA-VV, EphA2-T-cell engager-armed vaccinia virus; PE, phycoerythrin; scFv, single-chain variable fragment.
EphA2-TEs expression does not impair the ability of VV to replicate and induce tumor cell lysis
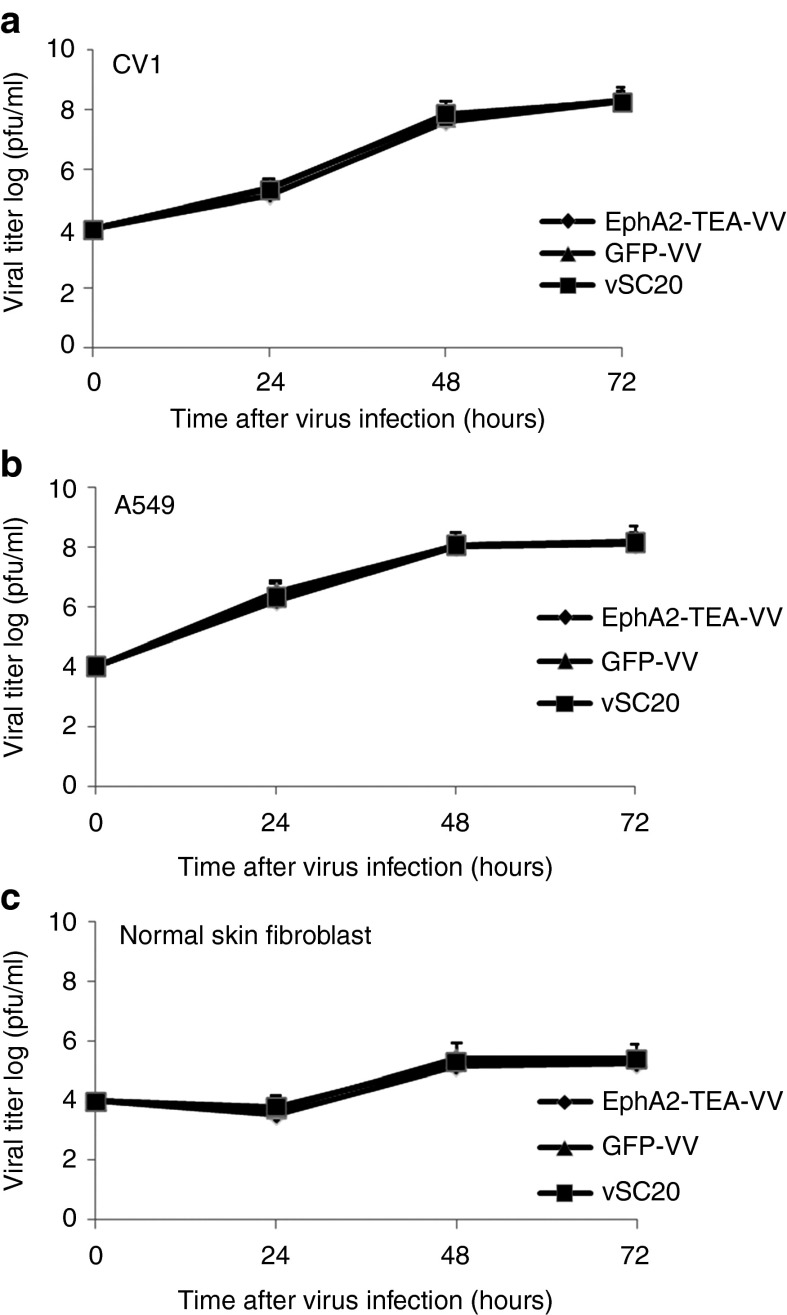
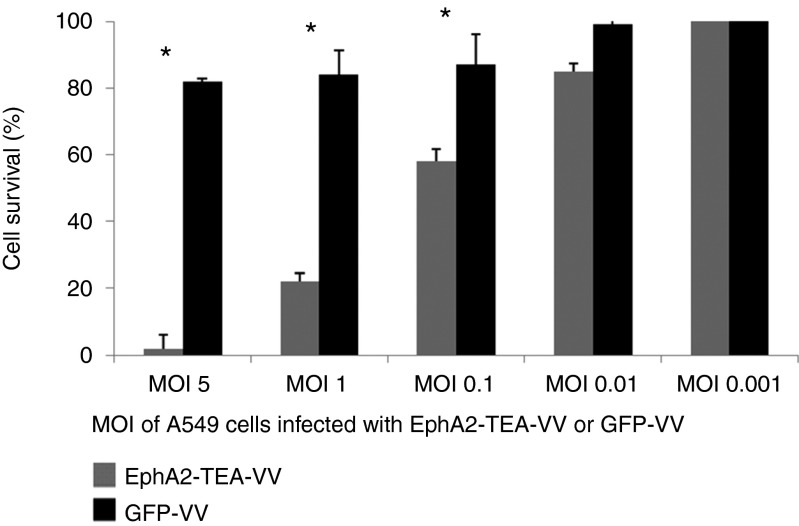
To demonstrate that EphA2-TEs do not impair the ability of EphA2-TEA-VV to replicate, CV-1 cells, A549 tumor cells, and normal human skin fibroblasts were infected with EphA2-TEA-VV, GFP-VV, or parental VV (VSC20). Infection of CV-1 (Figure 2a) and A549 (Figure 2b) with EphA2-TEA-VV, GFP-VV, or VSC20 yielded similar amounts of virus at various time points. By contrast, all three viruses replicated poorly in normal human skin fibroblasts (Figure 2c). Thus, EphA2-TEs do not interfere with VV replication. Next, we compared the ability of EphA2-TEA-VV or GFP-VV to induce tumor cell lysis in the absence of human T cells. A549 cells were transduced with EphA2-TEA-VV or GFP-VV at increasing MOIs (0, 0.01, 0.1, 1, or 5) and 48 hours post virus infection, A549 cell viability was determined using MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) assay. A549 cells were killed with increasing MOIs regardless of the VV used (Figure 3a). There was no difference between EphA2-TEA-VV and GFP-VV, indicating that the expression of EphA2-TEs does not interfere with the ability of VV to induce tumor cell lysis.
Figure 2.
In vitro replication of EphA2-TEA-VV in CV-1 cells and normal human cells. The replication of EphA2-TEA-VV, GFP-VV, and vSC20 in (a) CV-1, (b) A549 tumor cells, and (c) normal human fibroblast are shown. Cells were infected at a multiplicity of infection of 0.1, and after 1, 2, or 3 days, viral titers were determined using plaque assays in CV-1 cells. EphA2-TEA-VV, EphA2-T-cell engager-armed vaccinia virus; pfu, plaque-forming units.
Figure 3.
