Abstract
Respiratory syncytial virus (RSV) is a major cause of infectious lower respiratory disease in infants and the elderly. As there is no vaccine for RSV, we developed a genetic vaccine approach that induced protection of the entire respiratory tract from a single parenteral administration. The approach was based on adenovirus vectors derived from newly isolated nonhuman primate viruses with low seroprevalence. We show for the first time that a single intramuscular (IM) injection of the replication-deficient adenovirus vectors expressing the RSV fusion (F0) glycoprotein induced immune responses that protected both the lungs and noses of cotton rats and mice even at low doses and for several months postimmunization. The immune response included high titers of neutralizing antibody that were maintained ≥24 weeks and RSV-specific CD8+ and CD4+ T cells. The vectors were as potently immunogenic as a human adenovirus 5 vector in these two key respiratory pathogen animal models. Importantly, there was minimal alveolitis and granulocytic infiltrates in the lung, and type 2 cytokines were not produced after RSV challenge even under conditions of partial protection. Overall, this genetic vaccine is highly effective without potentiating immunopathology, and the results support development of the vaccine candidate for human testing.
Introduction
Respiratory syncytial virus (RSV) is a major cause of disease and hospitalization in infants and young children worldwide,1 resulting in >3.4 million hospitalizations and >200,000 deaths globally.2 Medically attended RSV pediatric disease in the USA exceeds $1 billion in direct medical costs annually.3 RSV infections also cause significant mortality and morbidity in the elderly and other high-risk adults.4,5 Synagis immunoprophylaxis reduces hospitalization rates, but it is available only for infants with identified risk factors for severe RSV disease.1,6 Thus, development of a vaccine would benefit both infant and adult populations.
Clinical trials of a formalin-inactivated RSV vaccine (FI-RSV) in infants did not protect against infection, but increased disease severity.7 Over the subsequent 50 years, multiple vaccine strategies have been investigated in preclinical and limited clinical testing.1,8 These vaccines generally have not progressed to clinical evaluation or have met with limited success in human trials. Progress has been hampered by limited immunogenicity, induction of Th2-biased immunity, or unacceptable levels of adverse events. Natural RSV infection does not induce long-term protection,9 possibly due to the ability of RSV to suppress or evade host immunity.10 While not long-lived, the ability of maternally transferred antibodies and passive administration of antibody products to protect infants demonstrates the importance of neutralizing antibody in protection against RSV disease.11,12,13 These results define features of an effective RSV vaccine: exclusion of immunosuppressive RSV proteins, induction of potent neutralizing antibodies, and prevention of memory immune responses producing type 2-associated and proinflammatory cytokines, which correlate with RSV disease potentiation. Thus, an effective RSV vaccine will combine an antigen and a delivery system that induce potent neutralizing antibodies and Th1-biased cellular immune responses. Replication-defective adenovirus–vectored vaccines have induced antibody responses as well as CD8+ and Th1 T-cell responses in clinical vaccine trials.14,15 Surprisingly, replication-deficient adenovirus vectors have not been well tested for RSV, limited to serotype 5 vectors in mouse protection models only.16,17,18 Thus, the true potential for replication-deficient adenovirus–vectored vaccines for RSV has not been evaluated in preclinical or clinical testing.
A limitation of Ad5 vaccine vectors is that 40–90% of the global population has systemic neutralizing antibody from natural infection. While vaccine trial volunteers possessing high titers of Ad5 neutralizing antibodies generated significant antigen-specific humoral and cellular responses, the magnitude and frequency of T-cell responses and innate immune responses were lower than those observed in Ad5-seronegative volunteers.14,15 Alternative vectors based on serotypes with low seroprevalence have been engineered, but they were generally less potent than Ad5-based vectors.19,20,21,22 Only two nonhuman primate adenovirus vectors have been comparable to Ad5.23,24,25
We have isolated novel and genetically distinct adenoviruses from wild gorillas (data not shown) that have low human seroprevalence. As the RSV fusion (F0) glycoprotein is relatively conserved across RSV A and B strains26 and preclinical and clinical data with Synagis demonstrate that RSV F-specific antibody is effective independent of RSV strain,13 the adenoviruses were engineered to be replication-defective and express RSV F0. The GC44.F0, GC45.F0, and GC46.F0 vectors, evaluated as candidate vaccines in cotton rat and mouse models, elicited protective antibody and T-cell immunity. Detailed evaluation of GC46.F0 immunogenicity showed a single intramuscular (IM) immunization protected both the upper and lower respiratory tracts from RSV challenge with no evidence of vaccine-enhanced disease. Antibody responses were durable and broadly protective. Thus, a vaccine design has been identified which will not be hampered by pre-existing immunity in the human population and which will rapidly generate effective and safe immunity, allowing development of a universal RSV vaccine for use in young infants.
Results
A single dose of GC44.F0, GC45.F0, or GC46.F0 was immunogenic and protective
Cotton rats were immunized with GC44.F0, GC45.F0, GC46.F0, Ad5.F0, and RSV. Each adenovirus vector elicited neutralizing antibody responses at high and low doses (Figure 1a) with only animals immunized with 107 particle units (PU) of GC45.F0 having IC50 titers significantly less than RSV-immunized controls. Neutralizing antibody titers trended higher at the higher dose with significant differences between 107 and 109 PU doses in the GC45.F0-immunized cotton rats. RSV titers in the lung were examined 5 days after challenge, and RSV was not detected in cotton rats immunized with RSV or any adenovirus vector (Figure 1b). In a parallel experiment, BALB/c mice immunized with 107 or 109 PU GC44.F0, GC45.F0, or GC46.F0 had no RSV in the lung tissue at 5 days post-RSV challenge except one of five mice in the 107 PU GC45.F0 group (Figure 1c).
Figure 1.
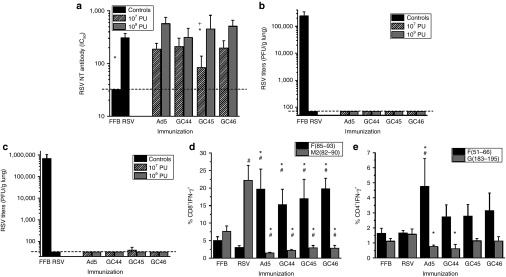
GC44.F0, GC45.F0, and GC46.F0 elicit antibody and T-cell responses which protected against RSV challenge. (a,b) Cotton rats and (c–e) mice were immunized intramuscularly with 107 or 109 PU adenovirus-F vector and challenged intranasally with RSV A2 6 weeks later. (a) Neutralizing serum antibody titers (IC50) were measured 4 weeks postimmunization by plaque reduction neutralization test assay. Limit of detection = 20, denoted by dashed line. (b,c) RSV titers in the lungs at 5 days postchallenge were measured by plaque assay. Limit of detection = 70 PFU/g lung, denoted by dashed line. (d,e) RSV-specific IFN-γ production by (d) CD8+ and (e) CD4+ T cells was evaluated at day 5 postchallenge. N = 5 animals per group. Statistical significance determined by one-way analysis of variance, where P < 0.05. *Statistically different from RSV-immunized animals; +Statistically different between doses of same immunogen; #Statistically different from FFB-immunized animals. FFB, final formulation buffer; IFN, interferon; NT, neutralizing; PFU, plaque-forming units; PU, particle units; RSV, respiratory syncytial virus.
