Abstract
The objective of this study was to evaluate ocular tolerance, safety, and effect on intraocular pressure (IOP) of a topically administered small interfering RNA; SYL040012, on healthy volunteers. The study was an open-label, controlled, single-center study comprised of two intervals that enrolled 30 healthy subjects having IOP below 21 mmHg. SYL040012 was administered to one eye as a single dose to six subjects during interval 1. During interval 2 two different doses of SYL040012 were administered to one eye on a daily basis to two separate groups of 12 subjects each, over a period of 7 days. The contralateral eye was evaluated but not administered and served as control for the tolerance study. SYL040012 was well tolerated locally. No local or systemic adverse events related to the product developed in response to any of the doses studied. SYL040012 was not detected in plasma at any time point. Administration of SYL040012 over a period of 7 days reduced IOP values in 15 out of 24 healthy subjects regardless of the dose used. IOP decrease was statistically significant in response to one of the doses tested and responsiveness to SYL040012 seemed to be greater in individuals with higher baseline IOP.
Introduction
RNA interference (RNAi) is a technology based on the principle that specifically designed, chemically synthesized, small RNA fragments can mediate specific mRNA degradation in the cytoplasm, thus inhibiting the synthesis of specific proteins.1,2,3,4 Compounds based on this technology can be rationally designed to block expression of any target gene, including genes for which traditional small molecule inhibitors cannot be found.5 Examples of successful in vivo use of RNAi with a view to developing therapeutics include reduction of virus-load in animal models infected with hepatitis B virus by poly-conjugate targeted delivery of cholesterol conjugated siRNAs to hepatocytes6 or ocular neuroprotection induced by a siRNA targeting caspase-2.7 RNAi has rapidly progressed and several compounds are already in advanced phases of clinical trials, such as RTP801 (Quark Pharmaceuticals, Fremont, PA; phase II) for treating age-related macular degeneration8 or ALN-RSV01 (Alnylam Pharmaceuticals, Cambridge, MA; phase II) for treating respiratory syncytial virus infection.9 SYL040012 is the first compound based on RNAi and administered in eye drops to be tested in humans.
Glaucoma is a syndrome characterized by progressive optic neuropathy and irreversible visual field loss. Glaucoma is the main cause of blindness in industrialized countries.10 Risk factors for developing glaucoma include elevated intraocular pressure (IOP), family history, ethnic background, and older age.11 Lowering IOP has shown to reduce the progression of nerve damage and therefore therapeutic management of glaucoma includes medications or surgeries that decrease IOP. The preferred IOP-lowering agents are locally applied prostaglandins and/or β-blockers.12,13 Prostaglandins lower IOP extremely well and are systemically safe but can have associated ocular side effects14 such as darkening of the iris color, lash growth, periocular pigmentation, and hyperemia. Less frequent ocular side effects of this drug class are intraocular inflammation, cystoid macular edema, and reactivation of ocular corneal herpes viral infections.15 In addition, prostaglandin analogs are contraindicated during pregnancy because of the potential risk of premature labor. β-blockers on the other hand are very well tolerated locally but are absorbed via conjunctival epithelium, lacrimal channel, nasal mucosa, and gastrointestinal tract into the systemic circulation. Circulating β-blockers are absorbed by systemic tissues such as lungs and heart where they cause undesired effects.16 Systemic side effects of β-blockers include among others bradycardia, hypotension, dizziness, bronchospasm, slow heart rate, depression, and fatigue. These systemic side effects associated with chronic use of topical β-blockers limit their use in populations in which β-blockers are contraindicated, such as patients with asthma, chronic obstructive pulmonary disease, or diabetes.16
SYL040012 is a double-stranded oligonucleotide that specifically inhibits synthesis of β2-adrenergic-receptor (ADRB2) via RNAi without affecting expression of other receptors of the adrenergic family. SYL040012 is a new chemical entity that decreases IOP by reducing local expression of ADRB2 after topical ocular instillation in animal models.17,18
This study of the safety, tolerability, and bioavailability of SYL040012 is the first step in the clinical development of SYL040012 for treatment of elevated IOP associated to glaucoma.