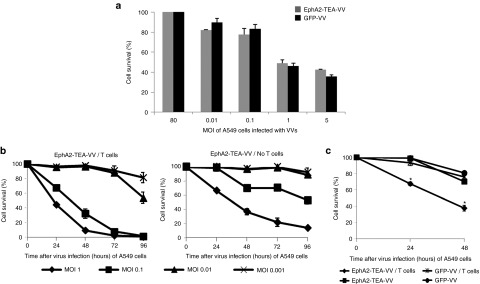
Lytic activity of EphA2-TEA-VV or GFP-VV against EphA2-positive A549 tumor cells. (a) A549 tumor cells were infected with increasing doses (multiplicity of infection (MOI) of 0.01, 0.1, 1, or 5) of EphA2-TEA-VV or GFP− VV. Cell viability at 48 hours postinfection was determined using MTS assays. EphA2-TEA-VV or GFP-VV exhibited comparable tumor lytic activity against A549 cells in the absence of human T cells. (b) Human T cells enhanced the oncolytic activity of EphA2-TEA-VV against EphA2-positive A549 cells. A549 tumor cells were infected with increasing MOIs (0.001, 0.01, 0.1, or 1) of EphA2-TEA-VV. Infected A549 cells were either cultured alone or in the presence of CD4/CD8 bead-isolated human T cells (T cells: A549 tumor cells = 5:1). Cell viability was determined using MTS assays at 24, 48, 72, and 96 hours postinfection. (c) A549 tumor cells were infected with EphA2-TEA-VV or GFP-VV at an MOI of 0.1. Infected A549 cells were either cultured alone or in the presence of CD4/CD8 bead-isolated human T cells (T cells: A549 cells = 5:1). Cell viability at 24 or 48 hours postinfection was determined using MTS assays (EphA2-TEA-VV vs. GFP-VV, *P < 0.05). EphA2-TEA-VV, EphA2-T-cell engager armed vaccinia virus.
EphA2-TEA-VVs redirect human T cells to EphA2-positive A549 cells
To determine whether EphA2-TEA-VVs redirect human T cells to A549 cells, cells were infected with EphA2-TEA-VV at increasing MOIs (MOI 0.001, 0.01, 0.1, or 1). Next, human unstimulated T cells isolated from PBMCs using CD4/CD8 microbeads were added to A549 cells at a T-cell to A549 ratio of 5:1. At 24, 48, 72, or 96 hours post virus infection, A549 viability was determined using MTS assay. A549 cells infected only with EphA2-TEA-VVs served as controls. EphA2-TEA-VV by itself induced cell killing in a dose-dependent manner. However, even at the highest MOI tested, 15% of tumor cells were still alive 96 hours postinfection. Adding human T cells to the culture significantly (P < 0.05) increased antitumor effects with all tumor cells being killed within 96 hours postinfection at MOIs of 0.1 and 1 (Figure 3b).
To confirm that the enhanced lytic activity of EphA2-TEA-VV depends on the secretion of EphA2-TEs, A549 cells were infected with EphA2-TEA-VV or GFP-VV at an MOI of 0.1. Human T cells were added as described above, and 24 or 48 hours post virus infection, A549 cell viability was determined using MTS assay. Only EphA2-TEA-VV displayed enhanced oncolytic activity in the presence of human T cells at 24 (EphA2-TEA-VV vs. GFP-VV, 75 vs. 100%) and 48 hours (EphA2-TEA-VV vs. GFP-VV, 35 vs. 81%) (Figure 3c). This finding was confirmed for a panel of EphA2-positive cancer cell lines (H1299, H1975, U373, and LM7) (Supplementary Figure S2A).27
EphA2-TEA-VVs activate T cells
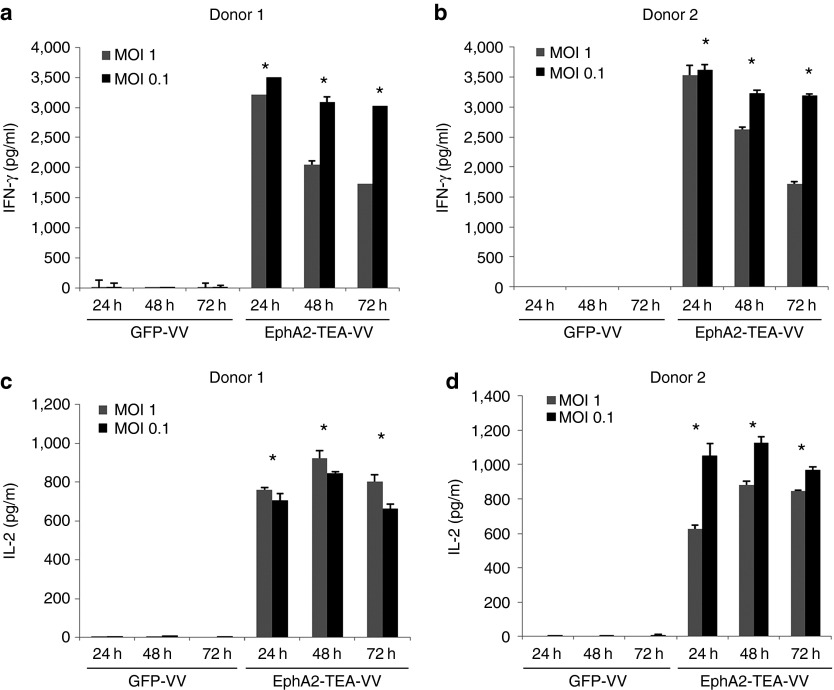
To determine whether EphA2-TEs secreted by EphA2-TEA-VV not only redirect T cells to tumor cells but also activate human T cells, A549 cells were infected with EphA2-TEA-VV or GFP-VV at an MOI of 1 or 0.1. Unstimulated human PBMCs were added as described above, and 24 or 48 hours post virus infection, cell culture media were collected to determine the presence of proinflammatory cytokines using enzyme-linked immunosorbent assay. Unstimulated human PBMCs were activated by EphA2-TEs as judged by the production of proinflammatory cytokines such as interferon-γ (IFN-γ) and interleukin-2 (IL-2) in the cell culture supernatant of EphA2-TEA-VV–infected A549 and T cells, compared with that of GFP-VV–infected A549 and T cells (P < 0.05). T cells produced little to no IFN-γ and IL-2 in response to GFP-VV–infected A549 cells (Figure 4). These results were confirmed for EphA2-positive cell lines H1299 and U373 (Supplementary Figure S2B) and indicate that T-cell activation depends on the expression of EphA2-TEs by tumor cells.
Figure 4.
EphA2-TEA-VV activates human T cells. A549 cells were infected with EphA2-TEA-VV or GFP-VV at a multiplicity of infection (MOI) of 0.1 or 1. Infected A549 cells were cultured in the presence of human PBMCs (PBMCs: A549 cell ratio = 5:1). After 24, 48, or 72 hours, supernatants were collected, and (a,b) interferon-γ (IFN-γ) and (c,d) interleukin-2 (IL-2) production was determined using enzyme-linked immunosorbent assay (EphA2-TEA-VV vs. GFP-VV, *P < 0.05). EphA2-TEA-VV, EphA2-T-cell engager armed vaccinia virus; PBMCs, peripheral blood mononuclear cells.