RSV-specific T-cell responses were examined by intracellular interferon-γ (IFN-γ) staining of peptide-stimulated lung lymphocytes. In BALB/c mice immunized with GC44.F0, GC45.F0, GC46.F0, or Ad5.F0, significantly greater percentages of CD8+ T cells stimulated with F85–93 peptide produced IFN-γ than did cells from RSV-immunized mice (Figure 1d). F51–66-specific CD4+ T-cell responses were greater in adenovirus vector-immunized mice with levels in Ad5.F0-immunized mice statistically greater than in RSV-immunized animals (Figure 1e). The propensity of RSV to induce weak memory T-cell responses is demonstrated by the minimal responses in peptide-stimulated CD8+ and CD4+ T cells from RSV-immunized mice where only the CD8 M282–90-specific is greater in RSV-immunized mice than in final formulation buffer (FFB)-immunized negative control mice (Figure 1d,e). The lack of responses in the adenovirus vector-immunized mice to the RSV M282–90 CD8 (Figure 1d) and the G183–195 CD4 (Figure 1e) peptides confirms that the F85–93 CD8 and F51–66 CD4 responses are vaccine-induced and not primary responses to the RSV challenge. Thus, the GC44.F0, GC45.F0, and GC46.F0 vectors induced protective neutralizing antibody titers comparable to RSV immunization with higher cellular responses.
GC44, GC45, and GC46 have low seroprevalence in humans
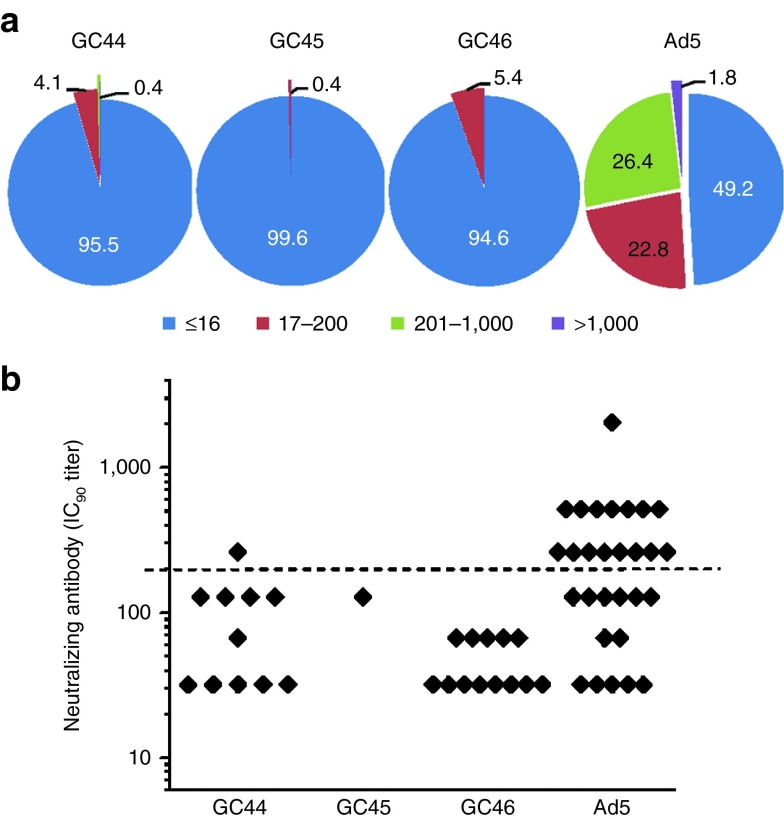
The prevalence of pre-existing immunity to the GC vectors was estimated for the US population by viral neutralization assays based on luciferase transgene activity.27 Human serum samples from five regions of the USA were tested for GC44 (n = 245), GC45 (n = 245), and GC46 (n = 240) neutralization.27 Greater than 94% of the serum samples were negative (IC90 titers ≤16) for neutralization of GC44, GC45, and GC46 with seroprevalence frequencies of 4.5, 0.4, and 5.4%, respectively (Figure 2a). In addition, the IC90 titers of the positive sera were low. Titers for GC45 and GC46 neutralization were all <200 and only one sample had a GC44 IC90 titer >200 (Figure 2b). Titers <200 may not be inhibitory in vivo.23 In comparison, 50.8% were positive for neutralization of Ad5, with 55.2% of the positive samples having endpoint titers >200 (Figure 2b).
Figure 2.
Pre-existing immunity to GC44, GC45, and GC46 was rare and weak. Seroprevalence in human sera was determined by neutralization assay. (a) Frequency of neutralizing titers to Ad5, GC44, GC45, and GC46. (b) IC90 titers of seropositive (IC90 > 16) samples. For Ad5, endpoint titrations were performed on 37 randomly chosen seropositive samples. Dashed line = IC90 of 200, a predicted threshold for inhibition in vivo. Each serum sample was assayed in triplicate.
GC46.F0 protected upper and lower respiratory tracts without enhanced disease in the cotton rat model
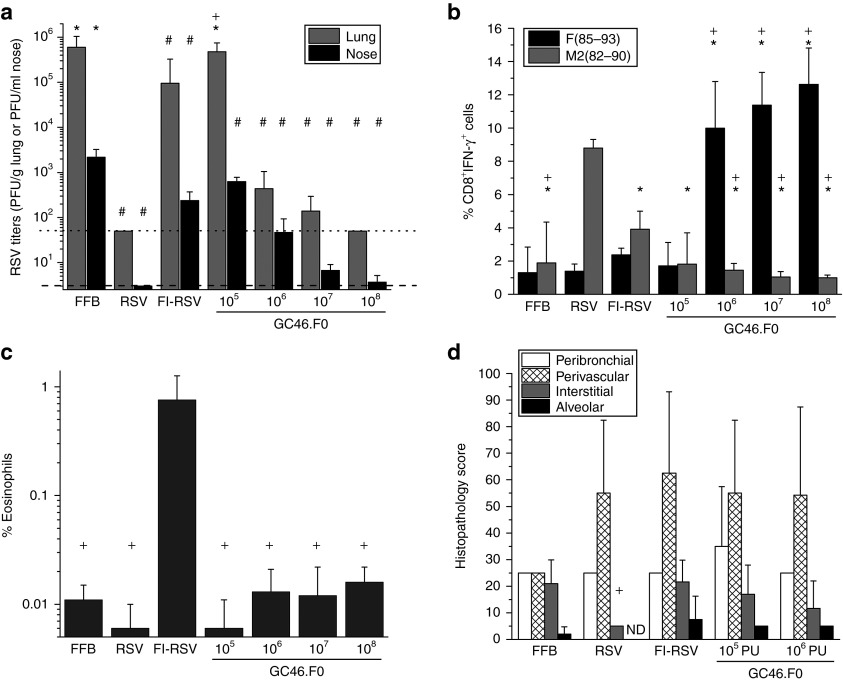
Based on low seroprevalence in humans and the strong induction of protective antibody and T-cell responses in cotton rats and mice, GC46.F0 was chosen for further analysis. The cotton rat model is considered a sensitive model for RSV challenge studies and detection of RSV vaccine-enhanced disease by histology.28 Based on prior cotton rat immunizations (Figure 1), cotton rats were immunized with a protective dose of 109 PU and a low dose of 106 PU and controls were immunized with FFB, RSV, or two IM injections of FI-RSV Lot 100. Sera were collected at day 28 (prior to second FI-RSV injection) and at day 56 (prior to challenge), and neutralizing antibody activity was assessed by plaque reduction neutralization test. At days 28 and 56 postimmunization, neutralizing antibody IC50 titers in cotton rats immunized with 109 PU GC46.F0 were the same as titers in RSV-immunized cotton rats (Figure 3a). Animals immunized with 106 PU GC46.F0 had significantly higher titers than FFB- and FI-RSV–immunized cotton rats and significantly lower titers than GC46.F0 109 PU or RSV-immunized groups. At day 5 postchallenge with 105 plaque-forming units (PFU) of RSV A/Long, RSV titers in the lungs of GC46.F0-, RSV-, and FI-RSV–immunized cotton rats were reduced to undetectable levels and were significantly lower than in FFB-immunized cotton rats with no statistically significant differences between these four immunization groups (Figure 3b). The nasal RSV viral loads in the 109 PU GC46.F0 and RSV groups were reduced ~1,000-fold to undetectable levels, while immunization with the low dose of GC46.F0 partially protected the nose from RSV challenge (significantly lower than the FFB-immunized group).
Figure 3.
