Abstract
Glaucoma is a progressive ocular syndrome characterized by degeneration of the optic nerve and irreversible visual field loss. Elevated intraocular pressure (IOP) is the main risk factor for glaucoma. Increased IOP is the result of an imbalance between synthesis and outflow of aqueous humor (AH). Blocking β2 adrenergic receptor (ADRB2) has shown to reduce IOP by decreasing production of AH at the ciliary body (CB). SYL040012 is a siRNA designed to specifically silence ADRB2 currently under development for glaucoma treatment. Here, we show that SYL040012 specifically reduces ADRB2 expression in cell cultures and eye tissues. The compound enters the eye shortly after administration in eye drops and is rapidly distributed among structures of the anterior segment of the eye. In addition, SYL040012 is actively taken up by cells of the CB but not by cells of systemic organs such as the lungs, where inhibition of ADRB2 could cause undesirable side effects. Moreover, SYL040012 reduces IOP in normotensive and hypertensive animal models and the effect appears to be long lasting and extremely well tolerated both locally and systemically.
Introduction
Glaucoma is a chronic condition characterized by progressive optic nerve damage and visual impairment frequently leading to blindness.1 It is the second leading cause of blindness worldwide; and the prevalence of the disease increases with age.2 The major risk for developing glaucoma is elevated intraocular pressure (IOP)3 and fluctuations in IOP, even within normal ranges, significantly contribute to visual field loss.4 Increased IOP is caused by an imbalance in the production and drainage of aqueous humor (AH), the clear liquid that fills the anterior and posterior chambers of the eye. AH is secreted by the ciliary epithelium lining in the ciliary body (CB) into the posterior chamber; it circulates around the lens and through the pupil into the anterior chamber. The roles of AH include delivering essential nutrients to the cells of the eye and IOP maintenance. AH leaves the eye through one of two pathways at the anterior chamber: the conventional pathway passing through the trabecular meshwork or the nonconventional route passing through uveoscleral pathway.5,6
Current glaucoma treatment cannot restore vision-loss caused by disease progression, but is focused on IOP reduction.4 Controlling IOP has been shown to protect against damage to the optic nerve in glaucoma.5,7
β-Blockers are among the most prescribed drugs for glaucoma.8 Topical ocular application of β-blockers reduces IOP by decreasing AH production at the CB. Unfortunately, topically administered β-blockers are also absorbed via conjunctival epithelium, lachrymal channel, nasal mucosa, and gastrointestinal tract into systemic circulation inducing systemic adverse reactions such as brachycardia, hypotension, precipitation of heart failure, respiratory failure, dyspnea, cough, bronchospasm, and conduction arrhythmias.9,10,11
In the eye, adrenergic receptors have been located at blood vessels that irrigate the CB and trabecular meshwork, where their main effect is vasoconstriction, although their involvement in AH secretion has also been described. Studies in rabbits' eyes have shown high density of β-adrenergic receptors in conjunctival, corneal and ciliary process epithelia. β-adrenergic receptors are also present in corneal endothelium, lens epithelium, choroid and extraocular muscle. Most β-adrenergic receptors detected in eye belong to the 2-type.12,13,14,15
RNA interference (RNAi) involves specific gene silencing by small molecules of double stranded RNA: small interference RNAs (siRNAs).16,17,18 This technology has emerged as a very powerful tool to develop new compounds aimed at blocking and/or reducing anomalous activities in defined proteins.19,20,21 RNAi is a very attractive approach to chronic conditions, since upon cessation of treatment the silenced protein has to be re-synthesized in order to recover its biological activity. Hence, the effects of compounds based on RNAi are in general more prolonged than those of conventional treatments.19,22
The eye is a relatively isolated tissue compartment; this particularity provides several advantages to the use of siRNA-based therapies. Local delivery of compounds to the eye limits systemic exposure and reduces the amount of compound needed. It allows for local silencing of a gene while reducing the likelihood of wide spread silencing outside the eye. In addition, the immune system has a limited access to the eye; therefore immune responses to the compound are less likely to occur.23 Finally, the eye has lower content in RNases than other tissues, allowing for an increased stability of RNA-based compounds.
We have developed SYL040012, a chemically synthesized double-stranded siRNA able to selectively inhibit synthesis of β2-adrenergic receptor, which shows promising results in animal models for treatment of elevated IOP.
Results
In vitro silencing effect of SYL040012 and rSYL040012
ADRB2 mRNA levels were analyzed by qPCR at different time points (24, 48, and 72 hours) after transfecting human cell cultures (BxPC3 or MDA-MB-231) with either 100 nmol/l SYL040012 or a scrambled siRNA; the above mentioned cell lines were chosen due to their constitutive expression of ADRB2. A decrease varying between 50–70% of basal mRNA levels of ADRB2 was observed, depending on the time-point. In both cell lines, maximal effect of SYL040012 was observed between 24 and 48 hours after transfection and it reached ~50% of basal levels. In BxPC3 cells, basal ADRB2 levels recovered ~72 hours after transfection, whereas recovery in MDA-MB-231 cells was slower. Transfection of a scrambled RNA sequence did not modulate ADRB2 levels demonstrating specificity of the effect (Figure 1a).
Figure 1.
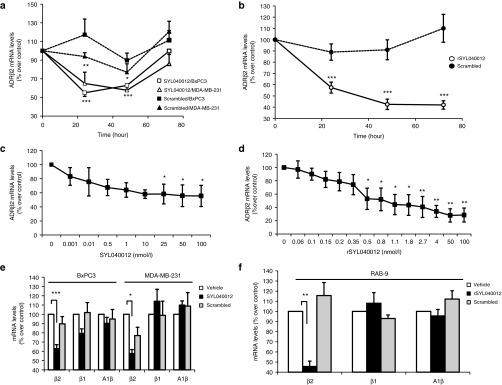
In vitro efficacy of SYL040012 and rSYL040012 in human and rabbit cells. (a) Time course inhibition of ADRB2 in human cells: BxPC3 and MDA-MB-231 cells were transfected with either 100 nmol/l SYL040012 or 100 nmol/l of a scrambled sequence siRNA, RNA was isolated and expression of ADRB2 was analyzed 24, 48, and 72 hours after transfection by qPCR. (b) Time course inhibition of ADRB2 in rabbit cells: RAB-9 cells were transfected with either 100 nmol/l of rSYL040012 or 100 nmol/l of a scrambled sequence siRNA, RNA was isolated and expression of ADRB2 was analyzed 24, 48, and 72 hours after transfection by qPCR. (c) Dose-dependent inhibition of ADRB2 in response to SYL040012 in BxPC3 cells 48 hours after transfection. (d) Dose-dependent inhibition of ADRB2 in response to rSYL040012 in RAB-9 cells 48 hours after transfection. (e) Expression of adrenergic receptors β2, β1, and αB1 in human cells (BxPC3 and MDA-MB-231) in response to 100 nmol/l SYL040012, the same dose of a scrambled sequence or phosphate-buffered saline (PBS) (vehicle). (f) Expression of adrenergic receptors β2, β1, and αB1 in rabbit cells (RAB-9) in response to 100 nmol/l rSYL040012, the same dose of a scrambled sequence or PBS (vehicle). Results show means ± SEM of five independents experiments. Statistical significance was calculated by unpaired Student's t-tests and was as follows: *P < 0.05; **P < 0.005; ***P < 0.001 versus time 0 (a–d) or versus vehicle (e–f).
SYL040012 is specifically designed to silence human ADRB2, the human target sequence differing in two nucleotides to the corresponding rabbit sequence. In vitro studies demonstrated that SYL040012 was not able to induce knock-down of the rabbit gene, therefore, a surrogate compound (rSYL040012) was synthetized in order to analyze SYL040012 efficacy in a relevant animal model. This surrogate was designed against the same region as the human compound, thus both compounds differ only in these two nucleotides. This approach has been widely used in development of oligonucleotides.24
Rabbit RAB-9 cells were transfected with either rSYL040012 at 100 nmol/l or a scrambled sequence at the same dose. ADRB2 mRNA levels were analyzed at the same time-points as in human cell cultures. Results show a significant decrease in ADRB2 expression 24 hours after transfection; mRNA levels continued to decrease over time and hadn't recovered to basal levels 72 hours after transfection (Figure 1b).