To confirm that T-cell activation depends on the presence of EphA2 on the cells surface of tumor cells, we took advantage of K562, which are EphA2 negative, and K562-EphA2, which were genetically modified to express EphA2. A549 tumor cells were infected with EphA2-TEA-VV or GFP-VV at an MOI of 5. After 24 hours, supernatants were collected and added to a coculture of human PBMCs with K562-EphA2 or K562 (effector cell: tumor cell = 5:1). Only the combination of K562-EphA2 and supernatants of EphA2-TEA-VV–infected A549 cells induced T-cell activation as judged by IFN-γ and IL-2 secretion, demonstrating that T-cell activation and tumor cell recognition not only depends on the presence of EphA2-TEs but also on the expression of EphA2 on tumor cells (Supplementary Figure S3A). This was confirmed in a cytotoxicity assay (Supplementary Figure S3B).
EphA2-TEA-VVs induce bystander killing of noninfected tumor cells
We next investigated the ability of EphA2-TEA-VVs to induce bystander killing of tumor cells. First, A549 cells were transduced with EphA2-TEA-VVs or GFP-VVs at various MOIs. The cell culture medium was collected and filtered with a 0.22 µm filter after 48 hours of culture. Then, noninfected A549 cells were cocultured with unstimulated PBMCs in the presence of the cell culture medium collected from the culture of VV-infected A549 cells. Forty-eight hours later, tumor killing was measured using MTS assay. A549 cells were only killed in the presence of cell culture medium of EphA2-TEA-VV–infected A549 cells, indicating bystander killing of tumor cells (Figure 5).
Figure 5.
EphA2-TEs induce bystander killing of tumor cells. A549 cells were infected with EphA2-TEA-VV or GFP-VV at various multiplicity of infections (MOIs), and after 24 hours, supernatants were collected and used in a coculture assay of unstimulated peripheral blood mononuclear cells (PBMCs) and A549 cells (PBMCs: A549 cells ratio = 5:1). After 48 hours, tumor cell killing was measured by MTS assay (EphA2-TEA-VV vs. GFP-VV, *P < 0.05). EphA2-TEs, EphA2-T-cell engagers; EphA2-TEA-VV, EphA2-T-cell engager-armed vaccinia virus.
EphA2-TEA-VVs have enhanced antitumor activity in A549 xenograft tumor model
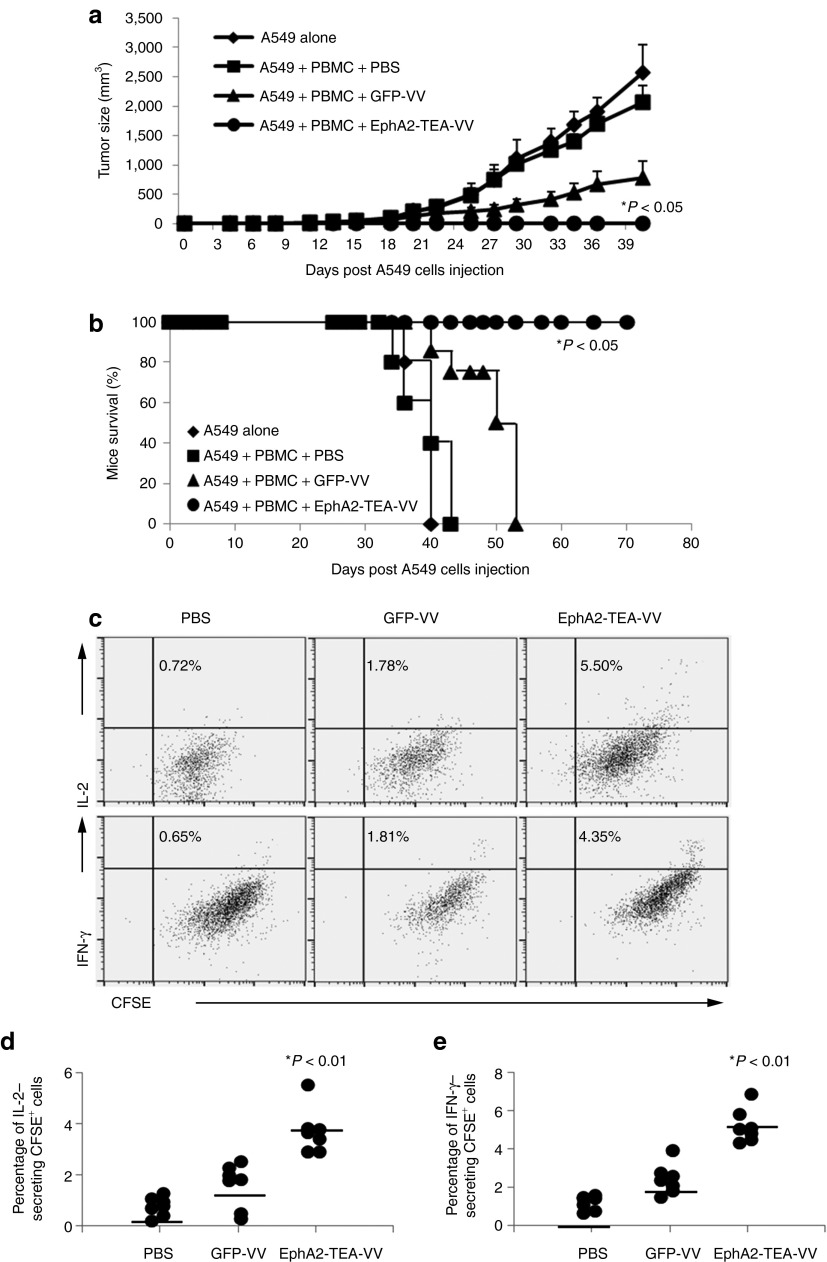
To investigate the antitumor effects of EphA2-TEA-VV in vivo, we initially used a subcutaneous A549 tumor model. To establish A549 tumors, 2 × 106 A549 cells were mixed with unstimulated PBMCs from healthy donor and inoculated subcutaneously (s.c.) into the right flank of SCID-Bg mice, followed by an intraperitoneal (i.p.) injection of 1 × 108 plaque-forming units (pfu) of EphA2-TEA-VV or GFP-VV on day 0. A549/unstimulated PBMC–injected mice receiving phosphate-buffered saline (PBS) and mice that were only injected s.c. with A549 served as control. GFP-VVs moderately inhibited tumor growth compared with that in control mice. By contrast, mice that received EphA2-TEA-VVs showed a significant decrease in tumor growth, compared with mice that received GFP-VV or PBS (Figure 6a, EphA2-TEA-VV vs. GFP-VV P < 0.0001). This resulted in a significant increase in survival of mice that received both EphA2-TEA-VV and human PBMCs (Figure 6b, EphA2-TEA-VV vs. GFP-VV P < 0.001).
Figure 6.