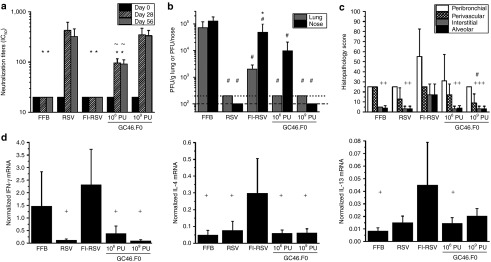
GC46.F0 induced protective immunity without enhanced immunopathology in cotton rats. Animals were immunized intramuscularly with GC46.F0 and challenged with 105 PFU RSV A/Long 8 weeks later. (a) Neutralizing serum antibody (IC50) responses were measured by plaque reduction neutralization test in sera obtained before immunization (week 0) and at 28 and 56 weeks after immunization. (b) RSV titers in the nose and lung measured by plaque assay at 5 days postchallenge. Limits of detection are 200 PFU/g lung and 100 PFU/nose as denoted by dotted and dashed lines, respectively. (c) Inflammation severity was measured by histopathology scoring of peribronchiolar, perivascular, interstitial, and alveolar regions 5 days postchallenge. (d) IFN- γ, IL-4, and IL-13 production was measured by quantitative reverse transcription PCR of lung tissue 5 days postchallenge. N = 5 cotton rats per group. Statistical significance determined by one-way analysis of variance, where P < 0.05. *Statistically different from RSV-immunized cotton rats; +Statistically different from FI-RSV–immunized cotton rats; #Statistically different from FFB-immunized cotton rats; ~Statistically different from 109 PU GC46.F0-immunized cotton rats. FFB, final formulation buffer; FI-RSV, formalin-inactivated respiratory syncytial virus vaccine; IFN, interferon; IL, interleukin; PFU, plaque-forming units; PU, particle units; RSV, respiratory syncytial virus.
At day 5 postchallenge, pulmonary inflammation was graded in four areas of tissue – peribronchiolar, perivascular, interstitial, and alveolar – to measure disease severity. The pathology scores for GC46.F0 immunization were the same or lower than for RSV and FFB immunization (Figure 3c). Cotton rats immunized with 109 PU GC46.F0 had significantly less perivasculitis than did those immunized with FFB or FI-RSV. Interstitial pneumonia and alveolitis were significantly greater in FI-RSV–immunized cotton rats, demonstrating vaccine-enhanced disease in this model (Figure 3c). The increased interstitial pneumonia and alveolitis in FI-RSV–immunized cotton rats paralleled greater levels of the type 2 cytokines: interleukin-4 and interleukin-13 (Figure 3d), all hallmarks of vaccine-enhanced disease in the RSV cotton rat model.29 Production of the inflammatory cytokine IFN-γ was also significantly increased in FI-RSV and FFB-immunized cotton rats (Figure 3d) with IFN-γ levels reflecting the degree of RSV replication (Figure 3b). The histopathology and cytokine mRNA assessments are consistent with the conclusion that immunization with GC46.F0 did not predispose for enhanced disease in the cotton rat model.
GC46.F0 induced durable protective immunity in the cotton rat model
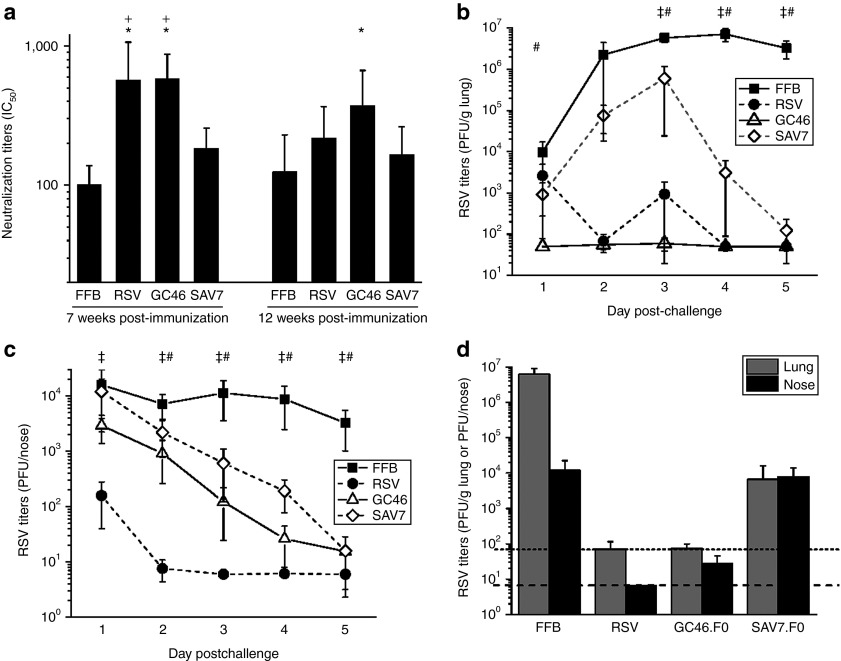
Cotton rats were immunized with GC46.F0, RSV, or FI-RSV, sera were collected periodically after immunization, and the animals were challenged at 24 weeks postimmunization. RSV-specific neutralizing antibody responses peaked by 4 weeks postimmunization in all groups (Figure 4a). While IC50 antibody titers began to decrease by week 16 postimmunization in FI-RSV–immunized animals, antibody titers were maintained in GC46.F0- and RSV-immunized cotton rats through at least 24 weeks after immunization. The cotton rats were challenged with 106 PFU of RSV A2, and viral titers in the lung and nose measured at day 5 postchallenge. Lungs of GC46.F0- and RSV-immunized animals were fully protected against challenge with no RSV detected after challenge (Figure 4b). To assess protection of the nose, GC46.F0- and RSV-immunized groups were compared with the FI-RSV–immunized group. The nasal RSV titers in the two dose groups immunized with GC46.F0 and in the RSV group were significantly lower than the titers in the FI-RSV group (Figure 4c). Full protection of the nasal tissues was observed in 60% of RSV-immunized cotton rats and in 18% of animals immunized with 109 PU GC46.F0. The results in the cotton rat model demonstrate that GC46.F0 generated protective immunity without disease potentiation and protection was durable, characterized by stable neutralizing antibody titers across 24 weeks.
Figure 4.
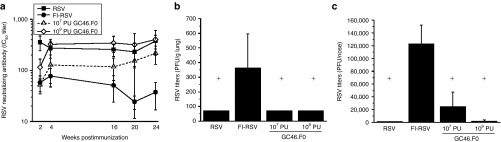
GC46.F0 induces durable immunity that is protective. Cotton rats were immunized with 107 or 109 PU GC46.F0. (a) Neutralizing serum antibody (IC50) titers were measured by plaque reduction neutralization test assay at the indicated times after immunization. (b,c) Animals were challenged with 106 PFU RSV A2 24 weeks postimmunization. RSV titers in the (b) lung and (c) nose were measured 5 days after challenge by plaque assay. N = 4 to 6 cotton rats per group. Statistical significance determined by one-way ANOVA where P < 0.05. *Statistically different from RSV-immunized cotton rats; +Statistically different from FI-RSV–immunized cotton rats. FI-RSV, formalin-inactivated respiratory syncytial virus vaccine; PFU, plaque-forming units; PU, particle units; RSV, respiratory syncytial virus.
GC46.F0 induced broadly reactive neutralizing antibodies
Neutralization assays for the in vivo experiments described above were performed with the lab-adapted RSV strains A2 or Long. Sera from cotton rats immunized with GC46.F0 were characterized for breadth of neutralizing activity against RSV B strains and clinical isolates. The mean IC50 titers were comparable for lab-adapted strains RSV A2, RSV B 18537, and RSV B West Virginia (WV), the parental strain for B1 (Figure 5a). The IC50 titers of the sera from animals immunized with 109 PU of GC46.F0 were 508 ± 156, 743 ± 129, and 423 ± 69, respectively (Figure 5a). In contrast to lab-adapted strains, naturally circulating viruses may be more difficult to neutralize.30,31 Neutralization of RSV clinical isolates was determined in a microneutralization assay. In this assay format, both RSV A2 and WV were neutralized by sera from RSV- and GC46.F0-immunized cotton rats and not by sera from FFB-immunized animals (Figure 5b). The RSV G sequences have been determined for the clinical isolates. Phylogenetic comparisons of these sequences demonstrated that the seven RSV isolates are genetically distinct.32,33 Microneutralization analyses demonstrated that all seven clinical isolates were neutralized by GC46.F0 sera (Figure 5c). Thus, GC46.F0 induces broad and potent serum neutralizing activity.