Compounds based on RNAi depend on the activity of endogenous RNAi machinery. One of the pitfalls of RNAi is that this endogenous system can be saturated when big amounts of exogenous RNA molecules are added.25 With the aim of assessing the effect of different doses of siRNAs targeting ADRB2, BxPC3, and RAB-9 cells were transfected with increasing doses of SYL040012 or rSYL040012 (0.001–100 nmol/l). Total RNA was isolated 24 hours after transfection and ADRB2 mRNA levels determined by qPCR (Figure 1c,d). A significant decrease in ADRB2 levels was observed at a 0.5 nmol/l dose in both cell lines. These decreases became more pronounced with dose until 10 nmol/l; beyond this point further dose increase did not achieve significantly higher knock-down of the target gene. Using these data, the inhibitory concentration 50 (IC50) value was calculated to be 9.2 nmol/l in BxPC3 cells and 1.99 nmol/l in RAB-9 cells.
Specificity of a compound for its target is crucial to minimize side effects in the clinical setting. Consequently, the effect of SYL040012 on other receptors of the adrenergic family was analyzed. mRNA levels of adrenergic receptors ADRB2, adrenergic receptor β1 (ADRB1), and adrenergic receptor α1B (ADRA1B) were assessed in BxPC3 and RAB-9 cells after transfection with either 100 nmol/l SYL040012 or rSYL040012. Figures 1e,f show that both siRNAs were able to selectively decrease ADRB2 mRNA levels in human and rabbit cell lines without significantly affecting the mRNA levels of ADRB1 or ADRA1B.
Finally, in order to assess if a decrease in ADRB2 levels had an effect on cell viability a 3-(4,5-dimethythiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay was performed at the same dose and time points mentioned above. SYL040012 did not cause a significant effect on cell viability over time (Table 1). This result indicates that a decrease in ADRB2 mRNA in response to SYL040012 does not result in cell toxicity.
Table 1. Cell viability in MDA-MB-231 and BxPC3 cells following treatment with either 100 nmol/l SYL040012, 100 nmol/l of a scrambled sequence or PBS.
Stability of SYL040012 and rSYL040012
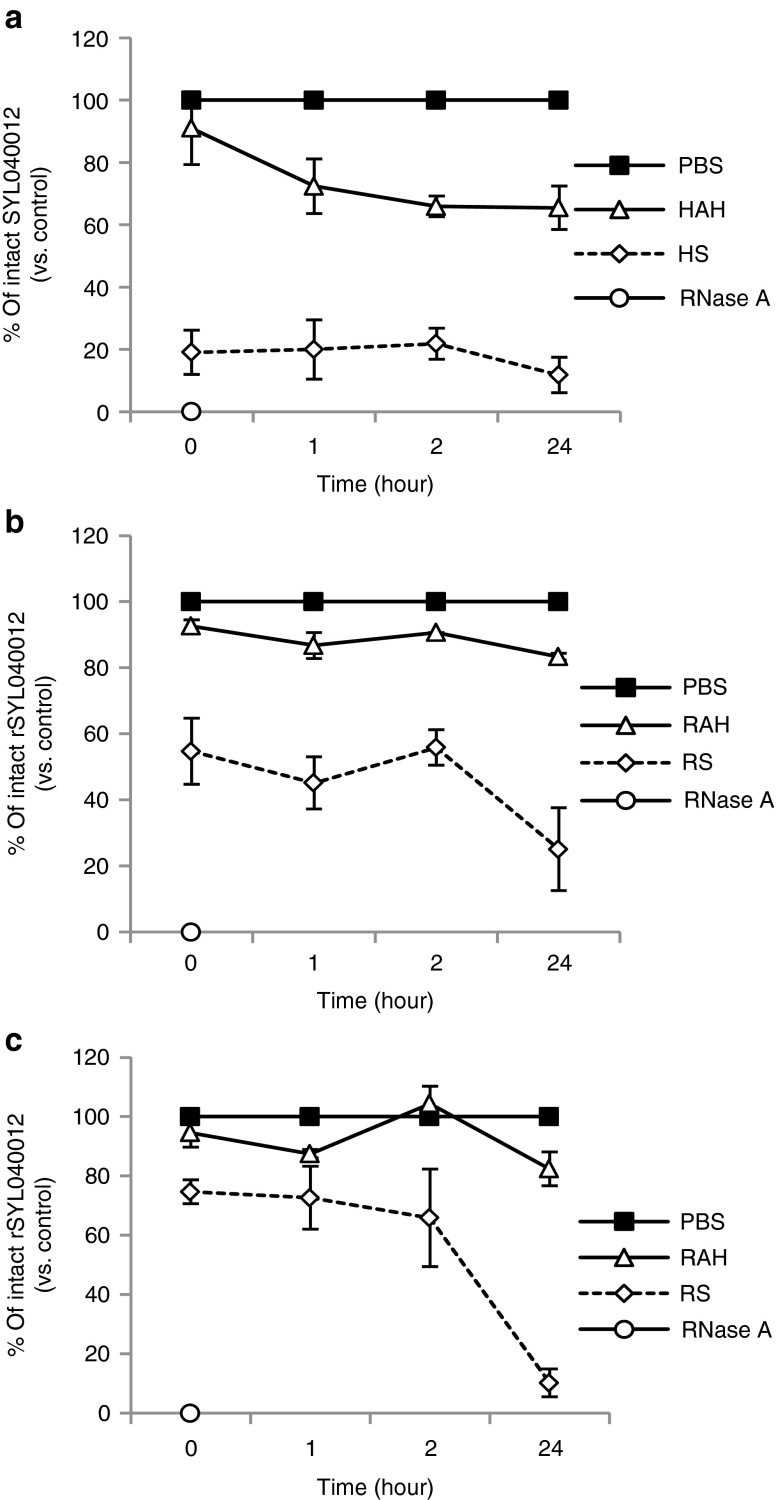
Naked siRNAs are very easily degraded by RNAses and cannot be systemically administered for therapeutic purposes without a delivery vehicle or chemical modifications that significantly prolong its half-life in serum. Locally applied siRNAs require somewhat different stability properties. In the case of the eye, sufficient stability for the siRNA to exert its action within the eye is desirable but a reduced half-life in serum usually reduces the likelihood of systemic side effects. Figure 2 shows the stability of rSYL040012 in human (A) and rabbit (B) serum, respectively, and AH. In both cases, SYL040012 stability rapidly decreases in serum, being the rate of decrease in human serum greater than in rabbit serum. In contrast, the compound has a significantly higher half-life in AH; ~60% of the compound is still intact in human AH 24 hours after incubation, whereas 80% of the compound remains in rabbit AH. Again, as observed in serum, the rate of decrease of the intact compound in human AH is greater than the rate of decrease observed in rabbit AH. Stability of the surrogate rSYL040012 was also assessed in rabbit serum and AH to ensure that this compound had similar stability properties to the human compound. Figure 2C shows that ~80% of rSYL040012 remains intact 24 hours after incubation in rabbit AH and <20% remains intact after 24 hours incubation in rabbit serum.
Figure 2.
Stability of SYL040012 in biological fluids. Stability of SYL040012 and rSYL040012 was assessed in human and rabbit aqueous humor (AH) and serum by spiking freshly obtained samples of each of the biological fluids with a 20 μmol/l solution of the siRNA. Samples were thereafter incubated during 1, 2, or 24 hours at 37 ºC and following incubation separated by electrophoresis (a) Stability of SYL040012 in human AH (HAH) and human serum (HS). (b) Stability of SYL040012 in rabbit AH (RAH) and rabbit serum (RS). (c) Stability of rSYL040012 in RAH and RS. Results are represented as percentage of the initial amount. Data represent means ± SEM of three independent analyses.
Ocular and systemic biodistribution of SYL040012
An important step in any interference study is to optimize conditions of siRNA delivery in vivo. The ability to detect phenotypic changes or loss of function in a target cell population depends on the efficiency with which the siRNA is delivered into the target tissue.