Administration of EphA2-TEA-VV in combination with peripheral blood mononuclear cells (PBMCs) results in an enhanced antitumor response and improved survival in A549 subcutaneous (s.c.) tumor model. 2 × 106 A549 cells were mixed with 1 × 107 unstimulated PBMCs and inoculated s.c. into the right flank of SCID mice on day 0, immediately followed by intraperitoneal (i.p.) injection of 1 × 108 plaque-forming units (pfu) EphA2-TEA-VV, GFP-VV, or phosphate-buffered saline (PBS). (a) Tumor size was measured using a caliper (EphA2-TEA-VV n = 13 vs. GFP-VV n = 12, *P < 0.001). (b) Kaplan–Meier survival showed a significant survival advantage of mice treated with EphA2-TEA-VVs plus PBMCs (EphA2-TEA-VV n = 13 vs. GFP-VV n = 12, *P < 0.0001). (c–e) 2 × 106 A549 cells were mixed with 1 × 107 CFSE-labeled unstimulated PBMCs and inoculated s.c. into the right flank of SCID mice on day 0, immediately followed by i.p. injection of 1 × 108 pfu EphA2-TEA-VV, GFP-VV, or PBS. Five days later, tumor tissues were dissected, and single cells suspensions were prepared for intracellular staining of interferon-γ (IFN-γ) and interleukin-2 (IL-2). (c) Representative fluorescence-activated cell sorting analysis of CFSE+ cells. (d,e) Summary data of all mice showing a significant increase of IFN-γ (n = 7, EphA2-TEA-VV vs. GFP-VV, 5.19 ± 0.94 vs. 2.43 ± 1.19%, P < 0.01) or IL-2 (n = 7, EphA2-TEA-VV vs. GFP-VV, 3.70 ± 0.88 vs. 1.60 ± 0.86%, P < 0.01) secreting PBMCs in EphA2-TEA-VV treated mice. EphA2-TEA-VV, EphA2-T-cell engager armed vaccinia virus.
To determine whether EphA2-TEA-VV activated T cells within tumor, mice were injected with an admixture of A549 cells and carboxyfluorescein succinimidyl ester-labeled PBMCs followed by an i.p. injection of 1 × 108 pfu of EphA2-TEA-VV or GFP-VV on day 0. Tumor infiltrating cells were isolated on day 5 and subjected to FACS analysis. Only EphA2-TEA-VV induced PBMC activation as judged by intracellular staining for IFN-γ (n = 7, EphA2-TEA-VV vs. GFP-VV, 5.19 ± 0.94 vs. 2.43 ± 1.19%, P < 0.01) and IL-2 (n = 7, EphA2-TEA-VV vs. GFP-VV, 3.70 ± 0.88 vs. 1.60 ± 0.86%, P < 0.01) (Figure 6c). However, there was no difference in the mean fluorescence intensity of carboxyfluorescein succinimidyl ester between EphA2-TEA-VV– and GFP-VV–injected animals, indicating that the observed cytokine production was not sufficient to induce T-cell proliferation.
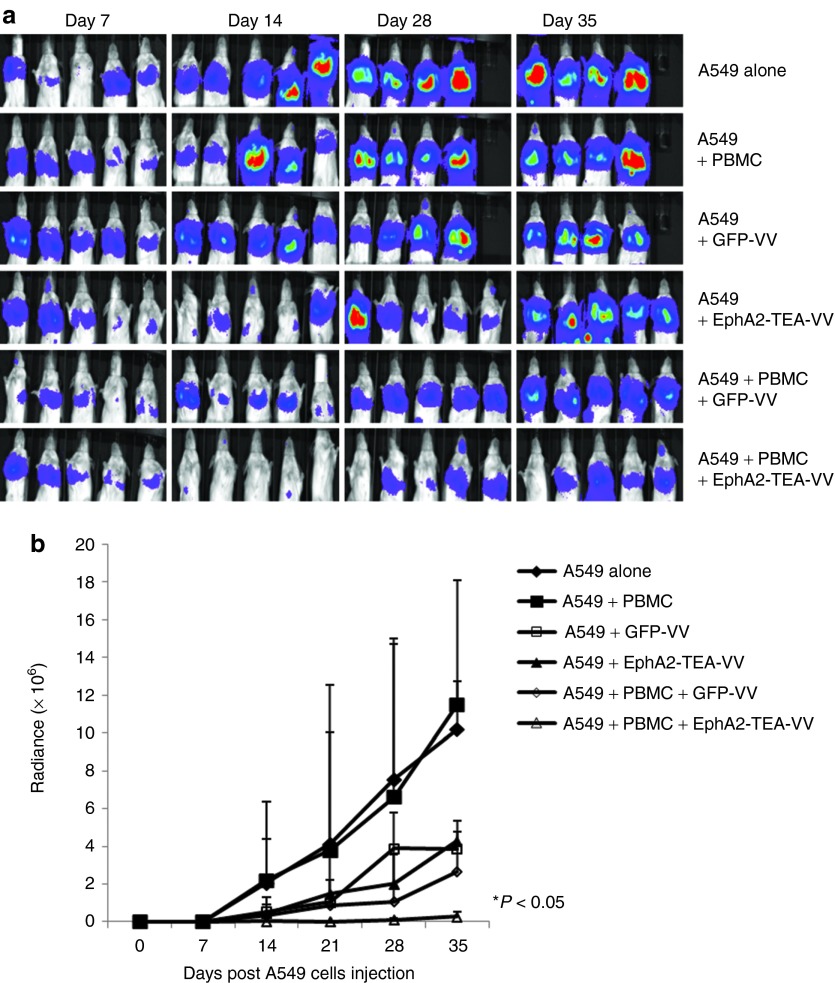
To further investigate the antitumor effects of EphA2-TEA-VV in a systemic model, luciferase-expressing A549 cells (A549.eGFP.ffluc) were injected intravenously (i.v.) into SCID-Bg mice on day 0. On day 7, an admixture of 10 × 106 untreated PBMCs and/or 1 × 108 pfu of EphA2-TEA-VVs or GFP-VVs were administered i.v. The mice that received only PBMCs served as control. Quantification of bioluminescent imaging showed that VVs or GFP-VVs plus PBMCs only moderately inhibited tumor growth compared with controls. By contrast, mice that received EphA2-TEA-VVs plus PBMCs showed a significant decrease in tumor growth, compared with mice that received VVs or PBMCs, or GFP-VVs plus PBMCs (Figure 7, EphA2-TEA-VV plus PBMCs vs. GFP-VV, EphA2-TEA-VV, and GFP-VV plus PBMCs, P < 0.05). Thus, in both animal models, EphA2-TEA-VV plus PBMCs resulted in enhanced antitumor activity in comparison with GFP-VV plus PBMCs.
Figure 7.
Administration of EphA2-TEA-VV in combination with peripheral blood mononuclear cells (PBMCs) results in an enhanced antitumor response in A549 intravenous (i.v.) lung cancer model. (a) 2 × 106 A549.eGFP.FFLuc were injected i.v. into SCID mice on day 0 and treated with a single injection of mixed 1 × 107 unstimulated PBMCs and 1 × 108 plaque-forming units EphA2-TEA-VV or GFP-VVs i.v. on day 7. Tumor progression was followed by in vivo bioluminescence imaging. Images of representative animals are shown. (b) Solid lines represent each group of mice (EphA2-TEA-VV n = 5 vs. GFP-VV n = 5, *P < 0.05). EphA2-TEA-VV, EphA2-T-cell engager armed vaccinia virus.