Figure 5.
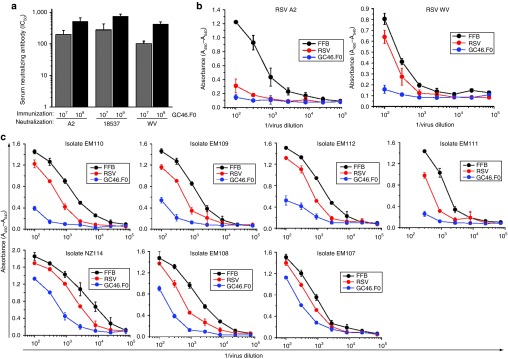
GC46.F0-induced antibody responses have a great breadth of reactivity. (a) The ability of cotton rat antisera generated in the experiment shown in Figure 1 was evaluated for the ability to neutralize lab-adapted RSV A2 and RSV B 18537 and WV strain viruses by plaque reduction neutralization test assay. N = 5 individual sera per group. (b,c) Cotton rat antisera (a pool of 10 animals per immunogen) were evaluated for the ability to neutralize lab strains (b) RSV A2 and RSV B WV and (c) seven genetically distinct RSV clinical isolates by microneutralization assay. FFB, final formulation buffer; RSV, respiratory syncytial virus; WV, West Virginia.
GC46.F0 protected upper and lower respiratory tracts without enhanced disease in the mouse model
Although human RSV does not replicate in murine respiratory tracts as well as in cotton rats, the mouse model has a few advantages, including ease of handling and the ability to readily conduct cellular immune response analyses. We used the BALB/c mouse model for broader assessments of dose, protection, cellular immune responses, and kinetics of RSV replication in the lung and nose. A sensitive approach for determining if immunization of animals potentiates for enhanced disease is to induce partial immunity, allowing the challenge virus to replicate and thereby increasing the antigen load in the lung. Therefore, a broad dose range of 105–108 PU of GC46.F0 was examined in mice to determine upper and lower respiratory tract protection as well as RSV disease potentiation. BALB/c mice were immunized, challenged with RSV 4 weeks later, and RSV viral loads determined in the lung and nasal tissues 5 days postchallenge. Viral loads in both tissues were significantly lower in GC46.F0-immunized groups, except the lung tissues from the lowest dose group, 105 PU (Figure 6a). RSV was not detectable in the lung tissue of animals immunized with 108 PU of GC46.F0 and only very low levels of RSV were detected in nasal tissue in two of five animals with a group mean viral load of 3.7 ± 1.5 PFU/ml in nose tissue. Similarly, the mean lung viral loads in the 107 and 106 PU GC46.F0-immunized groups were 1,000-fold lower than in the buffer group with three out of five and four out of six animals having no detectable RSV, respectively. The reduction in nasal virus load was also significant, with 325- and 46-fold reductions in the 107 and 106 PU GC46.F0-immunized groups, respectively. Neutralizing antibody titers could not be determined due to high background inhibition in preimmunization mouse sera (IC50 titers of 120 ± 39, n = 12). Immunization with GC46.F0 induced RSV F-specific CD8+ and CD4+ T cells in all groups. At day 5 postchallenge, levels of IFN-γ–producing CD8+ T cells were significantly greater in mice immunized with 106, 107, or 108 PU GC46.F0 than in FFB-, RSV-, or FI-RSV–immunized mice, but not in mice immunized with 105 PU GC46.F0 (Figure 6b). Similarly, CD4+ T-cell responses were higher in mice immunized with 106, 107, or 108 PU GC46.F0 than in FFB- or RSV-immunized mice and mice immunized with 105 PU GC46.F0 (Supplementary Figure S1). When compared with responses in RSV- and FI-RSV–immunized animals, the relative lack of RSV M2-specific CD8+IFN-γ+ T cells (Figure 6b) and RSV G-specific CD4+IFN-γ+ T cells (Supplementary Figure S1) in GC46.F0-immunized mice demonstrates that the RSV F-specific responses are vaccine-induced and not primary immune responses resulting from RSV challenge. These data demonstrate that IM immunization with GC46.F0 induced RSV-specific immunity that protected the entire airway against RSV challenge.
Figure 6.
GC46.F0 induced protective immunity in mice without vaccine-enhanced disease. Mice were immunized intramuscularly with GC46.F0 and challenged with 9 × 106 PFU RSV A2 4 weeks later. (a) Five days postchallenge, RSV titers in the nose and lung were measured by plaque assay. Limits of detection are 70 PFU/g lung and 3.3 PFU/ml of homogenized nose tissue and are represented as dotted and dashed lines, respectively. (b) T-cell responses were assessed by RSV-specific ICS at day 5 postchallenge with CD8+IFN-γ+ shown. (c) Pulmonary eosinophils (represented as the percentage of eosinophils in the leukocyte gate) were quantitated by flow cytometry at day 8 after challenge. (d) Inflammation severity was measured by histopathology scoring of peribronchiolar, perivascular, interstitial, and alveolar regions. N = 5 or 6 mice per group per time point. Statistical significance determined by one-way analysis of variance, where P < 0.05. *Statistically different from RSV-immunized mice; +Statistically different from FI-RSV–immunized mice. FFB, final formulation buffer; FI-RSV, formalin-inactivated respiratory syncytial virus vaccine; ICS, intracellular cytokine staining; IFN, interferon; ND, none detected; PFU, plaque-forming units; PU, particle units; RSV, respiratory syncytial virus.
While histological correlates of vaccine-enhanced disease have been defined in the cotton rat model, the mouse model is more amenable to detailed phenotypic evaluation of cellular immune responses associated with FI-RSV vaccine–enhanced disease. Therefore, pulmonary histopathology was evaluated for eosinophilic infiltrates at 8 days postchallenge in BALB/c mice, and leukocytes isolated from lungs were stained for CD3, CD4, CD8, Gr1, and CCR3. Eosinophils, defined as CD3−CD4−CD8−Gr1+CCR3+, were significantly elevated only in FI-RSV–immunized mice with minimal eosinophil recruitment in lungs of mice immunized with any dose of GC46.F0 tested (Figure 6c). The lungs from GC46.F0 immunized animals that had the greatest RSV viral loads, i.e., the 105 and 106 PU groups, were assessed for inflammation and compared with lungs from RSV- and FI-RSV–immunized animals. In the histopathology assessment, the magnitude of pulmonary inflammation was similar (Figure 6d), but a greater frequency of eosinophils were noted in the peribronchiolar and perivascular infiltrates in FI-RSV–immunized mice with only mononuclear cells present in RSV- and GC46.F0-immunized mice (data not shown), consistent with the detection of eosinophils by flow cytometry (Figure 6c). Thus, immunization with low doses of GC46.F0 which provided only partial protection against challenge did not predispose for allergic inflammation in BALB/c mice.
