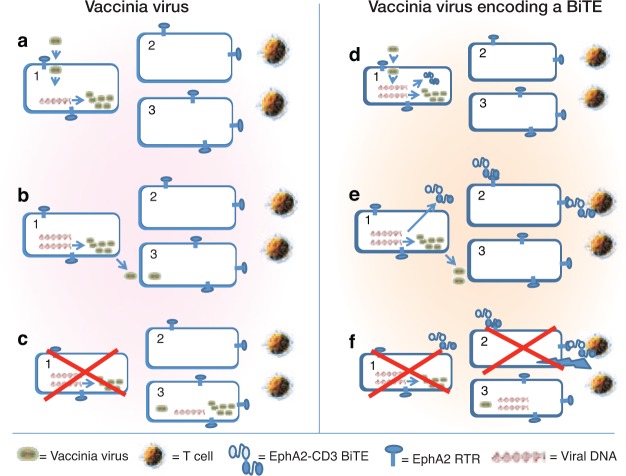
Two of the most exciting new areas of cancer therapy are the use of oncolytic viruses and immune-stimulating monoclonal antibodies. Each approach has shown great promise in animal models and some successes in early clinical trials, but there remain significant barriers to highly effective therapy. To date, the two technologies have been developed independently of each other, for the most part. However, in this issue of Molecular Therapy, Yu et al.1 describe experiments that combine the strengths of both approaches by creating an oncolytic vaccinia virus (VV) that has the ability to home to tumors where it can replicate and induce tumor lysis but also has the ability to secrete a bispecific T-cell engager (BiTE) that can bind T cells to tumor cells and additionally induce immune-mediated tumor cell destruction (Figure 1).
Figure 1.
Mechanism of action of a vaccinia vector vs. a vaccinia-BiTE vector. (a–c) Vaccinia vector. (a) After viral infection of tumor cell 1, the viral DNA directs production of new virus. (b) New virus that is produced and released can infect some of the neighboring tumor cells (cell 3). (c) The originally infected tumor cell (cell 1) is killed through viral oncolysis. Cell 3 is now infected and will undergo the same cycle of viral release and death with some neighbor cell killing until the process is extinguished by the immune system. The uninfected tumor cell 2 is not damaged. (d–f) Vaccinia virus encoding a bispecific T-cell engager (BiTE). (d) After viral infection of tumor cell 1 the viral DNA directs production of new virus plus production of BiTEs. (e) New virus produced and released can infect some of the neighboring tumor cells (cell 3). Soluble BiTEs are also released that can link the EphA2 surface receptor to T cells on cell 2. (f) The originally infected tumor cell (cell 1) is killed through viral oncolysis. Cell 3 is now infected and will undergo the same cycle of viral release and death with some neighbor cell killing until the process is extinguished by the immune system. In this case, however, the uninfected tumor cell 2 is now killed by T cell–mediated lysis. A bystander effect is thus created, amplifying the effects of the VV used alone.
Oncolytic viruses, which are capable of selective or preferential replication within tumor cells, have been studied intensively over the past 15 years.2 A large variety of such viruses have been studied in both the preclinical and clinical settings. Both naturally occurring viruses with intrinsic tumor selectivity (such as reovirus, vesicular stomatitis virus, and Newcastle disease virus) and viruses genetically engineered to enhance tumor specificity (such as adenovirus, herpes simplex virus, measles virus, polio virus, and VV) are being developed.3 One of the most promising approaches has been the use of oncolytic VVs,4 as this viral backbone is capable of rapid replication and spread that leads to profound localized tissue damage, and it has evolved to disseminate through the bloodstream and to induce a targeted immune response. Its safety was demonstrated during the smallpox-eradication program, and strains highly selective for tumor replication have been reported.5,6 This viral backbone is the area of focus of the paper by Yu et al.1
An important determinant in the success of any oncolytic virus is its interaction with the immune system. Not unexpectedly, injection of a virus induces strong innate and acquired immune responses with the goal of eradicating the virus. This immunologically mediated destruction of the virus and virally infected cells limits the ability of the virus to persist and spread, thus restricting its oncolytic potential and antitumor efficacy. It is therefore not surprising that the immune system was initially seen as a barrier that needed to be overcome in oncolytic viral therapy. However, it has more recently become clear that the relationship of the immune system to oncolytic virus therapy is far more complex.7 The key observation was that destruction of tumor cells in a highly immunogenic environment saturated with high levels of exogenous (viral) and endogenous danger signals was an ideal environment in which to generate and attract polyclonal antitumor CD8+ T cells. Further experimentation has confirmed that this induction of antitumor immune responses is important, and in some cases is the most important mechanism by which oncolytic viruses eliminate tumors.8
Finding the optimal balance between induction of antitumor immune responses and minimal inhibition of antiviral responses has thus become a goal of the field. Some of the most promising results in this area have come from studies using modified VVs.4 VV has been extensively used in immunotherapy studies. It has a large cloning capacity for cytokine or antigen expression, a long track record of safety when used as a vaccine, and a well-defined immune response. As such, a variety of transgenes designed to enhance the antitumor immune response induced by oncolytic VV have been examined. The most successful approach to date has been insertion of the gene encoding the immune-activating cytokine granulocyte–macrophage colony-stimulating factor (GM-CSF).9 Secretion of GM-CSF by tumor cells has long been known to reduce tumor formation in mice,10 probably through its ability to attract and activate dendritic cells. Genetic incorporation of GM-CSF into irradiated tumor cells has been used in the vaccine setting and is being tested in clinical trials with some success.11 With the same rationale, the GM-CSF gene was engineered into a modified VV and developed as an oncolytic agent (JX-594).9 JX-594 has moved to clinical trials, where it has shown potential in hepatocellular carcinomas12 and is now in phase III clinical testing. Other immune-activating cytokines and chemokines—including interferon-β (refs. 6, 8) and CCL5 (ref. 13)—have also been introduced into VVs and have shown enhanced efficacy in preclinical models.