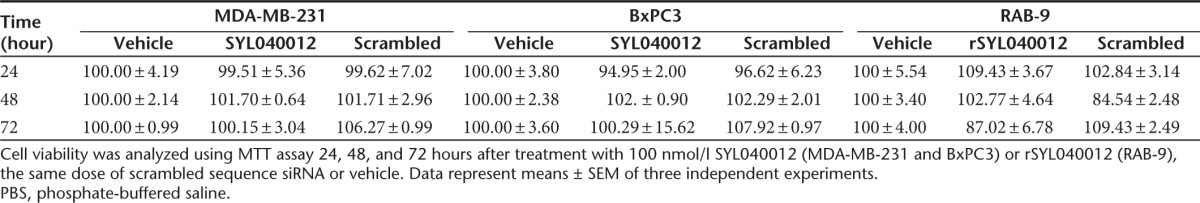
In order to investigate whether SYL040012 can access relevant structures of the anterior chamber of the eye and to assess systemic exposure of the compound following topical ocular instillation, we performed rabbit biodistribution studies were executed. In addition, detection of the 5′-phosphorylated antisense strand of the compound was performed. 5′ phosphorylation of the antisense strand of siRNAs takes place in the cytoplasm of the cell once the compound has escaped from the endosomes and serves therefore as a marker for intracellular delivery of the siRNA.26 The results of the biodistribution study show that, following topical ocular administration of a single dose of 60 nmol/eye SYL040012, the highest content of intact SYL040012 was found in the cornea 5 minutes after administration. At the same time point ~20% of the amount found in cornea was found in CB and iris, respectively; indicating that the compound enters the eye and reaches these structures very rapidly. Very small amounts of the compound were found in the AH 5 minutes after administration; this indicates that either SYL040012 is very rapidly cleared from AH or that it reaches the CB and iris avoiding the AH. Significantly less quantity of intact SYL040012 was found in lacrimal gland, ~25% of the amounts found in iris and CB (Figure 3a). Five minutes after topical ocular instillation the highest amount of 5′P-SYL040012 was found in the CB; the compound was also efficiently taken up by the cells of the cornea and iris and to a minor extent by cells of the lachrymal gland (Figure 3b). Thirty minutes after ocular instillation the intact compound could still be detected in cornea, iris, CB and lacrimal gland, although the quantities were significantly reduced (Figure 3c). The relative quantities of the intracellular distribution of the compound were the same 30 minutes after administration than those observed 5 minutes after administration (Figure 3d). It is of interest that the compound decrease in the extracellular compartment in each of the ocular structures analyzed was greater than the decrease in the intracellular compartment. This observation indicates that the degradation rate of SYL040012 is different when the compound is inside the cells than when it is in the extracellular compartment. This difference was of significant magnitude in the CB, where the relative quantity of 5′P-SYL040012 versus the total amount of SYL040012 increased from 5% at 5 minutes after administration to 13.5% at 30 minutes. An increase of this ratio was also observed in iris (from 2.5% to 5.6%) in contrast to the observations made in the rest of the ocular structures; where the relative amount of 5′-P-SYL040012 versus the total amount of compound decreased over time. The results also show that only trace amounts of intact SYL040012 reached the back of the eye segment, as indicated by the low content of compound in vitreous humor (Figure 3).
Figure 3.
Ocular biodistribution of SYL040012 in rabbits. Quantification of intact (a,c) SYL040012 and (b,d) 5′-P-SYL040012 in ocular structures. Animals were administered with a single dose of 60 nmol/eye SYL040012 and killed (a,b) 5 and (c,d) 30 minutes after administration. Data represent means ± SEM of three animals per group. AH, aqueous humor; C, cornea; CB, ciliary body; I, iris; LG, lachrymal gland; VH, vitreous humor.
Very low amounts of intact SYL040012 duplex (1.10 ± 0.49 ng/g) were detected in plasma and even lower amounts were detected in systemic organs such as lungs, liver or kidney at the time points studied (Table 2). No 5′P-SYL040012 was detected in any of the systemic tissues or in plasma, indicating that the trace amounts present in systemic tissues were not taken up by the cells and were therefore not biologically active.
Table 2. Systemic biodistribution of SYL040012.
In vivo efficacy of rSYL040012
As a first step to demonstrate in vivo efficacy of rSYL040012 New Zealand White Rabbits were treated with two products currently used in the clinic to manage glaucoma: 2% dorzolamide hydrochloride and 0.005% latanoprost. A single drop (40 µl) of each of the compounds was instilled into the eyes of two separate groups of rabbits over a period of 4 consecutive days. IOP measurements were obtained every hour for 8 hours, starting 1 hour after the last administration. All compounds caused an IOP decrease of 20–35%, depending on the compound, and this effect lasted ~6 hours (Table 3). These experiments confirmed the suitability of this animal model due to its ability to respond to IOP regulators.
Table 3. Maximal IOP decrease and mean time effect of rSYL040012, latanoprost and dorzoalamide in NZW rabbits.
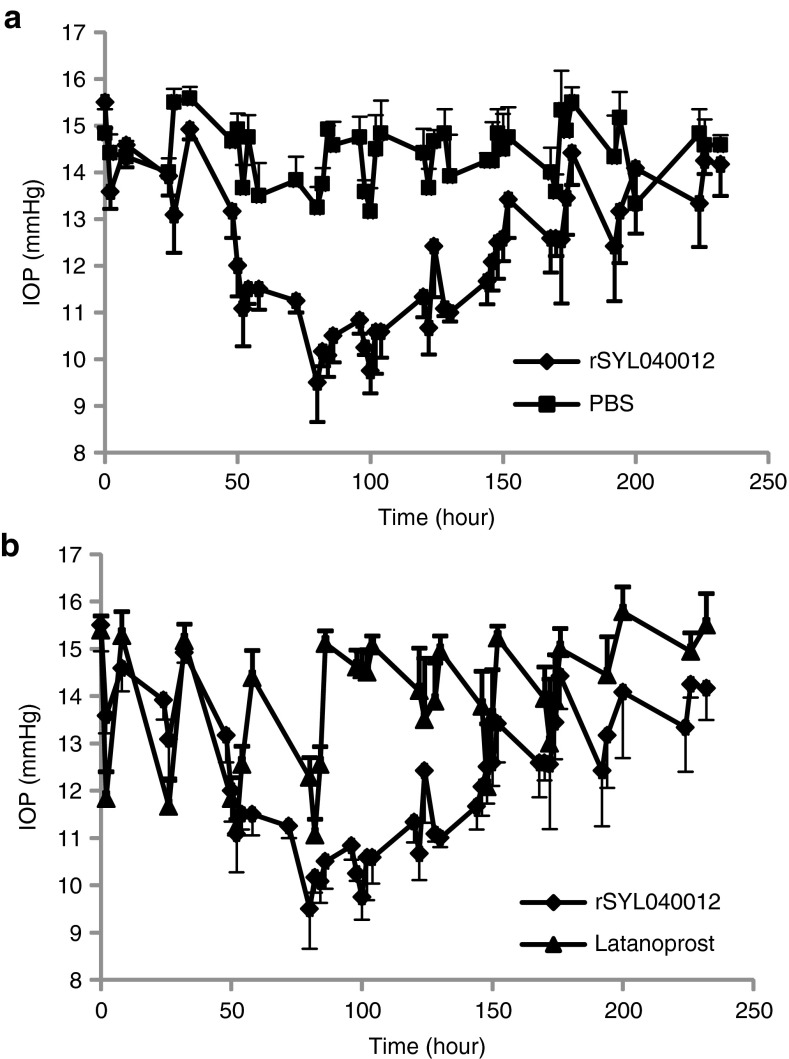
To assess the effect of rSYL040012 on IOP, New Zealand White rabbits received a topical administration of either 20 nmol/day of the compound or phosphate-buffered saline (PBS) over a period of 4 consecutive days. Figure 4a shows that there was 21.81% ± 1.55 IOP decrease when compared with the vehicle-treated group. The effect of rSYL040012 on IOP was detectable 2 days after the first administration and values remained below basal levels until ~2 days after the last administration (Figure 4b).
Figure 4.