GC46.F0 immunization affected the RSV challenge virus differently in the lung and nose
The reduction of RSV viral load at 5 days postchallenge in GC46.F0-immunized animals could be due to prevention of RSV infection or to more rapid clearance of RSV from the tissues. BALB/c mice were immunized with 108 PU of the adenovirus vectors GC46.F0, to induce high neutralizing antibody titers, or SAV7.F0, to induce low neutralizing antibody titers (data not shown). One set of mice were challenged with RSV A2 at 7 weeks postimmunization, and RSV viral loads were determined every 24 hours from day 1 through 5 postchallenge to characterize the protective response. In addition, to determine the durability of mucosal protection, separate groups of mice were challenged at 12 weeks postimmunization and viral loads determined at 5 days postchallenge. Prior to challenge, the GC46.F0 and RSV groups had comparable serum neutralizing antibody titers, and neutralizing antibody titers induced by SAV7.F0 were significantly lower (Figure 7a). When challenged at 7 weeks postimmunization, RSV was not detectable 24 hours postchallenge in the lungs of GC46.F0-immunized mice, whereas RSV was present (2649 ± 4973 PFU/g lung tissue) in all animals immunized intranasally with RSV (Figure 7b). The viral loads in these two groups remained low; on day 2, RSV was not detectable in 40 and 80% of RSV- and GC46.F0-immunized animals, respectively. Similarly, on day 3, RSV was not detectable in 60% of lung samples from both groups, and RSV was not detectable in the lungs from both groups on days 4 and 5 postchallenge. SAV7 immunization resulted in a different pattern of pulmonary RSV replication. Viral loads increased from day 1 through 3 postchallenge and then declined to undetectable levels in four out of five animals at day 5 postchallenge, consistent with a lack of protective antibody and with clearance mediated by T cells. Adenovirus vector immunization did not prevent the intranasal challenge dose of 5 × 106 PFU from establishing infection of the nose as evidenced by viral loads comparable to buffer immunization at 24 hours postchallenge (Figure 7c). However, viral loads were significantly reduced from day 2 through 5 postchallenge with only one nose sample having detectable RSV on day 5 in the GC46.F0 group. Protection against RSV challenge was also determined at 12 weeks postimmunization. At 5 days postchallenge, the viral load in GC46.F0-immunized animals was near the limit of detection and equal to the viral load in RSV-immunized mice (Figure 7d). The nasal RSV titers in GC46.F0-immunized mice challenged at 12 weeks postimmunization were comparable to challenge at 7 weeks postimmunization: 28 ± 17 and 15 ± 21 PFU/nose, respectively. In contrast, the RSV titer in nasal tissues in animals immunized with SAV7.F0 12 weeks before challenge was 200-fold higher than upon challenge at 7 weeks and when compared with titers in GC46.F0-immunized animal challenged at 7 or 12 weeks after immunization. Thus, GC46.F0 induced high titers of neutralizing antibody and mucosal protection that was durable through at least 3 months. Taken together, these data demonstrate that GC46.F0 immunization prevented RSV replication in the lung and increased clearance in nasal tissues.
Figure 7.
GC46.F0 induces protective immunity rapidly, clears RSV infection, and is durable. Mice were immunized intramuscularly with 106 PFU RSV A2, 108 particle units (PU) GC46.F0, or 108 PU SAV7.F0. (a) Serum was obtained at 7 and 12 weeks postimmunization, and neutralizing IC50 titers determined by plaque reduction neutralization test assay. N = 10 mice per group at each time point. *Statistically different from FFB-immunized mice; +Statistically different from SAV7.F0-immunized mice by one-way analysis of variance (ANOVA). (b,c) A subset of mice was challenged with 5 × 106 PFU RSV A2 at 7 weeks postimmunization. Mice were euthanized at days 1, 2, 3, 4, and 5 postchallenge, and RSV titers in the (b) lung and (c) nose were measured by plaque assay. N = 5 mice per group per time point, and limits of detection are 70 PFU/g lung and 6.7 PFU/nose. Statistical significance was determined by one-way ANOVA, where P < 0.05. ‡Statistically difference between FFB- and RSV-immunized mice; #Statistically difference between FFB-immunized mice and GC46.F0- or SAV7.F0-immunized mice. (d) A subset of mice was challenged with 5 × 106 PFU RSV A2 at 12 weeks postimmunization, then euthanized at day 5 postchallenge. RSV titers in the lung and nose were determined by plaque assay. Limits of detection are 70 PFU/g lung and 6.7 PFU/nose as denoted by dotted and dashed lines, respectively. N = 5 mice per group. FFB, final formulation buffer; PFU, plaque-forming units; RSV, respiratory syncytial virus.
Discussion
In this report, we describe the development of a novel nonhuman adenovirus vector, GC46, expressing the RSV F protein and demonstrate the generation of protective immunity with a single IM injection in two animal models. This immunization induced significant neutralizing serum antibody responses which were durable and had broad activity. Furthermore, the GC46.F0 vaccine elicited RSV F-specific CD4+ and CD8+ T cells. These antibody and T-cell responses were sufficient to protect both the upper and lower respiratory tracts against live RSV challenge with no evidence of vaccine-enhanced immunopathology in either cotton rat or mouse models.
Vaccine-induced antibodies must have strong neutralization activity to safely inhibit RSV infection, as illustrated by the poor neutralization capacity of FI-RSV–induced antibodies.34 The passively administered antibody Synagis was shown to protect cotton rats against RSV when the Synagis concentration in serum was 35 µg/ml.13 To relate our plaque reduction neutralization test assay to this protective activity in cotton rats, we determined that the IC50 titer of 40 µg/ml of Synagis for RSV A2 was 89 ± 5 (data not shown). In this report, the IC50 titers in GC46.F0-immunized cotton rats were in the range of 200–400, demonstrating that the vaccine-induced antibodies had significant neutralizing activity.
In animal models, a vaccine candidate does not need to generate protective responses better than RSV infection because the most effective immunization against RSV is infection by RSV. Immunity in humans induced by RSV infection is effective but short-lived. Immunization of adults with an Ad26 vector expressing a human immunodeficiency virus Env gene induced antibody responses that persisted for the duration of the study (1 year).35 The durability and breadth of the antibody responses induced by GC46.F0 in these animal models suggests that the vaccine has the potential to serve as a universal RSV vaccine in humans.
The mechanisms of RSV protection from GC46.F0 immunization are currently unknown, yet it is probably that both humoral and cellular responses play a role. First, there was an inverse relationship between the dose of GC46.F0 and RSV viral loads in both animal models. As the dose of GC46.F0 was increased, the plaque reduction neutralization test titers increased and nasal viral loads were reduced, but the magnitude of the T-cell responses did not change. In addition, lung and nasal protection persisted in GC46.F0-immunized animals, consistent with long-lived antibody responses. Second, the differences in RSV replication from day 1 through 5 postchallenge in immunized mice were consistent with antibody and cellular mechanisms of protection. RSV infection of the lung was blocked and viral replication was severely restricted by immunization with GC46.F0. This observed prevention of infection was probably mediated by antibody. The effect of GC46.F0 immunization on RSV replication in nasal tissue differed from the effect in lung tissue. Peak viral load occurred immediately postchallenge (24 hours) with continuous clearance of RSV from nasal tissues after the first 24 hours, consistent with a cellular mechanism of protection. Taken together, these data suggest that immunity induced by GC46.F0 prevented infection as well as contributed to clearance of RSV. Defining the contributions of B-cell and T-cell immunity to protection and characterization of the antibody repertoire induced by GC46.F0 immunization are active areas of research.
As with other nonhuman primate adenovirus vectors, there will be T-cell epitopes conserved between GC46 and human adenoviruses in structural proteins, particularly hexon. Thus, it is expected that many people with natural exposure to adenoviruses will have CD4+ T cells that recognize epitopes in the GC46 vector. However, the impact of this is unclear in the context of a pediatric vaccine. The recipients of the RSV vaccine will not have pre-existing T cells to human adenovirus and they may have neutralizing antibodies, passively acquired from their mothers. These adenovirus antibodies will not neutralize the GC46 RSV vaccine vector. In addition, the construction of the GC46 vector for clinical testing includes the deletion of E4 which is done to improve safety and reduce the likelihood of recombination leading to replication-competent adenovirus, to make room for larger antigen inserts, and to reduce the transcription and production of adenovirus structural proteins that require E4.