However, because mediators such as GM-CSF, IFN-β, and CCL5 are generalized activators of the immune system and have relatively little direct antitumor effect, they have at least three potential limitations. First, their efficacy is dependent on the development or enhancement of endogenous antitumor responses (which may be absent or compromised in many tumor patients). Second, the inflammation they produce may enhance viral clearance. Third, these activators may potentially trigger protumor immune effects (e.g., GM-CSF has been shown to induce the production of myeloid-derived suppressor cells). It would therefore be an advance if a VV cargo could be developed that would specifically induce antitumor immune responses without stimulating innate or acquired antiviral immune responses.
It is in this context that Yu et al.1 report experiments describing a VV designed to secrete a BiTE antibody (summarized in Figure 1). BiTEs consist of a tumor-targeting single-chain variable antibody fragment (scFv) translated in tandem with another scFv directed against the T-cell activation ligand, CD3 (ref. 14). These bispecific antibodies bind to both tumor-specific epitopes and the T-cell receptor (TCR) on nearby lymphocytes. Crosslinking of the TCR initiates an immunological synapse and triggers T-cell activation with subsequent release of proteases and cytokines that kill tumor cells. The T cells do not need to be preactivated, costimulation does not appear to be necessary, and serial lysis by engaged T cells has been observed. They are thus extremely potent, with in vitro activity in the picomolar range. BiTEs directed against CD19 (to target B-cell malignancies) have shown success in clinical trials.15 Other targets on tumor cells being explored include EpCAM, EGFRvIII, and EphA2 (refs. 14, 16). Despite great promise, however, there are still significant challenges in the administration of BiTes, including (i) the need for continuous intravenous administration by infusion pumps, (ii) difficulties in the manufacturing of these biological agents from mammalian producer cells, and (iii) troublesome systemic side effects (e.g., flulike symptoms and some worrisome central nervous system effects).
Yu et al.1 hypothesized that an oncolytic VV encoding a BiTE targeting the tumor surface antigen EphA2 would result in enhanced antitumor efficacy due to oncolysis plus bystander immune killing. They therefore generated a double-deleted VV (lacking the TK and VGF genes) expressing an EphA2-CD3 BiTE or control green fluorescent protein (GFP) by cloning these genes into the TK gene locus under the control of the F17R late promoter to allow sufficient viral replication before T-cell activation. Their tumor target was the human A549 lung cancer cell line, which expresses EphA2. They found that the BiTE did not interfere with either viral replication or the ability of the virus to induce A549 tumor cell lysis with no T cells present. Supernatant from the VV-BiTE–infected cells, but not VV-GFP–infected cells, was able to activate the T cells and induce T cell–mediated killing of uninfected A549 cells. Importantly, A549 lung cancer cells infected with the VV-BiTE in the presence of human T cells were killed more efficiently than A549 cells infected with the VV-GFP virus, establishing the bystander effect. Finally, the effect of the VV-BiTE virus was studied in two animal models. First, A549 cells were mixed with human peripheral blood mononuclear cells (PBMCs) and injected into the flanks of immunodeficient mice. Second, A549 cells were injected intravenously (to form lung tumors). Seven days later, the mice were injected intravenously with PBMCs alone, VVs alone, or an admixture of PBMCs with VVs. In both cases, systemic administration of VV-BiTEs, but not VV-GFP, resulted in significantly increased survival compared with controls.