Discussion
Oncolytic VVs have demonstrated promising results in the treatment of cancer in preclinical models and early clinical trails.1,2,3,4,5,6,7,8,9,10 However, complete clinical responses were rarely observed, highlighting the need for further improvement of oncolytic VV therapy. In this study, we generated a novel T-cell engager-armed oncolytic VV (TEA-VV) designed to engage T cells for cancer therapy. To our knowledge, this is the first example to evaluate a TEA oncolytic VV in preclinical cancer models.
To enhance the antitumor efficacy of VVs, oncolytic VVs have been engineered to express a variety of transgenes encoding cytokines, chemokines, prodrug-converting enzymes, and antiangiogenic agents.3,4,5,6,7,8,9,10,28,29,30,31,32,33 One major aim of these modifications was to induce bystander killing of tumor cells that are not infected and/or killed by oncolytic VVs. For example, expression of a prodrug-converting enzyme by VVs is capable of converting a systemically administrated nontoxic prodrug to an active pharmacological agent within the tumor. Such an oncolytic VV (vvDD-CDSR) is currently under investigation in an early phase clinical trail. The most successful transgene so far has been the cytokine granulocyte macrophage colony-stimulating factor (GM-CSF).3,4,5,6,7,8,9 The GM-CSF–expressing VV JX-594 has shown exciting results in phase I/IIa clinical trials targeting hepatocellular cancers as local therapy and a variety of solid tumors as systemic therapy. Based on these results, JX-594 is currently being evaluated in global phase III clinical trials. However, the exact mechanism of how GM-CSF enhances the antitumor activity of VVs remains elusive. For example, whereas some studies have shown that GM-CSF activates innate immunity and induces the generation of an adaptive antitumor response, other studies have highlighted the role of GM-CSF in inducing proliferation of monocyte-derived suppressor cells, which suppress antitumor immune responses.34 Compared with these approaches, our TEA-VV platform provides a straightforward mechanism by which TEA-VV exert its potent effects by utilizing adaptive immunity in a tumor antigen-specific manner. TEs expressed by TEA-VV are capable of engaging endogenous T cells and tumor cells, resulting in antigen-specific tumor lysis. In addition, we show that TEs induce the secretion of proinflammatory cytokines in vitro and in vivo that should reverse the immune inhibitory milieu, which exist in the majority of solid tumors.16,17
Although EphA2-TEA-VV induced the production of IFN-γ and IL-2, we did not observe significant T-cell proliferation in vitro and in vivo. However, EphA2-TEA-VVs induced significant T-cell proliferation if the cell culture medium was supplemented with 100 U/ml human IL-2 (Supplementary Figure S4). Thus, the amount of IL-2 produced by T cells after EphA2-TEA-VV-activation (1 ng/ml of IL-2, equivalent to ~1 U/ml, Figure 4c,d) is not sufficient to induce T-cell proliferation. Currently, TEA-VVs only provide T-cell activation by binding to CD3. Additional genetic modification of TEA-VVs with transgenes that encode costimulatory molecules or cytokines35,36,37,38,39,40,41 should result in TEA-VVs that not only activate T cells but also induce robust T-cell proliferation.
Limitations of systemic cancer treatments are their adverse effects, pharmacological half-life, and/or limited tissue distribution. For example, although BiTEs have been shown to be effective for the treatment of CD19-positive malignancies, BiTEs have a very short half-life, necessitating continuous infusion.18,19,20,21,22,23,24,25,26,42 In addition, BiTEs do not actively accumulate at tumor sites, and systemic adverse events have been reported including within the central nervous system. These limitations could be potentially overcome by the use of oncolytic virus as a delivery system, because transgene expression from the VVs will occur mainly within the tumor.34 Following virus infection of a cell, a cascade of virus protein, called early or late protein, is produced under the transcriptional control of early or late promoter individually. Generally, late promoters are activated after DNA replication of the virus in the cell, therefore allowing transgene expression selectively within the tumors. In this study, we choose the late vaccinia viral promoter F17R, because studies have demonstrated that the F17R promoter is only activated after VV infection in tumor cells.43 Thus tumor selective expression of transgene from VV will be further enhanced by the use of F17R promoter. The late expression of the T-cell engager will also allow for sufficient viral replication before T-cell activation and mediated tumor lysis.
We evaluated the therapeutic effects of EphA2-TEA-VVs in two murine xenograft models. EphA2-TEA-VVs plus human PBMCs induced complete remissions in all treated animals in the s.c. A549 model. Because the ability of human PBMCs to migrate to s.c. tumor sites in mice is limited, we injected s.c. an admixture of tumor cells and PBMCs as routinely done for the preclinical evaluation of BiTEs.18,19,20,21 To evaluate the antitumor activity of EphA2-TEA-VVs after i.v. administration of PBMCs, we used the systemic A549 lung cancer model. While in the systemic model, EphA2-TEA-VVs plus human PBMCs had potent antitumor response, all tumors progressed. This difference in efficacy in the two models is most likely due to several reasons. First, the systemic A549 model is a very aggressive tumor model, and we treated mice on day 7 after tumor cell injection. Second, TEA-VV exerts its antitumor effects in part by activating human T cells, which in the used xenograft model (SCID-Beige mice) persist only for 7–10 days postinfusion.44 As discussed above, introduction of additional transgenes into TEA-VVs to provide costimulatory signals and/or cytokines are potential strategies to improve T-cell activation and persistence in vivo.35,36,37,38,39,40,41 While we choose a xenograft model to demonstrate the benefit of arming VVs with TEs, we are currently developing an immune competent animal model to perform detailed mechanistic studies. Insight from this model will most likely enable to further improve the antitumor activity of TEA-VVs.
In conclusion, our findings provide preclinical evidence for the therapeutic potential of TEA-VVs. In this study, our EphA2-TEA-VVs activated human PBMCs and enhanced antitumor activity in preclinical models. Our T-cell engager arming strategy may be applicable to a broad range of oncolytic viruses currently under investigation, including oncolytic adenoviral vector, herpes simplex virus, reovirus, myxoma virus, poliovirus, vesicular stomatitis virus, measles virus, and Newcastle disease virus.
Materials and Methods
Tumor cell lines. The non-small-cell lung cancer cell line A549 was purchased from the American Type Culture Collection (Manassas, VA). A549 cell line expressing the eGFP-Firefly luciferase (A549.eGFP.ffluc) fusion gene was generated as previously described with a retrovirus vector encoding the eGFP.ffluc.44 Cell lines were grown in Roswell Park Memorial Institute (Thermo Scientific HyClone, Waltham, MA; Lonza, Basel, Switzerland) supplemented with 10% fetal calf serum ((FCS) HyClone, Logan, UT) and 2 mmol/l GlutaMAX-I (Invitrogen, Carlsbad, CA). Cells were maintained in a humidified atmosphere containing 5% CO2 at 37°C. The “Characterized Cell Line Core Facility” at MD Anderson Cancer Center, Houston, TX, performed cell line validation.