In vivo efficacy of rSYL040012 in rabbits. (a) Intraocular pressure (IOP) lowering effect of rSYL040012: two groups of New Zealand White (NZW) rabbits were treated with either rSYL040012 (20 nmol/eye/day) or phosphate-buffered saline (PBS) over a period of 4 consecutive days. IOP was assessed every 2 hours up to 8 hours after each administration, the same schedule was followed on days 5–10 but no compounds were given. (b) Comparison of the IOP lowering effect of rSYL040012 and latanoprost: two groups of NZW rabbits were treated with either 20 nmol/eye/day rSYL040012 or 40 µl of 0.005% latanoprost. IOP was assessed as described above. Data represent means ± SEM of at least four animals per group performed in three independent experiments.
The mean time effect of each compound in response to one daily administration during 4 consecutive days was calculated as the difference between the half maximal time-point of recovery and the time at which the half maximal effect on IOP was achieved. The results of these calculations are shown in Table 3, and illustrate the difference in mean time effect of SYL040012 (91.6 hours) versus latanoprost (5.36 hours) and dorzolamide (4.75 hours). In addition, analysis of IOP evolution when rSYL040012 was administered in two cycles of 4 days separated from each other by a drug-free period of 48 hours showed that the decrease in IOP was maintained during the drug-free period.
To evaluate the IOP-lowering effect of rSYL040012 in conditions closer to pathological sceneries observed in glaucoma, an oral water overloading model in New Zealand White Rabbits was used. This model has previously been described by several authors.27,28,29,30 The main advantage of this model over other experimental models of ocular hypertension is that administration of irritant compounds or techniques that are traumatic to the eye are avoided, leaving ocular structures intact and allowing a normal response to hypotensive drugs.30
The first experiment was a dose-range finding in which four different doses of rSYL040012 (10 nmol, 20 nmol, 40 nmol, and 60 nmol/eye/day) were administered a total of three times: 48, 24, and 2 hours before hypertension induction. All treatments were applied in both eyes and IOP was measured before hypertension induction and every 20 minutes up to 120 minutes after oral overloading. Analysis of the results showed a statistically significant effect of treatment over time (P < 0.0001). Differences between each of the doses and PBS were analyzed using a one-way analysis of variance with a Dunnett's post-hoc test. Figure 5a shows that rSYL040012 provides significant protection against the rise of IOP at all doses tested (P < 0.01 versus saline in all cases).The maximum mean ΔIOP value (IOP after water-loading − IOP before water-loading) in animals treated with rSYL040012 was 6.6, 8.2, 4.8, and 4.3 mmHg for doses of 10, 20, 40, and 60 nmol/eye/day, respectively versus a maximum ΔIOP in control animals (treated with vehicle) of 15.55 mmHg.
Figure 5.
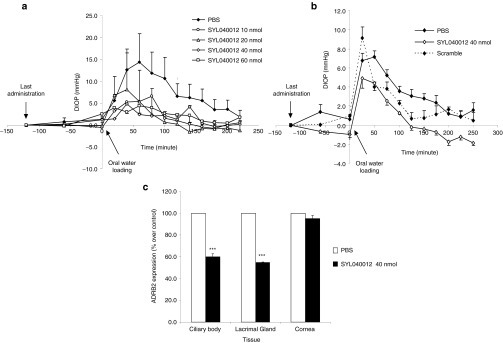
Efficacy of rSYL040012 in a rabbit model of high intraocular pressure induced by oral water overloading. (a) Dose response of rSYL040012 on intraocular pressure (IOP): animals were administered either rSYL040012 at one of the following doses: 10, 20, 40, or 60 nmol/eye/day or phosphate-buffered saline (PBS) over a period of 4 days. 120 minutes after the last dose ocular, hypertension was induced by oral water overloading. IOP was assessed coinciding with the last dosing, 60 minutes and immediately before oral water overloading and a total of 10 times with a 25-minute interval between measurements after oral water overloading. Data represent means ± SEM of two animals per group. (b) Specificity of SYL040012 on IOP: animals were administered either 40 nmol/eye/day of SYL040012, the same dose of a scrambled siRNA or PBS as mentioned above. Oral water overloading and IOP measurements were performed as mentioned in a. Data represent means ± SEM of 12 animals for SYL040012; 11 animals for PBS and 2 animals for the scrambled siRNA. (c) Decrease of ADRB2 levels in animals treated with rSYL040012. Animals were treated as stated in b and immediately after the last IOP measurement they were killed, eyes were enucleated and cornea, lachrymal gland and ciliary body were isolated. Total RNA was extracted and expression of ADRB2 was analyzed by real time PCR. Data represent means ± SEM of at least three animals per group. Statistical significance was calculated by unpaired Student's t-tests and was as follows: ***P < 0.001 versus PBS.
In order to confirm the efficacy and specificity of rSYL040012 on IOP, a larger group of animals was treated with a dose of 40 nmol/eye/day over a period of 4 consecutive days; on the fourth day, ocular hypertension was induced by water loading. As seen in Figure 5b, water loading caused an increase in IOP of ~7 mmHg during the first hour after hypertension induction in animals treated with PBS. Analysis of the results showed an overall significant effect of treatment (P < 0.0001). Further analysis performed by comparing IOP values at each time point indicate that treatment with rSYL040012 significantly reduced ΔIOP values within the first hour compared with PBS-treated animals (P < 0.05 versus PBS). The effect of rSYL040012 was specific since treatment with a scrambled sequence siRNA had no effect on IOP (P > 0.05 versus PBS).
To assess if the IOP decrease was accompanied by a decrease in ADRB2 mRNA levels in relevant ocular structures the animals used in the water loading experiments were killed 24 hours after the last administration and mRNA levels of ADRB2 were analyzed in the following ocular structures: cornea, lacrimal gland, and CB by qPCR. Figure 5c shows that there was a statistically significant decrease in ADRB2 mRNA levels in lachrymal gland and CB 24 hours after the last administration of rSYL040012 (P < 0.001).
Safety of SYL040012 in cynomolgus monkeys
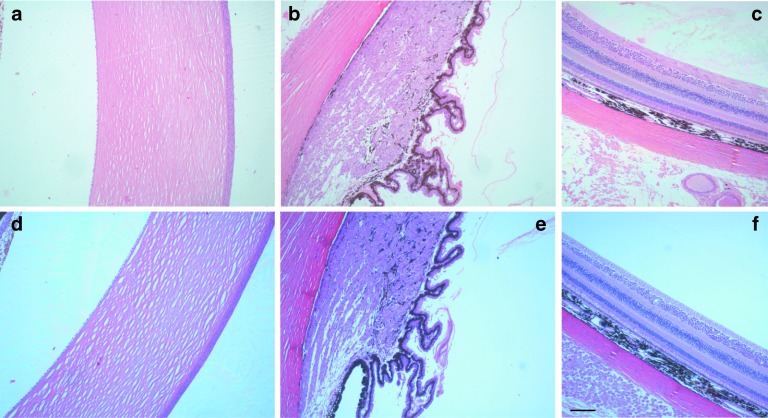
GLP compliance 28-day toxicity studies were performed in cynomolgus monkeys, this animal model was chosen because the target sequence of SYL040012 is identical in humans and cynomolgus monkeys. Using an animal model that has the same target sequence as humans would allow for the detection of adverse events caused either by exaggerated pharmacology or by the action of these molecules on unintended targets (i.e., off-target effects, immunotoxicity). Animals received topical ocular administration of SYL040012 at three different doses (72, 144, and 288 nmol/eye/day) over a period of 28 days and were observed twice a day for the presence of clinical signs and/or alterations to the eye. In addition, ophthalmoscopic examinations and electrocardiograms were performed before beginning treatment at different time-points during the administration schedule. Table 4 summarizes the parameters analyzed in the study, the time-points at which these analyses were performed and the observations made during the study. A pharmacokinetic profile of SYL040012 in plasma was also obtained at days 1 and 28 of administration; the results obtained indicate that SYL040012 was not detected above the detection limit (44.2 ng/ml) at any time point. At the end of the study animals were killed and histopathology studies were performed. Figure 6 shows representative microphotographs of monkeys eyes treated with either PBS or the highest dose of SYL040012 (288 nmol/eye/day). Analysis of the eyes revealed no macro- or microscopic alterations in response to any of the SYL040012 doses. In addition, the results of this study showed no alterations in any of the parameters analyzed (Table 4); therefore, SYL040012 was found to be safe and well tolerated at all doses used.