There are some limitations to this study. The authors studied only one BiTE target and one tumor cell target. Also, the animal models used were artificial in that PBMCs were coinjected with either the tumor cells or the virus. A more realistic model would require VV-BiTE treatment in mice in which the tumors are infiltrated with human T cells. It is also not clear how effectively BiTEs will activate tumor-infiltrating lymphocytes that show profound hypofunction and proximal defects in their TCR signaling machinery.17
However, even given these limitations, the findings by Yu et al.1 show the promise of combining two promising and previously separate new forms of anticancer therapy. By using the VV as a “carrier” and localized expression factory, it should be possible to overcome some of the limitations of systemically injected BiTEs by ensuring very high local concentrations, which should minimize systemic side effects and may enhance efficacy. On the other hand, because the BiTEs should induce only minimal antiviral effects (perhaps due to local cytokine production), the oncolytic effects of the VV should be undiminished and perhaps allow persistence. Further exploration of the merger of these two new promising antitumor technologies seems warranted.
References
- Yu F, Wang X, Guo ZS, Bartlett DL, Gottschalk SM., and, Song X-T. T-cell engager-armed oncolytic vaccinia virus significantly enhances antitumor therapy. Mol Ther. 2014;22:102–111. doi: 10.1038/mt.2013.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eager RM., and, Nemunaitis J. Clinical development directions in oncolytic viral therapy. Cancer Gene Ther. 2011;18:305–317. doi: 10.1038/cgt.2011.7. [DOI] [PubMed] [Google Scholar]
- Guo ZS, Thorne SH., and, Bartlett DL. Oncolytic virotherapy: molecular targets in tumor-selective replication and carrier cell-mediated delivery of oncolytic viruses. Biochim Biophys Acta. 2008;1785:217–231. doi: 10.1016/j.bbcan.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirn DH., and, Thorne SH. Targeted and armed oncolytic poxviruses: a novel multi-mechanistic therapeutic class for cancer. Nat Rev Cancer. 2009;9:64–71. doi: 10.1038/nrc2545. [DOI] [PubMed] [Google Scholar]
- McCart JA, Ward JM, Lee J, Hu Y, Alexander HR, Libutti SK.et al. (2001Systemic cancer therapy with a tumor-selective vaccinia virus mutant lacking thymidine kinase and vaccinia growth factor genes Cancer Res 618751–8757. [PubMed] [Google Scholar]
- Kirn DH, Wang Y, Le Boeuf F, Bell J., and, Thorne SH. Targeting of interferon-beta to produce a specific, multi-mechanistic oncolytic vaccinia virus. PLoS Med. 2007;4:e353. doi: 10.1371/journal.pmed.0040353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestwich RJ, Ilett EJ, Errington F, Diaz RM, Steele LP, Kottke T.et al. (2009Immune-mediated antitumor activity of reovirus is required for therapy and is independent of direct viral oncolysis and replication Clin Cancer Res 154374–4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LC, Lynn RC, Cheng G, Alexander E, Kapoor V, Moon EK.et al. (2012Treating tumors with a vaccinia virus expressing IFNβ illustrates the complex relationships between oncolytic ability and immunogenicity Mol Ther 20736–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Oh JY, Park BH, Lee DE, Kim JS, Park HE.et al. (2006Systemic armed oncolytic and immunologic therapy for cancer with JX-594, a targeted poxvirus expressing GM-CSF Mol Ther 14361–370. [DOI] [PubMed] [Google Scholar]
- Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K.et al. (1993Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity Proc Natl Acad Sci USA 903539–3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFrancesco L. Landmark approval for Dendreon's cancer vaccine. Nat Biotechnol. 2010;28:531–532. doi: 10.1038/nbt0610-531. [DOI] [PubMed] [Google Scholar]
- Heo J, Reid T, Ruo L, Breitbach CJ, Rose S, Bloomston M.et al. (2013Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer Nat Med 19329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, O'Malley M, Urban J, Sampath P, Guo ZS, Kalinski P.et al.2011Chemokine expression from oncolytic vaccinia virus enhances vaccine therapies of cancer Mol Ther 19650–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi BD, Cai M, Bigner DD, Mehta AI, Kuan CT., and, Sampson JH. Bispecific antibodies engage T cells for antitumor immunotherapy. Expert Opin Biol Ther. 2011;11:843–853. doi: 10.1517/14712598.2011.572874. [DOI] [PubMed] [Google Scholar]
- Bargou R, Leo E, Zugmaier G, Klinger M, Goebeler M, Knop S.et al.2008Tumor regression in cancer patients by very low doses of a T cell-engaging antibody Science 321974–977. [DOI] [PubMed] [Google Scholar]
- Hammond SA, Lutterbuese R, Roff S, Lutterbuese P, Schlereth B, Bruckheimer E.et al. (2007Selective targeting and potent control of tumor growth using an EphA2/CD3-bispecific single-chain antibody construct Cancer Res 673927–3935. [DOI] [PubMed] [Google Scholar]
- Vazquez-Cintron EJ, Monu NR., and, Frey AB. Tumor-induced disruption of proximal TCR-mediated signal transduction in tumor-infiltration CD8+ lymphocytes inactivates antitumor effector phase. J Immunol. 2010;185:7133–7140. doi: 10.4049/jimmunol.1001157. [DOI] [PMC free article] [PubMed] [Google Scholar]