The unique immunological state of infant humans provides challenges to vaccination of the youngest babies.36 Although it is not known whether adenovirus-vectored vaccines will be effective in infants, adenovirus vectors have been shown to induce protective levels of rabies antibodies in neonatal animals.37 Another significant hurdle to overcome in development of a pediatric RSV vaccine is the legacy of vaccine-enhanced disease observed in the FI-RSV clinical trials.7 While the precise molecular mechanism(s) underlying the generation of this immunopathologic immunity may not be fully elucidated, well-defined correlates of FI-RSV–enhanced RSV disease in children are induction of antibody responses with poor neutralization capacity34 and enhanced eosinophil and/or neutrophil recruitment to the lung,7,38 suggesting Th2-biased responses.7,38,39 GC46.F0 induces strong neutralizing antibody responses, T-cell responses which do not produce type 2 cytokines or cause illness, and immune responses which do not augment eosinophilic pulmonary infiltrates following RSV challenge. This demonstration of protective immunity that lacks disease-enhancing correlates in two animal models demonstrates the feasibility of GC46.F0 for RSV vaccination.
The potency of the immune response elicited by GC46.F0 is unprecedented. A single IM immunization was sufficient to induce durable immunity which protected both the upper and lower respiratory tracts. To our knowledge, this is the first report of protection of the upper respiratory tract with one dose of a non-mucosally administered vaccine. Overall, critical to the safe and effective immunity generated by GC46.F0 immunization may be the unique induction of a balanced immune response, composed of high titers of neutralizing antibody and type 1-associated CD4 and CD8 T cells. Taken together, these observations support the continued evaluation of GC46.F0 as a universal RSV vaccine that may be administered safely to populations with urgent medical needs.
Materials and Methods
Viruses and cells. HEp-2, Vero, and A549 cells and RSV A (A2, VR-1540; Long, VR-26) and RSV B (WV, VR-1400; 9320, VR-955; 18537, VR-1540) strains were purchased from the American Type Culture Collection (Manassas, VA). RSV stocks were grown in HEp-2 cells with Eagle's minimal essential media supplemented with 10% fetal bovine serum (10% Eagle's minimal essential medium (EMEM)). FI-RSV was prepared using RSV A2 and HEp-2 cells as previously described.7 RSV clinical isolates were provided by Martin Moore (Emory University, Atlanta, GA; ref. 32) and JoAnn Kirman (Malaghan Institute, Wellington, New Zealand; ref. 33) and expanded in HEp-2 cells. E1-deleted vectors cloned from adenoviruses isolated from wild gorillas, designated GC44, GC45, and GC46, and from Ad5 were engineered to express firefly luciferase (GC44.L, GC45.L, GC46.L, Ad5.L) or full-length RSV F0 protein (GC44.F0, GC45.F0, GC46.F0, Ad5.F0) using published methods.27 An E1-deleted vector based on a monkey adenovirus, SAV7 (ATCC VR-201; ATCC; ref. 40), was similarly constructed. The E1-deletion rendered the viral vectors replication-deficient. Adenovirus stocks were produced in 293-ORF6 cells41 and purified into FFB as previously described.27 Post-purification vector yields >50,000 PU/cell were readily obtained. Pre-purification yields of GC46 expressing RSV F.0 averaged 137,333 ± 30,742 PU/cell.
Adenovirus seroprevalence. A549 cells, grown in Dulbecco's modified essential media with 10% fetal bovine serum (10% Dulbecco's modified eagle medium), were infected with luciferase-expressing vectors (Ad5.L, GC44.L, GC45.L, and GC46.L) at a multiplicity of infection determined to produce 10,000 relative luciferase units (multiplicity of infection = 2,000–4,000 PU). Neutralization activity in human sera (New York Biologics, South Hampton, NY) was determined as previously detailed,27 mixing diluted sera with the optimal multiplicity of infection of each virus. Neutralization (IC90) titers were defined as the maximum serum dilution which inhibited adenovirus activity (i.e., luciferase expression) by 90%. For endpoint titrations in Ad5 seropositive specimens, 37 seropositive samples (IC90 > 16) were randomly chosen and tested.
Immunization and challenge. Five- to 8-week old Cotton rats (Harlan Sprague Dawley, Frederick, MD or Sigmovir, Gaithersburg, MD) or 5- to 6- week old mice (Harlan Sprague Dawley) were immunized with 106 PFU RSV intranasally under isoflurane anesthesia. Animals were immunized with FFB, FI-RSV, or RSV F-expressing adenovirus by IM injection. Cotton rats were challenged intranasally with 106 PFU (in 0.1 ml) live RSV while mice were challenged with 4 × 106 to 9 × 106 PFU RSV in 0.1 ml.
RSV titers. At days 5–8 postchallenge, animals were euthanized, and the lungs and noses were removed and quick-frozen in 10% EMEM. RSV titers were then determined by plaque assay as previously described42 with minor modification. Noses were ground with mortar and pestle as originally described, but lungs were ground using a GentleMACS (Miltenyi Biotech, Cambridge, MA) dissociator. After formalin fixation, cell monolayers were stained with Giemsa histologic stain (Azer Scientific, Morgantown, PA) rather than hematoxylin and eosin. The data are expressed in PFU/g lung tissue or PFU/nose.
Neutralization assays. Neutralizing activity titers in sera from immunized animals were measured by plaque reduction neutralization test assay as previously described except for using Giemsa to stain the plates after formalin fixation.42 Serum samples were heat-treated to inactivate complement, and no exogenous complement was used. Neutralization of RSV clinical isolates was evaluated by microneutralization (microNT) assay as follows. Triplicate serial threefold dilutions of RSV clinical isolate stocks were made in serum-free EMEM in U-bottom microtiter plates, and an equal volume of antiserum (diluted to IC90 titers) was added to each well, pipetting to mix. After incubating at 37 °C for 1 hour, 20 µl antisera-RSV mix were transferred to HEp-2 monolayers (>24-hour post-plating, ~90% confluent), and infected at room temperature for 1 hour. After adding 0.1 ml 10% EMEM to each well, plates were incubated at 37 °C for 48 hour. Media was removed, and cells were gently washed with phosphate-buffered saline–Tween (0.05% vol/vol). Cells were fixed with 80% acetone/phosphate-buffered saline (vol/vol) at 4 °C for 15 minutes, then plates were blocked with phosphate-buffered saline–1% bovine serum albumin (wt/vol) at room temperature for 1 hour. Plates were washed 3× with phosphate-buffered saline–Tween using an ELx405 Select automated plate washer (BioTek, Winooski, VT). Infected cells were detected using mouse anti-RSV F monoclonal antibody (Millipore, Billerica, MA), followed by HRP-labeled goat anti-mouse IgG (Jackson ImmunoResearch, West Grove, PA). The reaction was developed with TMB substrate reagent (R&D Systems, Minneapolis, MN), stopped by the addition of 2 NH2SO4, and plates were read at A450 and A540 on a SpectroMax 340 PC plate reader (Molecular Devices, Sunnyvale, CA). Data are represented as the A450–A540 value for each virus dilution.
Flow cytometry analysis. To evaluate vaccine-induced RSV F-specific T-cell function, intracellular cytokine analysis was performed on peptide-stimulated lung lymphocytes 5 days after RSV challenge. Ground lung tissue was passed over a 100 micron filter, and the cell suspension was overlaid on mouse Fico-Lyte separation media (Atlanta Biologicals, Lawrenceville, GA). The mononuclear cell band was isolated, washed in 10% EMEM, counted, and resuspended to 2 × 106 cells/ml. Aliquots of cells (1 × 106) were stimulated with 2 µg/ml CD4 and CD8 peptides in the presence of 1 µg each CD28 and CD49d costimulatory antibodies and 0.7 µl GolgiStop (BD Pharmingen, San Jose, CA). To measure RSV F-specific responses, one aliquot was stimulated with the CD4 peptide F51–6638 and CD8 peptide F85–93.43 To measure RSV-specific responses induced solely by RSV challenge, one aliquot was stimulated with the RSV G183–195 CD4 peptide39 and the RSV M282–90CD8 peptide.44 Controls included one aliquot from each mouse stimulated with no peptide and PMA-ionomycin–stimulated cells. The cells were incubated at 37 °C for 5 hours, then fixed, permeabilized, and stained for intracellular cytokine analysis according to the CytoFix/CytoPerm Plus kit protocol (BD Pharmingen). Cells were stained with LIVE/DEAD aqua-fluorescent reactive dye (Invitrogen, Grand Island, NY) and with antibodies to anti-CD3-APC-Cy7 (BD Pharmingen), anti-CD4-eFluor450 (eBiosciences, San Diego, CA), anti-CD8-PerCP-Cy5.5 (eBiosciences), and anti-IFN-γ-PE (BD Pharmingen). The cells were analyzed on a Becton Dickinson FACSCalibur E1610 upgraded to acquire eight fluorescent spectra from three lasers for multi-color analysis (Cytek, Fremont, CA). FlowJo software (TreeStar, Ashland, OR) was used for off-line analysis.