Table 4. Test, readouts and frequency of the readouts performed on the 28-day toxicity study in cynomolgus monkeys.
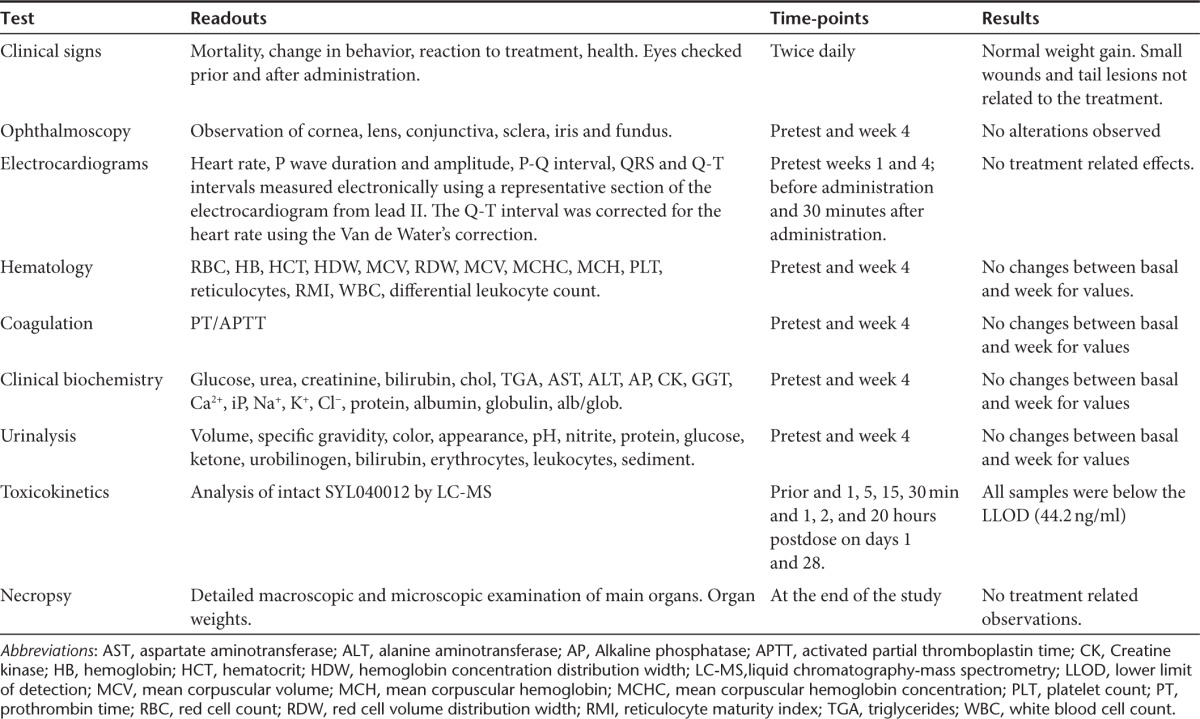
Figure 6.
Microscopic features of cynomolgus monkeys' eyes treated with phosphate-buffered saline (PBS) or SYL040012 over a period of 28 days. Histomorphologic assessment of (a,d) cornea, (b,e) ciliary body, and (c,f) retina treated with either (a–c) PBS or (d–f) 288 nmol/eye/day SYL040012 after 28 days of treatment. Representative photographs are shown. Bar represents 100 nm.
Discussion
Glaucoma is one of the leading causes of blindness worldwide.31 2.65% of global population over 40 years old suffered from glaucoma in 2010.2 Current treatments for increased IOP associated to glaucoma have relatively few ocular side effects but may have systemic side effects if the compound reaches the bloodstream.9,32,33 Treatments that are systemically better tolerated, such as prostaglandins, have many local tolerance issues.34 This fact together with the required frequency of instillations in order to maintain adequate levels of IOP makes treatment compliance a challenge for patients.3 Failure to comply with therapy cannot only allow for disease progression but can also have a reboot effect causing sudden increases in IOP that can be very damaging to the optic nerve. Treatments for glaucoma based on RNAi technology are very attractive since they should help to overcome the challenges of difficult treatment compliance.35 siRNAs are specifically designed to silence expression of a target gene; thus, they are extremely specific molecules. In addition, naked RNAs are quickly degraded and cleared by the kidneys when they reach the bloodstream, thus reducing the likelihood of systemic side effects.23,36 Finally, the mechanism of action of siRNAs usually extends the efficacy of the compound from hours to days.19 This latter reason could have a direct impact on the number of instillations required to maintain constant levels of IOP.
In this report, we show that silencing ADRB2 in the anterior segment of the eye with siRNAs designed to specifically silence this gene is capable of reducing IOP in normotensive and hypertensive animal models. Target gene expression in animal models following a 40 nmol/eye/day administration over a period of 4 days is reduced around 50%, this being sufficient to cause a significant decrease in IOP. Given the similarity in size and distribution of ADRB2 of the human and rabbit eye, the dose used in the current study might serve as an indicator for the efficacious dose in humans.37 The results shown in the present report demonstrate that the efficacy of rSYL040012 in reducing IOP in animal models is similar to the effect of commercially available drugs such as dorzolamide or latanoprost.27,38 On the other hand, when commercial drugs are used, sustained IOP decrease relies on the continuous application of the drugs. This is not the case with rSYL040012, whose effect in animal models not only lasts 15 times longer than the effect of dorzolamide or latanoprost; but is also able to maintain these IOP levels even when the compound is not administered over a period of up to 72 hours. This feature is very attractive since it would protect against eventual optic damage caused by a reboot effect in IOP in cases of poor compliance with treatment.
There are several programs developing siRNAs for the treatment of eye diseases most of which focus on diseases of the back of the eye where the compounds are administered via intravitreal injections.39,40,41,42 Several of these programs have been discontinued due to lack of efficacy. In addition, the work of Kleinman and coworkers has shown that naked siRNAs 21 nucleotide or longer intravitreally administered suppress angiogenesis through unspecific activation of TLR3 and more recently that inhibition of angiogenesis is accompanied by induction of retinal degeneration via the same mechanism.43,44 These studies show significant vacuolization of the retinal pigmented epithelium, pigment loss, and disorganization of the overlying photoreceptor array following a week of daily administration of a dose of 2 µg of a 21-nt naked siRNA to mice. Here, we show that the safety studies performed by administering doses as high as 288 nmol/eye/day (4,320 µg/eye/day) of SYL040012 over a period of 28 days to cynomolgus monkeys indicate that there are no micro- or macroscopic alterations in the retina or in any other structure of the eye. These differences in retinal degeneration between mice and monkeys in response to naked siRNAs may be caused by biological and anatomical differences between the two species, or it might also be possible that the doses required to induce retinal toxicity cannot be achieved by topical administration of siRNAs. This latter fact is supported by the low content of intact SYL040012 found in the vitreous humor in biodistribution studies.
Topical drugs can enter the eye through one of two pathways; crossing the cornea to reach the AH or through the conjunctival-scleral pathway. Depending on the size and hydrophilic/lipophilic ratio of the molecule, the relative quantity that enters through each of the above mentioned routes varies significantly. Generally hydrophilic and large molecules are absorbed via the conjunctival route whereas small lipophilic drugs are mainly absorbed through the cornea.45,46,47 Topically applied macromolecules and nanoparticles absorbed via the paracellular route of the conjunctiva and sclera usually give a distribution pattern that resembles the one obtained in the current study.47,48 Several studies have tried to establish the rate-limiting size of a molecule to enter the eye. Inulin (~5 kDa) was one of the first macromolecules that were shown to enter the eye through noncorneal absorption routes.47 Subsequent studies by Bochot and coworkers showed that T16, a 16-mer oligothymidylate instilled in rabbits eyes is detected in cornea, sclera, conjunctiva and iris 10 minutes after administration and not detectable at any time-point in AH, vitreous humor or lens.49 Further studies performed by Hämäläinen and coworkers have specifically characterized the penetration routes through cornea, conjunctiva and sclera. Studies performed by this group have shown that the pore diameter in the apical rate-limiting cell layer of the corneal epithelium was 2.0 nm whereas the pores of the conjunctiva were between 3–4.9 nm; pores this large would allow diffusion of macromolecules such as lysozyme with a molecular weight of 14.000 Da and a diameter of 4.1 nm.50 Moreover, other studies show that molecules as large as 20 kDa such as dextrans or single chain antibodies (26 kDa) are absorbed and distributed into the eye when applied in eye drops without penetration enhancers.48,51 The low amount of SYL040012 found in AH, the rapid diffusion of the molecule into the iris/CB observed in the biodistribution studies and the physical properties of the molecule indicate that the most likely route of entrance for SYL040012 into the eye is through the conjunctiva.