Mononuclear cells. Blood samples from healthy donors were obtained in accordance to protocols approved by the Institutional Review Board of Baylor College of Medicine. Peripheral blood was processed over Ficoll gradients, and the resulting PBMCs were cultured in Roswell Park Memorial Institute 1640 (Thermo Scientific HyClone, Waltham, MA; Lonza, Basel, Switzerland) supplemented with 10% heat-inactivated FCS and 2 mmol/l GLUTAMAX.
Production of VV. We generated double-deleted VVs (vvDD, Western Reserve strain) expressing EphA2-scFv-CD3-scFv (EphA2-TEA-VV) or GFP (GFP-VV) by recombination with a pSEL shuttle plasmid encoding CD3 scFv18,19,20,21,22,23,24,25,26 or EphA2-scFv27 or GFP into the TK gene of the VSC20 strain of WR VV (Figure 1).45,46,47,48,49,50 The inserted TEs were expressed under the transcriptional control of the F17R late promoter to allow for sufficient viral replication before T-cell activation.34 All VVs used in this study were derived from the WR strain. Two low pathogenic mutants containing deletions in the TK and VGF genes (vvdd) have been previously described. Viral stocks were prepared as previously reported. Briefly, dividing CV-1 cells were infected with 200 pfu (MOI: ~0.0005) of VSC20, GFP-VV, or TEA-VV in 1 ml of minimum essential medium-2.5% FCS for 2 hours at 37°C. Dulbecco's modified Eagle's medium-10% FCS was added, and cells were incubated until harvesting at 24, 48, and 72 hours after infection. After a single freeze-thaw cycle, virus was quantified by plaque titering on CV-1 cells as described previously.50
Flow cytometry. For immunophenotyping, cells were stained with fluorescein-conjugated monoclonal antibodies (Becton Dickinson, San Jose, CA) directed against CD3-, CD4-, CD8-, or FITC-labeled EphA2 protein. Isotype controls were immunoglobulin G1–fluorescein isothiocyanate (IgG1-FITC; BD) and IgG1–phycoerythrin (IgG1-PE; BD). Cells were collected and washed once with PBS containing 1% FBS (Sigma, St. Louis, MO; FACS buffer) before the addition of antibodies. Cells were then incubated for 30 minutes on ice in the dark, washed once, and fixed in 0.5% paraformaldehyde/FACS buffer before analysis. For each sample, 10,000 cells were analyzed using FACSCalibur instrument (BD, Becton Dickinson, Mountain View, CA) with the Cell Quest Software (Becton Dickinson) or with FCS Express software (De Novo Software, Los Angeles, CA).
Enzyme-linked immunosorbent assay. Tumor cells were infected with either EphA2-TEA-VV or GFP-VV at different MOIs followed by coculturing with unstimulated PBMCs. At different time points, supernatants were collected and run on enzyme-linked immunosorbent assay with DuoSet enzyme-linked immunosorbent assay development kit (R&D systems, Minneapolis, MN) according to the manufacturer's guidelines.
MTS assay. Tumor cells were plated in a 96-well tissue culture plate at 1 × 104 cells per well (100 µl) and incubated overnight at 37°C. The tumor cells were infected with VVs at indicated MOIs in 2.5% FBS medium for 2 hours followed by culturing in complete medium. All samples were measured in triplicate. Unstimulated human PBMCs were incubated at 37°C for 2 hours to remove adherent cells. In some experiments, unstimulated nonadherent human PBMCs or CD4/8 microbead-selected T cells were added to the culture at effector: target ratio of 5:1. Plates were incubated at 37°C for 48 or 72 hours. The nonadherent human PBMCs or CD4/8 microbead-selected T cells were removed, and the wells were washed gently twice with PBS. The viability of tumor cells was then determined using a MTS formazan viability assay (Promega, Madison, WI). Optical density of the wells was read at 490 nm on a plate reader (Molecular Devices VERSAmax, Sunnyvale, CA (Molecular Devices)). The mean viability of the virus infected tumor cells for each viral dilution was calculated as a percentage relative to the control wells treated with media alone (100% survival) ± SEM.
A549 tumor models. All animal experiments followed a protocol approved by the Baylor College of Medicine Institutional Animal Care and Use Committee. Nine- to 12-week-old SCID Beige mice were purchased from Jackson, Bar Harbor, ME.
A549 i.v. lung model. 2 × 106 A549.eGFP.ffLuc cells in PBS were injected i.v. on day 0. To determine the antitumor efficacy of EphA2-TEA-VV, mice were treated with an i.v. administration of either 1 × 108 pfu GFP-VV or 1 × 108 pfu EphA2-TEA-VV mixed with 1 × 107 human unstimulated PBMCs on day 7 posttumor challenge. Animals receiving unstimulated PBMCs alone or PBS served as controls. Isoflurane anesthetized animals were imaged. A constant region-of-interest was drawn over the chest of the animal, and the intensity of the signal was measured as total photons/s/cm2/steradian. Mice were euthanized when they met euthanasia criteria (weight loss, signs of distress) in accordance with the Center for Comparative Medicine at Baylor College of Medicine.
A549 s.c. tumor model. 2 × 106 A549 cells alone or mixed with unstimulated PBMCs from healthy donors in 100 µl of PBS were injected s.c. on day 0, immediately followed by i.p. administration of either 1 × 108 pfu GFP-VV or 1 × 108 pfu EphA2-TEA-VV. Animals receiving PBS served as controls. Tumor size was measured using a caliper.
To monitor T cells within tumor tissue, 2 × 106 A549 cells were mixed with 1 × 107 carboxyfluorescein succinimidyl ester-labeled unstimulated PBMCs and inoculated s.c. into the right flank of SCID mice on day 0, immediately followed by i.p. injection of 1 × 108 pfu EphA2-TEA-VV, GFP-VV, or PBS. Five days later, tumor tissues were dissected, and single cells suspensions were prepared for intracellular staining of IFN-γ and IL-2.
Statistical analysis. All in vitro experiments were performed in triplicate. Measurement data were presented as mean ± SD. The differences between means were tested by nonparametric Mann–Whitney U test or Bonferroni's multiple comparison t-test. The significance level used was P < 0.05. For the mouse experiments, tumor radiance data were log-transformed and summarized using mean ± SD at baseline and multiple subsequent time points for each group of mice. Changes in tumor radiance from baseline at each time point were calculated and compared between groups using t-test. Survival determined from the time of tumor cell injection was analyzed by the Kaplan–Meier method and by the log-rank test.
SUPPLEMENTARY MATERIAL Figure S1. EphA2-specific T-cell engagers induce tumor cells killing. Figure S2. T cells recognize EphA2-TEA-VV–infected EphA2-positive H1299, H1975, U373, or LM7 tumor cells. Figure S3. T cells recognize EphA2-TEA-VV–nfected EphA2 expressing K562-EphA2 cells, but not parental, EphA2-negative K562 cells. Figure S4. EphA2-TEA-VVs induced significant T-cell proliferation in the presence of 100 U/ml human IL2. Supplementary Materials and Methods.