Granulocytic infiltrates in mouse lungs were quantitated by surface staining with anti-CD3-APC-Cy7, anti-CD4-eFluor450, and anti-CD8-PerCP-Cy5.5 to identify T cells, and anti-Gr1-PE-Cy7 and anti-CCR3-AX647 (both from BioLegend, San Diego, CA) to identify neutrophils (CD3−4−8−Gr1+CCR3−) and eosinophils (CD3−4−8−Gr1+CCR3+).
Cytokine production. IFN-γ, interleukin-4, interleukin-13, and eotaxin levels were measured in mouse lung supernatants using Quantikine kits (R&D Systems) according to kit protocols. Cotton rat cytokine responses were evaluated by quantitative reverse transcription PCR analyses of lung tissue as described.45
Histopathology. Lungs were formalin-fixed, and thin sections were stained with hematoxylin and eosin or with Giemsa. Sections were scored in a blinded fashion as previously described.29 Briefly, four parameters of pulmonary inflammation were evaluated: peribronchiolitis (inflammatory cell infiltration around the bronchioles), perivasculitis (inflammatory cell infiltration around the small blood vessels), interstitial pneumonia (inflammatory cell infiltration and thickening of alveolar walls), and alveolitis (cells within the alveolar spaces). Slides were scored blind on a severity scale of 0–4. The scores were subsequently converted to a histopathology scale of 0–100%.
Statistical analyses. Data sets were maintained in Excel and Origin spreadsheets. Statistical analyses were performed in Origin and included Student's t test, one-way analysis of variance using Tukey's test for means comparison, and nonlinear curve fitting with χ2 testing.
Ethics statement. All studies were conducted under the animal care and use protocol MP-GAL-01 approved by the Institutional Animal Care and Use Committee of GenVec (Gaithersburg, MD), and in compliance with policies as outlined in the “NIH Guide for the Care and Use of Laboratory Animals” and the Public Health Service “Policy on Humane Care and Use of Laboratory Animals.”
SUPPLEMENTARY MATERIAL Figure S1.GC46.F0 induced RSV F-specific CD4+ T cells.
Acknowledgments
We thank Grant Liao, Emmanuella Eastman, Grace Lee, Karen Tressler, Valerie Moore, and Damador EttyReddy for excellent technical assistance as well as Jorge Blanco and Kevin Yim (Sigmovir) for expert advice and histopathology review. The work was funded by NIH grant R43AI075686-01 and GenVec. T.R.J, D.R., D.E.B., and J.G.G. were employees of GenVec, a for-profit corporation, and hold stock interests in the company. B.S.G. and T.R.J. are named on a patent application for the F antigen construct expressed by the rAd vectors, and B.S.G. is supported by intramural funding from the National Institutes of Health, National Institute of Allergy and Infectious Diseases. The authors declared no conflict of interest.
Supplementary Material
References
- Collins PL, Graham BS. Viral and host factors in human respiratory syncytial virus pathogenesis. J Virol. 2008;82:2040–2055. doi: 10.1128/JVI.01625-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375:1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360:588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352:1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- Small TN, Casson A, Malak SF, Boulad F, Kiehn TE, Stiles J, et al. Respiratory syncytial virus infection following hematopoietic stem cell transplantation. Bone Marrow Transplant. 2002;29:321–327. doi: 10.1038/sj.bmt.1703365. [DOI] [PubMed] [Google Scholar]
- Hall CB. The burgeoning burden of respiratory syncytial virus among children. Infect Disord Drug Targets. 2012;12:92–97. doi: 10.2174/187152612800100099. [DOI] [PubMed] [Google Scholar]
- Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- Hurwitz JL. Respiratory syncytial virus vaccine development. Expert Rev Vaccines. 2011;10:1415–1433. doi: 10.1586/erv.11.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson FW, Collier AM, Clyde WA Jr, Denny FW. Respiratory-syncytial-virus infections, reinfections and immunity. A prospective, longitudinal study in young children. N Engl J Med. 1979;300:530–534. doi: 10.1056/NEJM197903083001004. [DOI] [PubMed] [Google Scholar]
- van Drunen Littel-van den Hurk S, Watkiss ER. Pathogenesis of respiratory syncytial virus. Curr Opin Virol. 2012;2:300–305. doi: 10.1016/j.coviro.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Prince GA, Horswood RL, Camargo E, Koenig D, Chanock RM. Mechanisms of immunity to respiratory syncytial virus in cotton rats. Infect Immun. 1983;42:81–87. doi: 10.1128/iai.42.1.81-87.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochola R, Sande C, Fegan G, Scott PD, Medley GF, Cane PA, et al. The level and duration of RSV-specific maternal IgG in infants in Kilifi Kenya. PLoS ONE. 2009;4:e8088. doi: 10.1371/journal.pone.0008088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S, Oliver C, Prince GA, Hemming VG, Pfarr DS, Wang SC, et al. Development of a humanized monoclonal antibody (MEDI-493) with potent in vitro and in vivo activity against respiratory syncytial virus. J Infect Dis. 1997;176:1215–1224. doi: 10.1086/514115. [DOI] [PubMed] [Google Scholar]
- Van Kampen KR, Shi Z, Gao P, Zhang J, Foster KW, Chen DT, et al. Safety and immunogenicity of adenovirus-vectored nasal and epicutaneous influenza vaccines in humans. Vaccine. 2005;23:1029–1036. doi: 10.1016/j.vaccine.2004.07.043. [DOI] [PubMed] [Google Scholar]
- Catanzaro AT, Koup RA, Roederer M, Bailer RT, Enama ME, Moodie Z, et al. Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 candidate vaccine delivered by a replication-defective recombinant adenovirus vector. J Infect Dis. 2006;194:1638–1649. doi: 10.1086/509258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, He J, Zheng X, Wu Q, Zhang M, Wang X, et al. Intranasal immunization with a replication-deficient adenoviral vector expressing the fusion glycoprotein of respiratory syncytial virus elicits protective immunity in BALB/c mice. Biochem Biophys Res Commun. 2009;381:528–532. doi: 10.1016/j.bbrc.2009.02.075. [DOI] [PubMed] [Google Scholar]
- Kohlmann R, Schwannecke S, Tippler B, Ternette N, Temchura VV, Tenbusch M, et al. Protective efficacy and immunogenicity of an adenoviral vector vaccine encoding the codon-optimized F protein of respiratory syncytial virus. J Virol. 2009;83:12601–12610. doi: 10.1128/JVI.01036-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JR, Kim S, Lee JB, Chang J. Single intranasal immunization with recombinant adenovirus-based vaccine induces protective immunity against respiratory syncytial virus infection. J Virol. 2008;82:2350–2357. doi: 10.1128/JVI.02372-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbink P, Lemckert AA, Ewald BA, Lynch DM, Denholtz M, Smits S, et al. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J Virol. 2007;81:4654–4663. doi: 10.1128/JVI.02696-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiver JW, Emini EA. Recent advances in the development of HIV-1 vaccines using replication-incompetent adenovirus vectors. Annu Rev Med. 2004;55:355–372. doi: 10.1146/annurev.med.55.091902.104344. [DOI] [PubMed] [Google Scholar]