Stability can be an issue when working with RNA-based compounds.52 Here, we show that both SYL040012 and rSYL040012 are degraded at a slower pace in human and rabbit AH than in human and rabbit serum, where the compound is very rapidly degraded. The biodistribution studies indicate that systemic exposure to SYL040012 is extremely low and that the amounts present in circulation and systemic organs are not actively being taken up by the cells. The stability properties and biodistribution pattern of SYL040012 support the use of a siRNA-based therapeutic approach for glaucoma since the compound is stable enough to exert its effect in the eye but is rapidly degraded when reaching systemic circulation.
Most β receptor blockers used in clinic for the management of glaucoma are nonselective β-blockers. β-receptors present in the CB are mainly ADRB212; thus, the specificity of SYL040012 for ADRB2 ensures that only these target receptors are affected avoiding alteration of other receptors that could be responsible for eventual side effects.
Finally, the safety studies performed show no alterations in electrocardiograms or breathing activity; therefore typical side-effect observed in response to systemic β-blockers are not observed in animal models and are not expected in humans.
In summary, the results shown in the current study support the specificity of SYL040012 to silence its target gene in the tissue where it is supposed to exert its action. Restriction of the site of action is sustained on the labile nature of the molecule in systemic circulation and on the absence of cell penetration of the trace levels found in systemic tissues. The efficacy studies performed in animal models demonstrate that due to the particular mechanism of action of siRNAs, the effect observed on IOP is significantly longer than that of other drugs currently used for glaucoma management. Finally, safety studies confirm that SYL040012 is safe and well tolerated when administered over extended periods of time. SYL040012 is currently undergoing clinical trials with the hope of confirming the features of this molecule observed in animal models.
Materials and Methods
siRNAs. The siRNAs used in all studies were synthetized by BioSpring (Frankfurt am Main, Germany) using standard phosphoroamidite chemistry and contained the following sequences (5′ to 3′): SYL040012-sense: CAUUGUGCAUGUGAUCCAGdTdT, SYL040012-antisense: CUGGAUCACAUGCACAAUGdTdT, rSYL040012-sense: CAU CGUGCACGUGAUCCAGdTdT, rSYL040012-antisense: CUGGAUCACGUGCAC GAUGdTdT, scrambled-sense: GGCUACGUCCAGGAGCGCAdTdT Scrambled-antisense: UGCGCUCCUGGACGUAGCCdTdT.
Animals. Adult male New Zealand White (NZW) Rabbits (Granja San Bernardo, Spain; Charles River Laboratories and Hypharm, France) ~2–3 months old were used for the efficacy and biodistribution studies. Rabbits were individually housed in standard cages in a controlled-temperature room with a 12 hours light/dark cycle with free access to food and water. Drugs and vehicles were administered in all cases in a dose volume of 40 µl/eye.
Cynomolgus monkeys (Macaca fascicularis; Dayling Pingnan, Guangxi, China), aged 2–3 years, bred in captivity were used for the 28-day toxicology study. The females were nulliparous and nonpregnant. Cynomolgus monkeys were housed in an ambient-controlled room with temperatures set at 20–24 °C, relative humidity of 40–70% and a 12 hours light/dark cycle. Animals received 180 g of standard monkey diet/day and fresh fruit every day; water was available ad libitum. Drugs and vehicles were administered in the following dose volumes: 72 nmol/eye/day in 40 µl; 144 nmol/eye/day in 2 applications of 40 µl; and 288 nmol/eye/day in 4 applications of 40 µl.
Animal studies were approved by the Institutional Review Boards of both Sylentis and the CROs where the studies were performed. Animals were handled according to the ARVO statement for use of Animals in Ophthalmic and Vision Research.
All animals were subjected to a basic ophthalmic examination during the week before the beginning and at the end of each study. The following parameters were observed: eyelid irritation/inflammation, tear production, pupil size, cornea appearance, and conjunctive irritation/inflammation.
Cell culture and transfections. BxPC3 (human pancreatic epithelial cell line), MDA-MB-231 (human mammary gland adenocarcinoma cell line) and RAB-9 (rabbit fibroblast cell line) cells were obtained from American Association of Culture Collection (Rockville, MD) and maintained in culture medium (BxPC3 cells: RPMI-1640 medium supplemented with 10% FBS; MDA-MB-231 cells: 10% FBS supplemented DMEM and RAB-9 cells: EMEM medium supplemented with 10% FBS, 1 mmol/l Sodium Pyruvate and 1% of Glutamine) in a humidified incubator under an atmosphere of 5% CO2/95% air at 37 ºC. For transfections cells were seeded at a density of 106.000 cells/cm2 for BxPC3 line; 200.000 cells/cm2 for MDA-MB-231 line and 90.000 cells/cm2 for RAB-9 line. When cell cultures reached ~90% confluence, cells were transfected with siRNAs using Lipofectamine 2000 (Invitrogen, Pasley, UK). Transfection efficiency was estimated by quantifying the amount of Block-it-Alexa fluor red fluorescent oligonucleotide (Invitrogen) present inside cells in control cultures 24 hours after transfection.
RNA isolation, retrotrascription, and qPCR. Total RNA was isolated from cell cultures or tissues using RNeasy RNA extraction kit (Invitrogen, Carlsbad, CA). A 4 µg of total RNA were retrotranscribed using High-Capacity cDNA Archive kit (Applied Biosystems, Foster City, CA) according to the manufacturer's instructions.
qPCR was performed using Stepone plus detection system (Applied Biosystems). A 500 ng of each cDNA were amplified in a TaqMan 2X Universal Master Mix under the following conditions: 95 °C for 10 minutes, followed by 40 cycles of 95 °C for 15 seconds, and 60 °C for 1 minutes using TaqMan chemistry and FAM-labeled reporter.
The data were analyzed using the ΔΔCT method of relative quantification.
Cell viability assays. Cell viability was assessed by the 3-(4,5-dimethythiazol-2-yl)-2,5-diphenyl tetrazolium bromide method and assayed at different time-points (24, 48, and 72 hours) after transfection in MDA-MB-231, BxPC3, and RAB-9 cells using the Cell Titer 96 Aqueous Non Radioactive Cell Proliferation Assay kit (Promega, Mannheim, Germany) following the manufacturer's instructions.
Stability studies. Stability of SYL040012 and rSYL040012 in rabbit and human serum and AH was assessed by incubating the siRNAs at a concentration of 20 µmol/l in the appropriate biological fluid at 37 ºC. At the selected time-points the siRNAs were loaded and separated on a TBE-UREA denaturing polyacrylamide gel (20% w/v polyacrylamide, 8 mol/l urea in 1X TBE). Gels were run for at least 8 hours at 100 V and 4 ºC, thereafter stained with SYBR safe and visualized on a UVI-DOC-UV light visualization system. Pictures of the gels were digitilized and the density of the bands was analyzed using Image J.
siRNA biodistribution studies. SYL040012 or PBS was topically applied to both eyes of New Zealand White rabbits in a dose volume of 40 µl. Blood samples were obtained immediately before killing the animals by an overdose of pentobarbital. Blood was collected in K2EDTA tubes containing 10% DEPC in PBS and immediately frozen in liquid N2. Following sacrifice the following structures and organs were harvested and processed for quantification of SYL040012: cornea, AH, CB, iris, lachrymal gland, vitreous humor, lungs, liver, kidney cortex, and kidney medulla.