Acknowledgments
We thank Cliona M. Rooney and Malcolm K. Brenner for helpful discussions and advice. This work was funded by NIH R01 CA148748. The work was done in Houston, TX, USA.
Supplementary Material
References
- Russell SJ, Peng KW, Bell JC. Oncolytic virotherapy. Nat Biotechnol. 2012;30:658–670. doi: 10.1038/nbt.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly E, Russell SJ. History of oncolytic viruses: genesis to genetic engineering. Mol Ther. 2007;15:651–659. doi: 10.1038/sj.mt.6300108. [DOI] [PubMed] [Google Scholar]
- Heo J, Reid T, Ruo L, Breitbach CJ, Rose S, Bloomston M, et al. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat Med. 2013;19:329–336. doi: 10.1038/nm.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitbach CJ, Burke J, Jonker D, Stephenson J, Haas AR, Chow LQ, et al. Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature. 2011;477:99–102. doi: 10.1038/nature10358. [DOI] [PubMed] [Google Scholar]
- Kim JH, Oh JY, Park BH, Lee DE, Kim JS, Park HE, et al. Systemic armed oncolytic and immunologic therapy for cancer with JX-594, a targeted poxvirus expressing GM-CSF. Mol Ther. 2006;14:361–370. doi: 10.1016/j.ymthe.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Hwang TH, Moon A, Burke J, Ribas A, Stephenson J, Breitbach CJ, et al. A mechanistic proof-of-concept clinical trial with JX-594, a targeted multi-mechanistic oncolytic poxvirus, in patients with metastatic melanoma. Mol Ther. 2011;19:1913–1922. doi: 10.1038/mt.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lun X, Chan J, Zhou H, Sun B, Kelly JJ, Stechishin OO, et al. Efficacy and safety/toxicity study of recombinant vaccinia virus JX-594 in two immunocompetent animal models of glioma. Mol Ther. 2010;18:1927–1936. doi: 10.1038/mt.2010.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo J, Breitbach CJ, Moon A, Kim CW, Patt R, Kim MK, et al. Sequential therapy with JX-594, a targeted oncolytic poxvirus, followed by sorafenib in hepatocellular carcinoma: preclinical and clinical demonstration of combination efficacy. Mol Ther. 2011;19:1170–1179. doi: 10.1038/mt.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senzer NN, Kaufman HL, Amatruda T, Nemunaitis M, Reid T, Daniels G, et al. Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J Clin Oncol. 2009;27:5763–5771. doi: 10.1200/JCO.2009.24.3675. [DOI] [PubMed] [Google Scholar]
- Adair RA, Roulstone V, Scott KJ, Morgan R, Nuovo GJ, Fuller M, et al. Cell carriage, delivery, and selective replication of an oncolytic virus in tumor in patients. Sci Transl Med. 2012;4:138ra77. doi: 10.1126/scitranslmed.3003578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollard CM, Aguilar L, Straathof KC, Gahn B, Huls MH, Rousseau A, et al. Cytotoxic T lymphocyte therapy for Epstein-Barr virus+ Hodgkin's disease. J Exp Med. 2004;200:1623–1633. doi: 10.1084/jem.20040890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollard CM, Gottschalk S, Leen AM, Weiss H, Straathof KC, Carrum G, et al. Complete responses of relapsed lymphoma following genetic modification of tumor-antigen presenting cells and T-lymphocyte transfer. Blood. 2007;110:2838–2845. doi: 10.1182/blood-2007-05-091280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14:1264–1270. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12:269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer-Weaver K, Anderson M, Malyguine A, Hurwitz AA. T cell tolerance to tumors and cancer immunotherapy. Adv Exp Med Biol. 2007;601:357–368. doi: 10.1007/978-0-387-72005-0_38. [DOI] [PubMed] [Google Scholar]
- Shafer-Weaver KA, Anderson MJ, Stagliano K, Malyguine A, Greenberg NM, Hurwitz AA. Cutting Edge: Tumor-specific CD8+ T cells infiltrating prostatic tumors are induced to become suppressor cells. J Immunol. 2009;183:4848–4852. doi: 10.4049/jimmunol.0900848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond SA, Lutterbuese R, Roff S, Lutterbuese P, Schlereth B, Bruckheimer E, et al. Selective targeting and potent control of tumor growth using an EphA2/CD3-Bispecific single-chain antibody construct. Cancer Res. 2007;67:3927–3935. doi: 10.1158/0008-5472.CAN-06-2760. [DOI] [PubMed] [Google Scholar]
- Lutterbuese R, Raum T, Kischel R, Hoffmann P, Mangold S, Rattel B, et al. T cell-engaging BiTE antibodies specific for EGFR potently eliminate KRAS- and BRAF-mutated colorectal cancer cells. Proc Natl Acad Sci USA. 2010;107:12605–12610. doi: 10.1073/pnas.1000976107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich M, Raum T, Lutterbuese R, Voelkel M, Deegen P, Rau D, et al. Regression of human prostate cancer xenografts in mice by AMG 212/BAY2010112, a novel PSMA/CD3-Bispecific BiTE antibody cross-reactive with non-human primate antigens. Mol Cancer Ther. 2012;11:2664–2673. doi: 10.1158/1535-7163.MCT-12-0042. [DOI] [PubMed] [Google Scholar]
- Choi BD, Kuan CT, Cai M, Archer GE, Mitchell DA, Gedeon PC, et al. Systemic administration of a bispecific antibody targeting EGFRvIII successfully treats intracerebral glioma. Proc Natl Acad Sci USA. 2013;110:270–275. doi: 10.1073/pnas.1219817110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack M, Riethmüller G, Kufer P. A small bispecific antibody construct expressed as a functional single-chain molecule with high tumor cell cytotoxicity. Proc Natl Acad Sci USA. 1995;92:7021–7025. doi: 10.1073/pnas.92.15.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löffler A, Kufer P, Lutterbüse R, Zettl F, Daniel PT, Schwenkenbecher JM, et al. A recombinant bispecific single-chain antibody, CD19 x CD3, induces rapid and high lymphoma-directed cytotoxicity by unstimulated T lymphocytes. Blood. 2000;95:2098–2103. [PubMed] [Google Scholar]
- Bargou R, Leo E, Zugmaier G, Klinger M, Goebeler M, Knop S, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321:974–977. doi: 10.1126/science.1158545. [DOI] [PubMed] [Google Scholar]
- Topp MS, Kufer P, Gökbuget N, Goebeler M, Klinger M, Neumann S, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. 2011;29:2493–2498. doi: 10.1200/JCO.2010.32.7270. [DOI] [PubMed] [Google Scholar]
- Nagorsen D, Bargou R, Ruttinger D, Kufer P, Baeuerle PA, Zugmaier G. Immunotherapy of lymphoma and leukemia with T-cell engaging BiTE antibody blinatumomab. Leuk Lymphoma. 2009;50:886–891. doi: 10.1080/10428190902943077. [DOI] [PubMed] [Google Scholar]