- Fattori E, Zampaglione I, Arcuri M, Meola A, Ercole BB, Cirillo A, et al. Efficient immunization of rhesus macaques with an HCV candidate vaccine by heterologous priming-boosting with novel adenoviral vectors based on different serotypes. Gene Ther. 2006;13:1088–1096. doi: 10.1038/sj.gt.3302754. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Gao G, Reyes-Sandoval A, Cohen CJ, Li Y, Bergelson JM, et al. Novel, chimpanzee serotype 68-based adenoviral vaccine carrier for induction of antibodies to a transgene product. J Virol. 2002;76:2667–2675. doi: 10.1128/JVI.76.6.2667-2675.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colloca S, Barnes E, Folgori A, Ammendola V, Capone S, Cirillo A, et al. Vaccine vectors derived from a large collection of simian adenoviruses induce potent cellular immunity across multiple species. Sci Transl Med. 2012;4:115ra112. doi: 10.1126/scitranslmed.3002925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes E, Folgori A, Capone S, Swadling L, Aston S, Kurioka A, et al. Novel adenovirus-based vaccines induce broad and sustained T cell responses to HCV in man. Sci Transl Med. 2012;4:115ra111. doi: 10.1126/scitranslmed.3003155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehy SH, Duncan CJ, Elias SC, Collins KA, Ewer KJ, Spencer AJ, et al. Phase Ia clinical evaluation of the Plasmodium falciparum blood-stage antigen MSP1 in ChAd63 and MVA vaccine vectors. Mol Ther. 2011;19:2269–2276. doi: 10.1038/mt.2011.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh EE, Brandriss MW, Schlesinger JJ. Immunological differences between the envelope glycoproteins of two strains of human respiratory syncytial virus. J Gen Virol. 1987;68 (Pt 8):2169–2176. doi: 10.1099/0022-1317-68-8-2169. [DOI] [PubMed] [Google Scholar]
- Kahl CA, Bonnell J, Hiriyanna S, Fultz M, Nyberg-Hoffman C, Chen P, et al. Potent immune responses and in vitro pro-inflammatory cytokine suppression by a novel adenovirus vaccine vector based on rare human serotype 28. Vaccine. 2010;28:5691–5702. doi: 10.1016/j.vaccine.2010.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy BR, Sotnikov A, Paradiso PR, Hildreth SW, Jenson AB, Baggs RB, et al. Immunization of cotton rats with the fusion (F) and large (G) glycoproteins of respiratory syncytial virus (RSV) protects against RSV challenge without potentiating RSV disease. Vaccine. 1989;7:533–540. doi: 10.1016/0264-410x(89)90278-8. [DOI] [PubMed] [Google Scholar]
- Prince GA, Curtis SJ, Yim KC, Porter DD. Vaccine-enhanced respiratory syncytial virus disease in cotton rats following immunization with Lot 100 or a newly prepared reference vaccine. J Gen Virol. 2001;82 Pt 12:2881–2888. doi: 10.1099/0022-1317-82-12-2881. [DOI] [PubMed] [Google Scholar]
- Mascola JR, Snyder SW, Weislow OS, Belay SM, Belshe RB, Schwartz DH, et al. Immunization with envelope subunit vaccine products elicits neutralizing antibodies against laboratory-adapted but not primary isolates of human immunodeficiency virus type 1. The National Institute of Allergy and Infectious Diseases AIDS Vaccine Evaluation Group. J Infect Dis. 1996;173:340–348. doi: 10.1093/infdis/173.2.340. [DOI] [PubMed] [Google Scholar]
- Martin NC, Pardo J, Simmons M, Tjaden JA, Widjaja S, Marovich MA, et al. An immunocytometric assay based on dengue infection via DC-SIGN permits rapid measurement of anti-dengue neutralizing antibodies. J Virol Methods. 2006;134:74–85. doi: 10.1016/j.jviromet.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Stokes KL, Chi MH, Sakamoto K, Newcomb DC, Currier MG, Huckabee MM, et al. Differential pathogenesis of respiratory syncytial virus clinical isolates in BALB/c mice. J Virol. 2011;85:5782–5793. doi: 10.1128/JVI.01693-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheson JW, Rich FJ, Cohet C, Grimwood K, Huang QS, Penny D, et al. Distinct patterns of evolution between respiratory syncytial virus subgroups A and B from New Zealand isolates collected over thirty-seven years. J Med Virol. 2006;78:1354–1364. doi: 10.1002/jmv.20702. [DOI] [PubMed] [Google Scholar]
- Murphy BR, Prince GA, Walsh EE, Kim HW, Parrott RH, Hemming VG, et al. Dissociation between serum neutralizing and glycoprotein antibody responses of infants and children who received inactivated respiratory syncytial virus vaccine. J Clin Microbiol. 1986;24:197–202. doi: 10.1128/jcm.24.2.197-202.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baden LR, Walsh SR, Seaman MS, Tucker RP, Krause KH, Patel A, et al. First-in-human evaluation of the safety and immunogenicity of a recombinant adenovirus serotype 26 HIV-1 Env vaccine (IPCAVD 001). J Infect Dis. 2013;207:240–247. doi: 10.1093/infdis/jis670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BS. Biological challenges and technological opportunities for respiratory syncytial virus vaccine development. Immunol Rev. 2011;239:149–166. doi: 10.1111/j.1600-065X.2010.00972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Xiang Z, Pasquini S, Ertl HC. The use of an E1-deleted, replication-defective adenovirus recombinant expressing the rabies virus glycoprotein for early vaccination of mice against rabies virus. J Virol. 1997;71:3677–3683. doi: 10.1128/jvi.71.5.3677-3683.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levely ME, Bannow CA, Smith CW, Nicholas JA. Immunodominant T-cell epitope on the F protein of respiratory syncytial virus recognized by human lymphocytes. J Virol. 1991;65:3789–3796. doi: 10.1128/jvi.65.7.3789-3796.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikiatkhachorn A, Chang W, Braciale TJ. Induction of Th-1 and Th-2 responses by respiratory syncytial virus attachment glycoprotein is epitope and major histocompatibility complex independent. J Virol. 1999;73:6590–6597. doi: 10.1128/jvi.73.8.6590-6597.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull RN, Minner JR, Mascoli CC. New viral agents recovered from tissue cultures of monkey kidney cells. III. Recovery of additional agents both from cultures of monkey tissues and directly from tissues and excreta. Am J Hyg. 1958;68:31–44. doi: 10.1093/oxfordjournals.aje.a119947. [DOI] [PubMed] [Google Scholar]
- Brough DE, Lizonova A, Hsu C, Kulesa VA, Kovesdi I. A gene transfer vector-cell line system for complete functional complementation of adenovirus early regions E1 and E4. J Virol. 1996;70:6497–6501. doi: 10.1128/jvi.70.9.6497-6501.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BS, Perkins MD, Wright PF, Karzon DT. Primary respiratory syncytial virus infection in mice. J Med Virol. 1988;26:153–162. doi: 10.1002/jmv.1890260207. [DOI] [PubMed] [Google Scholar]
- Chang J, Srikiatkhachorn A, Braciale TJ. Visualization and characterization of respiratory syncytial virus F-specific CD8(+) T cells during experimental virus infection. J Immunol. 2001;167:4254–4260. doi: 10.4049/jimmunol.167.8.4254. [DOI] [PubMed] [Google Scholar]
- Kulkarni AB, Morse HC 3rd, Bennink JR, Yewdell JW, Murphy BR. Immunization of mice with vaccinia virus-M2 recombinant induces epitope-specific and cross-reactive Kd-restricted CD8+ cytotoxic T cells. J Virol. 1993;67:4086–4092. doi: 10.1128/jvi.67.7.4086-4092.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco JC, Richardson JY, Darnell ME, Rowzee A, Pletneva L, Porter DD, et al. Cytokine and chemokine gene expression after primary and secondary respiratory syncytial virus infection in cotton rats. J Infect Dis. 2002;185:1780–1785. doi: 10.1086/340823. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.