Quantification of SYL040012 and 5′PSYL040012. Tissue and plasma exposure of SYL040012 was evaluated at Axolabs GmbH (Kulmbach, Germany) using a nondenaturing Anion Exchange (AEX)-HPLC combined with fluorescence detection. Tissue samples were homogenized by ultrasonic treatment in a denaturing cell lysis buffer (Epicentre MTH, Madison, WI). The homogenized tissues as well as the plasma samples were subsequently treated with proteinase K at 65 °C for 30 minutes.
A 10 pmol of a fluorescently labeled PNA strand that is partially complementary to the antisense strand of SYL040012 was added to an aliquot of plasma and tissue lysis solution. (PNA-Sequence: Atto425-OO-CAT TGT GCA TGT GAT CCA from Panagene, Seoul, South Korea). Samples were heated at 95 °C for 10 minutes to dissociate SYL040012 siRNA into the single strands and subsequently cooled to room temperature.
Finally, the samples were evaluated by nondenaturing AEX-HPLC analysis on a Dionex Ultimate 3000 HPLC system coupled to a Dionex RF2000 fluorescence detector (Idstein, Germany) without further processing. Aliquots corresponding to 4.5 µl plasma or up to 5 mg tissue containing between 0.16 and 500 fmol SYL040012 were analyzed. The duplex formed between the SYL040012 antisense strand as well as related metabolites (e.g., the 5′-phosphorylated antisense strand) with fluorescently labeled PNA probe were separated on a Dionex DNA Pac PA200 column (4 × 250 mm) applying a sodium chloride gradient from 180 to 450 mmol/l. Excess PNA eluted in the void volume of the HPLC system and no unspecific background signals were observed during gradient elution. All peak areas for duplex containing intact antisense strand as well as duplex containing metabolites were evaluated against an external calibration curve from the parent compound SYL040012 (0.16 up to 500 fmol on column).
IOP measurement. IOP was measured using the Applanation Tonometer TONO-PEN AVIA after topical application of 0.4% tetracaine + 0.4% oxibuprocaine to the cornea, to avoid animal discomfort. All measurements were performed in triplicate and the average of the three measurements was used.
Oral water-loading hypertension model. Four days before beginning each study, five IOP measurements were registered with 2-hour intervals between measurements. Rabbits were thereafter assigned to experimental groups randomizing the animals according to IOP values. Compounds were administered once a day over a period of 4 days in a dose volume of 40 µl per eye. PBS was used as a negative control. During the first 3 days of administration, basal IOP measurements were recorded before test item or vehicle administration. On the day of induction of ocular hypertension, basal IOP measurements were recorded before administration and 1 and 2 hours after administration. At this point, hypertension was induced by means of oral water-loading (60 ml/kg) in overnight fasted animals. Thereafter, IOP was measured a total of 10 times with a 25-minute interval between measurements. After the last measurement, animals were euthanized by an overdose of pentobarbital and the following ocular structures were collected for target gene knock-down analysis: CB, lachrymal gland, and cornea.
Acknowledgments
Efficacy studies in normotensive rabbits were performed in collaboration with Jesús Pintor and Asumpta Peral (Escuela de Óptica, Universidad Complutense de Madrid). The authors are grateful to Ascensión Cuesta, Amor Guerra, Verónica Hellín, Susana Monteiro, and Ana Cadete (Sylentis) for excellent technical assistance and to Beatriz Vargas (Sylentis) for discussion of the results. Human aqueous humor was kindly provided by Jesús Moreno-Montañés (Clínica Universidad de Navarra). Funding: 32/07 (2007–2008) from Comunidad Autónoma de Madrid to A.I.J. and Nanofarma (2006–2010) from CDTI to A.I.J. T.M., V.G., N.W., C.P., and A.I.J. are employed by Sylentis, a subsidiary of Zeltia. I.R. declare no conflict of interest.
References
- Quigley HA. Glaucoma. Lancet. 2011;377:1367–1377. doi: 10.1016/S0140-6736(10)61423-7. [DOI] [PubMed] [Google Scholar]
- Varma R, Lee PP, Goldberg I, Kotak S. An assessment of the health and economic burdens of glaucoma. Am J Ophthalmol. 2011;152:515–522. doi: 10.1016/j.ajo.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–13; discussion 829. doi: 10.1001/archopht.120.6.701. [DOI] [PubMed] [Google Scholar]
- Caprioli J, Varma R. Intraocular pressure: modulation as treatment for glaucoma. Am J Ophthalmol. 2011;152:340–344.e2. doi: 10.1016/j.ajo.2011.05.029. [DOI] [PubMed] [Google Scholar]
- Khaw PT, Shah P, Elkington AR. Glaucoma–1: diagnosis. BMJ. 2004;328:97–99. doi: 10.1136/bmj.328.7431.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel M, Picciani RG, Lee RK, Bhattacharya SK. Aqueous humor dynamics: a review. Open Ophthalmol J. 2010;4:52–59. doi: 10.2174/1874364101004010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M. Early Manifest Glaucoma Trial Group Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120:1268–1279. doi: 10.1001/archopht.120.10.1268. [DOI] [PubMed] [Google Scholar]
- Sidjanin DJ, McCarty CA, Patchett R, Smith E, Wilke RA. Pharmacogenetics of ophthalmic topical beta-blockers. Per Med. 2008;5:377–385. doi: 10.2217/17410541.5.4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JA, Frishman WH, Wu Sun S, Palmiero PM, Petrillo R. Cardiovascular and respiratory considerations with pharmacotherapy of glaucoma and ocular hypertension. Cardiol Rev. 2008;16:95–108. doi: 10.1097/CRD.0b013e318156ec64. [DOI] [PubMed] [Google Scholar]
- Nieminen T, Lehtimäki T, Mäenpää J, Ropo A, Uusitalo H, Kähönen M. Ophthalmic timolol: plasma concentration and systemic cardiopulmonary effects. Scand J Clin Lab Invest. 2007;67:237–245. doi: 10.1080/00365510601034736. [DOI] [PubMed] [Google Scholar]
- Zimmerman TJ. Topical ophthalmic beta blockers: a comparative review. J Ocul Pharmacol. 1993;9:373–384. doi: 10.1089/jop.1993.9.373. [DOI] [PubMed] [Google Scholar]
- Wax MB, Molinoff PB. Distribution and properties of beta-adrenergic receptors in human iris-ciliary body. Invest Ophthalmol Vis Sci. 1987;28:420–430. [PubMed] [Google Scholar]
- Elena PP, Denis P, Kosina-Boix M, Saraux H, Lapalus P. Beta adrenergic binding sites in the human eye: an autoradiographic study. J Ocul Pharmacol. 1990;6:143–149. doi: 10.1089/jop.1990.6.143. [DOI] [PubMed] [Google Scholar]
- Elena PP, Kosina-Boix M, Moulin G, Lapalus P. Autoradiographic localization of beta-adrenergic receptors in rabbit eye. Invest Ophthalmol Vis Sci. 1987;28:1436–1441. [PubMed] [Google Scholar]
- Trope GE, Clark B. Beta adrenergic receptors in pigmented ciliary processes. Br J Ophthalmol. 1982;66:788–792. doi: 10.1136/bjo.66.12.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- Tuschl T, Sharp PA, Bartel DP. Selection in vitro of novel ribozymes from a partially randomized U2 and U6 snRNA library. EMBO J. 1998;17:2637–2650. doi: 10.1093/emboj/17.9.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamore PD, Tuschl T, Sharp PA, Bartel DP. RNAi: double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell. 2000;101:25–33. doi: 10.1016/S0092-8674(00)80620-0. [DOI] [PubMed] [Google Scholar]