Results
Safety and tolerability
No ocular surface changes were observed at any time point. Treatment was well tolerated in response to either a single administration of 600 µg/eye/day of the compound or a repeated schedule of a daily administration of either 600 or 900 µg/eye/day SYL040012 over a period of 7 days. No adverse events were reported in response to a single dose of SYL040012 and six mild adverse events were reported in six separate subjects during the repeated administration schedule (interval 2; Table 1). Three of these events were determined to be unrelated to the compound, i.e., a muscle spasm due to a slight traumatic contusion, grade 1 conjunctival hyperemia in the nonadministered eye, and bilateral itching and difficulty focusing. All of these adverse events were observed in the 900 µg/eye/day dose group. The other three adverse events were: unilateral stinging in the administered eye, sporadic headache that lasted for 24 hours, and grade 1 conjunctival hyperemia in the administered eye. The first two adverse events were observed in the low dose group (600 µg/eye/day) whereas the latter adverse event was observed in the high dose group (900 µg/eye/day). The unilateral stinging started immediately after the second instillation and lasted 2 minutes. Grade 1 hyperemia was detected 22 hours after the second instillation and lasted throughout the period of administration; 24 hours after the last instillation hyperemia was undetectable. A comprehensive ocular examination was performed on the subjects who reported adverse events in the administered eye to rule out ocular surface damage. This examination showed no ocular changes and no underlying causes for the symptoms were identified. All events observed in the treated eye resolved spontaneously and only the muscle spasm due to an accident required medication.
Table 1. Adverse events observed after each administration schedule.
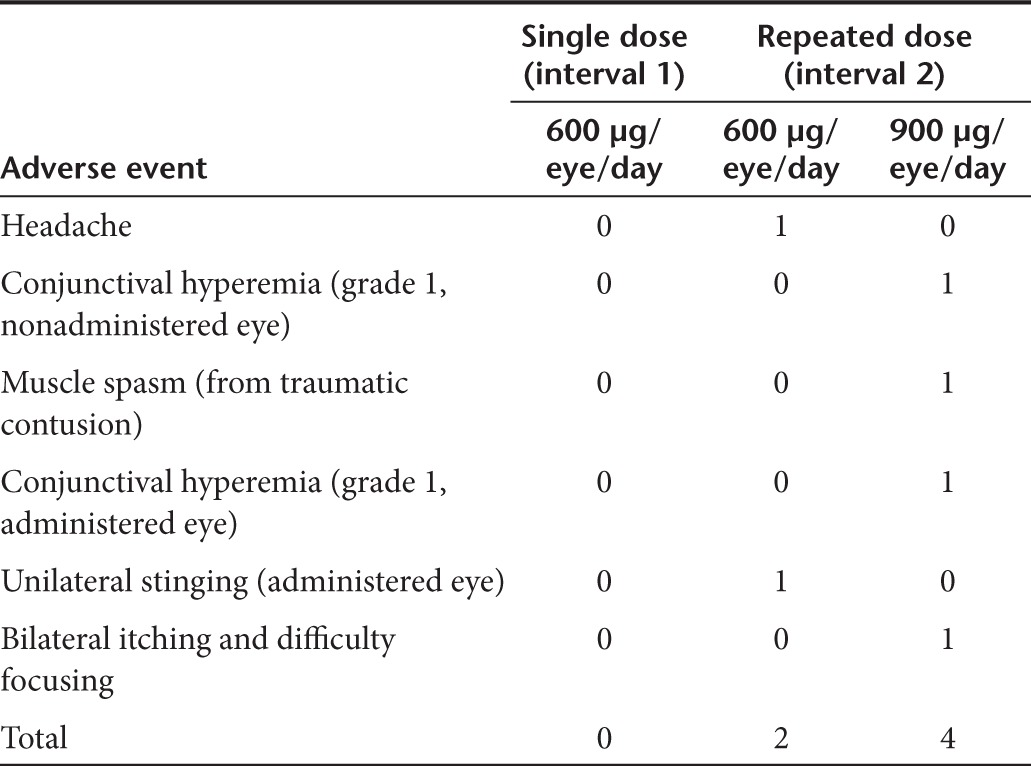
The comprehensive ocular examinations performed before the beginning of the study and at final examination indicated that ocular parameters did not differ significantly between both time-points (Table 2). There was a slight reduction in the number of corneal epithelial cells at final examination in interval 2; this observation was made in the untreated eye and was therefore ruled out to be related to SYL040012 administration.