- Chow KKH, Naik S, Kakarla S, Brawley VS, Shaffer DR, Yi Z, et al. T cells redirected to EphA2 for the immunotherapy of glioblastoma. Mol Ther. 2013;21:629–637. doi: 10.1038/mt.2012.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frentzen A, Yu YA, Chen N, Zhang Q, Weibel S, Raab V, et al. Anti-VEGF single-chain antibody GLAF-1 encoded by oncolytic vaccinia virus significantly enhances antitumor therapy. Proc Natl Acad Sci USA. 2009;106:12915–12920. doi: 10.1073/pnas.0900660106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tysome JR, Briat A, Alusi G, Cao F, Gao D, Yu J, et al. Lister strain of vaccinia virus armed with endostatin-angiostatin fusion gene as a novel therapeutic agent for human pancreatic cancer. Gene Ther. 2009;16:1223–1233. doi: 10.1038/gt.2009.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guse K, Sloniecka M, Diaconu I, Ottolino-Perry K, Tang N, Ng C, et al. Antiangiogenic arming of an oncolytic vaccinia virus enhances antitumor efficacy in renal cell cancer models. J Virol. 2010;84:856–866. doi: 10.1128/JVI.00692-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalikonda S, Kivlen MH, O'Malley ME, Eric Dong XD, McCart JA, Gorry MC, et al. Oncolytic virotherapy for ovarian carcinomatosis using a replication-selective vaccinia virus armed with a yeast cytosine deaminase gene. Cancer Gene Ther. 2008;15:115–125. doi: 10.1038/sj.cgt.7701110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, O'Malley M, Urban J, Sampath P, Guo ZS, Kalinski P, et al. Chemokine expression from oncolytic vaccinia virus enhances vaccine therapies of cancer. Mol Ther. 2011;19:650–657. doi: 10.1038/mt.2010.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson KB, Barra NG, Davies E, Ashkar AA, Lichty BD. Expressing human interleukin-15 from oncolytic vesicular stomatitis virus improves survival in a murine metastatic colon adenocarcinoma model through the enhancement of anti-tumor immunity. Cancer Gene Ther. 2012;19:238–246. doi: 10.1038/cgt.2011.81. [DOI] [PubMed] [Google Scholar]
- Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, et al. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. 2007;25:2546–2553. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- June CH, Bluestone JA, Nadler LM, Thompson CB. The B7 and CD28 receptor families. Immunol Today. 1994;15:321–331. doi: 10.1016/0167-5699(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Fishman M, Hunter TB, Soliman H, Thompson P, Dunn M, Smilee R, et al. Phase II trial of B7-1 (CD-86) transduced, cultured autologous tumor cell vaccine plus subcutaneous interleukin-2 for treatment of stage IV renal cell carcinoma. J Immunother. 2008;31:72–80. doi: 10.1097/CJI.0b013e31815ba792. [DOI] [PubMed] [Google Scholar]
- Kaufman HL, Lenz HJ, Marshall J, Singh D, Garett C, Cripps C, et al. Combination chemotherapy and ALVAC-CEA/B7.1 vaccine in patients with metastatic colorectal cancer. Clin Cancer Res. 2008;14:4843–4849. doi: 10.1158/1078-0432.CCR-08-0276. [DOI] [PubMed] [Google Scholar]
- Kaufman HL, Cohen S, Cheung K, DeRaffele G, Mitcham J, Moroziewicz D, et al. Local delivery of vaccinia virus expressing multiple costimulatory molecules for the treatment of established tumors. Hum Gene Ther. 2006;17:239–244. doi: 10.1089/hum.2006.17.239. [DOI] [PubMed] [Google Scholar]
- Li G, Wu X, Zhang F. Triple expression of B7-1, B7-2 and 4-1BBL enhanced antitumor immune response against mouse H22 hepatocellular carcinoma. J Cancer Res Clin Oncol. 2011;137:695–703. doi: 10.1007/s00432-010-0905-9. [DOI] [PubMed] [Google Scholar]
- Melero I, Shuford WW, Newby SA, Aruffo A, Ledbetter JA, Hellström KE, et al. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat Med. 1997;3:682–685. doi: 10.1038/nm0697-682. [DOI] [PubMed] [Google Scholar]
- Lee H, Park HJ, Sohn HJ, Kim JM, Kim SJ. Combinatorial therapy for liver metastatic colon cancer: dendritic cell vaccine and low-dose agonistic anti-4-1BB antibody co-stimulatory signal. J Surg Res. 2011;169:e43–e50. doi: 10.1016/j.jss.2011.03.067. [DOI] [PubMed] [Google Scholar]
- Kirn DH, Wang Y, Le Boeuf F, Bell J, Thorne SH. Targeting of interferon-beta to produce a specific, multi-mechanistic oncolytic vaccinia virus. PLoS Med. 2007;4:e353. doi: 10.1371/journal.pmed.0040353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dower K, Rubins KH, Hensley LE, Connor JH. Development of Vaccinia reporter viruses for rapid, high content analysis of viral function at all stages of gene expression. Antiviral Res. 2011;91:72–80. doi: 10.1016/j.antiviral.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakarla S, Chow KKH, Mata M, Schaffer D, Song XT, Wu M-F, et al. 2013Genetically modified T-cells redirected to the tumor stroma for the adoptive immunotherapy of solid tumors. Mol Ther 211611–1620.23732988 [Google Scholar]
- Guo ZS, Thorne SH, Bartlett DL. Oncolytic virotherapy: molecular targets in tumor-selective replication and carrier cell-mediated delivery of oncolytic viruses. Biochim Biophys Acta. 2008;1785:217–231. doi: 10.1016/j.bbcan.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Davis JJ, Zhu H, Dong F, Guo W, Ang J, et al. Redirecting adaptive immunity against foreign antigens to tumors for cancer therapy. Cancer Biol Ther. 2007;6:1773–1779. doi: 10.4161/cbt.6.11.4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne SH, Hwang TH, O'Gorman WE, Bartlett DL, Sei S, Kanji F, et al. Rational strain selection and engineering creates a broad-spectrum, systemically effective oncolytic poxvirus, JX-963. J Clin Invest. 2007;117:3350–3358. doi: 10.1172/JCI32727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik AM, Chalikonda S, McCart JA, Xu H, Guo ZS, Langham G, et al. Intravenous and isolated limb perfusion delivery of wild type and a tumor-selective replicating mutant vaccinia virus in nonhuman primates. Hum Gene Ther. 2006;17:31–45. doi: 10.1089/hum.2006.17.31. [DOI] [PubMed] [Google Scholar]
- Guo ZS, Naik A, O'Malley ME, Popovic P, Demarco R, Hu Y, et al. The enhanced tumor selectivity of an oncolytic vaccinia lacking the host range and antiapoptosis genes SPI-1 and SPI-2. Cancer Res. 2005;65:9991–9998. doi: 10.1158/0008-5472.CAN-05-1630. [DOI] [PubMed] [Google Scholar]
- McCart JA, Ward JM, Lee J, Hu Y, Alexander HR, Libutti SK, et al. Systemic cancer therapy with a tumor-selective vaccinia virus mutant lacking thymidine kinase and vaccinia growth factor genes. Cancer Res. 2001;61:8751–8757. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.