- Lu PY, Xie F, Woodle MC. In vivo application of RNA interference: from functional genomics to therapeutics. Adv Genet. 2005;54:117–142. doi: 10.1016/S0065-2660(05)54006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Fraga M, Martínez T, Jiménez A. RNA interference technologies and therapeutics: from basic research to products. BioDrugs. 2009;23:305–332. doi: 10.2165/11318190-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooddell CI, Rozema DB, Hossbach M, John M, Hamilton HL, Chu Q, et al. Hepatocyte-targeted RNAi therapeutics for the treatment of chronic hepatitis B virus infection. Mol Ther. 2013;21:973–985. doi: 10.1038/mt.2013.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behlke MA. Progress towards in vivo use of siRNAs. Mol Ther. 2006;13:644–670. doi: 10.1016/j.ymthe.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campochiaro PA. Potential applications for RNAi to probe pathogenesis and develop new treatments for ocular disorders. Gene Ther. 2006;13:559–562. doi: 10.1038/sj.gt.3302653. [DOI] [PubMed] [Google Scholar]
- Kornbrust D, Cavagnaro J, Levin A, Foy J, Pavco P, Gamba-Vitalo C, et al. Oligo safety working group exaggerated pharmacology subcommittee consensus document. Nucleic Acid Ther. 2013;23:21–28. doi: 10.1089/nat.2012.0399. [DOI] [PubMed] [Google Scholar]
- Grimm D, Streetz KL, Jopling CL, Storm TA, Pandey K, Davis CR, et al. Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways. Nature. 2006;441:537–541. doi: 10.1038/nature04791. [DOI] [PubMed] [Google Scholar]
- Nykänen A, Haley B, Zamore PD. ATP requirements and small interfering RNA structure in the RNA interference pathway. Cell. 2001;107:309–321. doi: 10.1016/s0092-8674(01)00547-5. [DOI] [PubMed] [Google Scholar]
- Gupta SK, Agarwal R, Galpalli ND, Srivastava S, Agrawal SS, Saxena R. Comparative efficacy of pilocarpine, timolol and latanoprost in experimental models of glaucoma. Methods Find Exp Clin Pharmacol. 2007;29:665–671. doi: 10.1358/mf.2007.29.10.1147765. [DOI] [PubMed] [Google Scholar]
- Hariton C, Marce D, Debon C. Transitory models of experimentally induced intraocular pressure changes in the rabbit. A reappraisal. J Pharmacol Methods. 1990;24:79–88. doi: 10.1016/0160-5402(90)90019-h. [DOI] [PubMed] [Google Scholar]
- Bonomi L, Tomazzoli L, Jaria D. An improved model of experimentally induced ocular hypertension in the rabbit. Invest Ophthalmol. 1976;15:781–784. [PubMed] [Google Scholar]
- Santafé J, Martínez de Ibarreta MJ, Segarra J, Melena J. The effect of topical diltiazem on ocular hypertension induced by water loading in rabbits. Gen Pharmacol. 1999;32:201–205. doi: 10.1016/s0306-3623(98)00196-7. [DOI] [PubMed] [Google Scholar]
- Kingman S. Glaucoma is the second leading cause of blindness globally. Bull World Health Organ. 2004;82:811–890. [PMC free article] [PubMed] [Google Scholar]
- Alm A, Camras CB, Watson PG. Phase III latanoprost studies in Scandinavia, the United Kingdom and the United States. Surv Ophthalmol. 1997;41 suppl. 2:S105–S110. doi: 10.1016/s0039-6257(97)80016-1. [DOI] [PubMed] [Google Scholar]
- Servat JJ, Bernardino CR. Effects of common topical antiglaucoma medications on the ocular surface, eyelids and periorbital tissue. Drugs Aging. 2011;28:267–282. doi: 10.2165/11588830-000000000-00000. [DOI] [PubMed] [Google Scholar]
- De Natale R, Le Pen C, Berdeaux G. Efficiency of glaucoma drug regulation in 5 European countries: a 1995-2006 longitudinal prescription analysis. J Glaucoma. 2011;20:234–239. doi: 10.1097/ijg.0b013e3181e0791c. [DOI] [PubMed] [Google Scholar]
- Aagaard L, Rossi JJ. RNAi therapeutics: principles, prospects and challenges. Adv Drug Deliv Rev. 2007;59:75–86. doi: 10.1016/j.addr.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soutschek J, Akinc A, Bramlage B, Charisse K, Constien R, Donoghue M, et al. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature. 2004;432:173–178. doi: 10.1038/nature03121. [DOI] [PubMed] [Google Scholar]
- Nathanson JA. Differential inhibition of beta adrenergic receptors in human and rabbit ciliary process and heart. J Pharmacol Exp Ther. 1985;232:119–126. [PubMed] [Google Scholar]
- Orihashi M, Shima Y, Tsuneki H, Kimura I. Potent reduction of intraocular pressure by nipradilol plus latanoprost in ocular hypertensive rabbits. Biol Pharm Bull. 2005;28:65–68. doi: 10.1248/bpb.28.65. [DOI] [PubMed] [Google Scholar]
- Ahmed Z, Kalinski H, Berry M, Almasieh M, Ashush H, Slager N, et al. Ocular neuroprotection by siRNA targeting caspase-2. Cell Death Dis. 2011;2:e173. doi: 10.1038/cddis.2011.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser PK, Symons RC, Shah SM, Quinlan EJ, Tabandeh H, Do DV, et al. Sirna-027 Study Investigators RNAi-based treatment for neovascular age-related macular degeneration by Sirna-027. Am J Ophthalmol. 2010;150:33–39.e2. doi: 10.1016/j.ajo.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Reich SJ, Fosnot J, Kuroki A, Tang W, Yang X, Maguire AM, et al. Small interfering RNA (siRNA) targeting VEGF effectively inhibits ocular neovascularization in a mouse model. Mol Vis. 2003;9:210–216. [PubMed] [Google Scholar]
- Shen J, Samul R, Silva RL, Akiyama H, Liu H, Saishin Y, et al. Suppression of ocular neovascularization with siRNA targeting VEGF receptor 1. Gene Ther. 2006;13:225–234. doi: 10.1038/sj.gt.3302641. [DOI] [PubMed] [Google Scholar]
- Kleinman ME, Kaneko H, Cho WG, Dridi S, Fowler BJ, Blandford AD, et al. Short-interfering RNAs induce retinal degeneration via TLR3 and IRF3. Mol Ther. 2012;20:101–108. doi: 10.1038/mt.2011.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman ME, Yamada K, Takeda A, Chandrasekaran V, Nozaki M, Baffi JZ, et al. Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. Nature. 2008;452:591–597. doi: 10.1038/nature06765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears M, Maurice DM, Mishima S. Ocular pharmacokinetics. Springer: Berlin; Pharmacology of the Eye. 1984;vol. 69:19–116. [Google Scholar]
- Urtti A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv Drug Deliv Rev. 2006;58:1131–1135. doi: 10.1016/j.addr.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Ahmed I, Patton TF. Importance of the noncorneal absorption route in topical ophthalmic drug delivery. Invest Ophthalmol Vis Sci. 1985;26:584–587. [PubMed] [Google Scholar]
- Huang AJ, Tseng SC, Kenyon KR. Paracellular permeability of corneal and conjunctival epithelia. Invest Ophthalmol Vis Sci. 1989;30:684–689. [PubMed] [Google Scholar]
- Bochot A, Mashhour B, Puisieux F, Couvreur P, Fattal E. Comparison of the ocular distribution of a model oligonucleotide after topical instillation in rabbits of conventional and new dosage forms. J Drug Target. 1998;6:309–313. doi: 10.3109/10611869808996838. [DOI] [PubMed] [Google Scholar]
- Hämäläinen KM, Kananen K, Auriola S, Kontturi K, Urtti A. Characterization of paracellular and aqueous penetration routes in cornea, conjunctiva, and sclera. Invest Ophthalmol Vis Sci. 1997;38:627–634. [PubMed] [Google Scholar]
- Ottiger M, Thiel MA, Feige U, Lichtlen P, Urech DM. Efficient intraocular penetration of topical anti-TNF-alpha single-chain antibody (ESBA105) to anterior and posterior segment without penetration enhancer. Invest Ophthalmol Vis Sci. 2009;50:779–786. doi: 10.1167/iovs.08-2372. [DOI] [PubMed] [Google Scholar]
- Hickerson RP, Vlassov AV, Wang Q, Leake D, Ilves H, Gonzalez-Gonzalez E, et al. Stability study of unmodified siRNA and relevance to clinical use. Oligonucleotides. 2008;18:345–354. doi: 10.1089/oli.2008.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]