Table 2. Ocular parameters at screening and final examination.
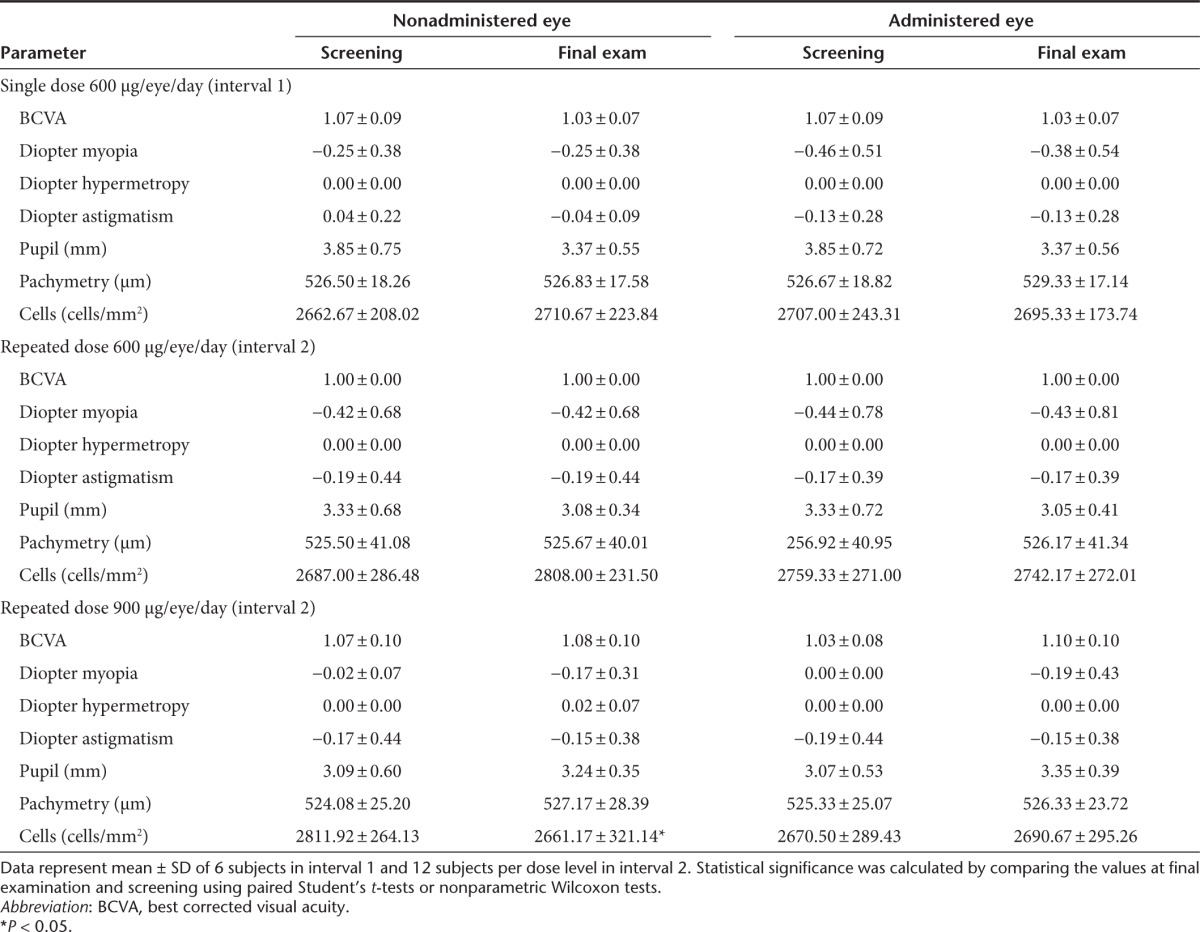
No significant changes were observed during the comprehensive clinical evaluations, monitoring of vital signs, or electrocardiograms. The results of vital sign monitoring at screening and final examination are shown in Table 3. It should be noted that there was a statistically significant reduction in diastolic blood pressure following the repeated-dose schedule of 600 µg/eye/day SYL040012. This slight decrease in diastolic pressure was not accompanied by changes in other parameters and the values observed were always within normal range. In addition, no reduction in diastolic blood pressure was observed in response to 900 µg/eye/day SYL040012. No relevant changes were observed in hematology, clinical biochemistry, and urinalysis parameters in response to any of the administration schedules of SYL040012.
Table 3. Vital signs recorded at screening and final examination.
SYL040012 was not detected (lower limit of quantification, 44.2 ng/ml) in any of the blood samples collected after a single instillation. To assess possible accumulation of the compound in plasma after repeated instillations, sample collection and quantification of SYL040012 during interval 2 was performed before the first instillation and 5 minutes after the seventh instillation. SYL040012 was not detected in any of the samples collected during interval 2. These results leave three possible avenues: (i) SYL040012 is present at concentrations below the lower limit of quantification; (ii) SYL040012 administered in eye drops does not reach systemic circulation; or (iii) SYL040012 reaches systemic circulation but is degraded and cleared within 5 minutes of administration.
Effect of SYL040012 on IOP
No significant differences in IOP were seen between values obtained at screening and those obtained following a single instillation of 600 µg/eye/day SYL040012. During interval 2, administration of SYL040012 on a repeated-dose schedule over a period of 7 days reduced IOP values in 15 out of 24 healthy subjects regardless of the dose used. Administration of 600 µg/eye/day SYL0420012 caused an overall statistically significant decrease in IOP after 4 days of administration; the post hoc data analysis showed a significant effect of SYL040012 on the measurements obtained at 15:00 hours (Figure 1a). Five volunteers who received the dose of 600 µg/eye/day showed a mean decrease in IOP values exceeding 20% on day 4 compared with values at screening. We performed a separate analysis in this subgroup and found an overall statistically significant effect on IOP; the post hoc analysis revealed that the differences were statistically significant at all time-points studied (Figure 1b). It is noteworthy that the basal IOP value in these five subjects was higher than the basal IOP values in other subjects (16.2 ± 2.9 mmHg versus 14.9 ± 2.8 mmHg, respectively). The dose of 900 µg/eye/day did not have a significant effect on the IOP curve after the same treatment period (P = 0.05) (Figure 1c).
Figure 1.
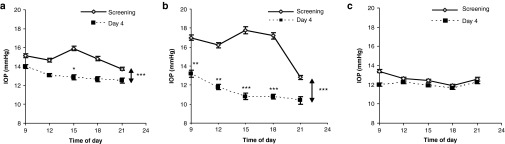
Intraocular pressure (IOP) curves in response to 600 or 900 μg/eye/day SYL040012. (a) IOP evolution in 12 healthy subjects in response to repeated administration of 600 µg/eye/day SYL040012; (b) IOP evolution in the subgroup of subjects that showed a decrease in IOP >20% in response to 600 µg/eye/day SYL040012 (n = 5). (c) IOP evolution in 12 healthy subjects in response to repeated administrations of 900 µg/eye/day SYL040012. Data represent mean ± SEM of 12 subjects in a and c and 5 subjects in b. Statistical significance was calculated by repeated measures two-way analysis of variance and Bonferroni's corrections were made for the subsequent pairwise comparisons and was as follows: ***P < 0.001; **P < 0.01; and *P < 0.05.
Discussion
The goal in medical management of glaucoma is lowering IOP to prevent vision loss. β-blockers are a mainstay in IOP decrease and are present in most marketed combination therapies for treating primary open-angle glaucoma. However, in the last decade prostaglandin analogues have replaced β-blockers as standard first-line treatment because of their safety profile and better patient compliance.12 Prostaglandin analogues are however not free from disadvantages; several of the marketed prostaglandins can have ocular tolerance issues and cause cosmetic changes in the eyes' appearance. These issues can impact patient compliance and this is particularly relevant when treatment is only required in one eye.15
The results presented in this study show that SYL040012, a siRNA that specifically decreases ADRB2, administered in eye drops is well tolerated in single or repeated schedules. No drug-related ocular events developed throughout either study interval. The adverse events observed in response to the repeated treatment schedule were minor, reversible and no direct relationship to the compound could be established. SYL040012 was not detected in plasma at any time after either a single or after a 7-day period repeated instillation. The absence of SYL040012 from plasma when the compound is applied in eye drops is not surprising, since RNases are abundantly present in blood and these enzymes are responsible for rapid degradation of the compound once it reaches the bloodstream.19 In addition, animal biodistribution studies performed using a newly developed bioanalysis method with a lower limit of detection of 0.25 ng/ml have shown that only traces of SYL040012 can be detected in blood after topical ocular instillation. This newly developed method not only detects SYL040012 but is also able to detect a metabolite of SYL040012 produced only when the compound is located inside the cells: SYL040012-5′-P. SYL040012-5′-P is only detected in ocular structures following topical ocular instillation of SYL040012 and is completely absent from systemic circulation or tissues, indicating that the compound is only active in cells located in the eye. These data support the observations made in humans and in addition could explain the absence of systemic side effects observed both in animal models and humans. In the present study a slight, but statistically significant, reduction in diastolic blood pressure was observed in response to 600 µg/eye/day SYL040012. As mentioned above, it is not likely that SYL040012 reaches any systemic organ when administered in eye drops, but the eventual impact of this observation will be clarified in subsequent placebo-controlled clinical trials. The low systemic exposure of SYL040012 is in agreement with previous observations for other oligonucleotides administered locally in the eye. GS-101, a phosphorothioate antisense oligonucleotide was not detected in plasma following administration of the compound in eye drops.20 Currently available treatments for glaucoma based on β-blockers reach the bloodstream at concentrations that can induce adverse effects. Both timolol and carteolol have been detected in plasma after ocular instillation;21,22 and side effects have been recorded following administration of β-blockers in eye drops.
The effect of SYL040012 on IOP was analyzed after a single or seven repeated instillations. It should be mentioned, that although IOP was measured, no effect was expected since this phase I study was performed in healthy volunteers with normal IOP values (<21 mmHg) and no control (placebo treated) group was available to compare the potential IOP-lowering effect of SYL040012. The results obtained in subjects treated according to a repeated-dose schedule showed an IOP decrease in 15 out of 24 subjects regardless of the dose. This decrease in IOP was statistically significant when compared with the basal IOP curve in response to the dose of 600 µg/eye/day after 4 days of administration. Among these 12 subjects, 5 showed a higher mean IOP decrease (31.5 ± 7.3%). This particular group of subjects, that was more responsive than others to SYL040012, had higher baseline IOP values (16.2 ± 2.9 mmHg) than the rest of the subjects in the trial (14.9 ± 2.8 mmHg) (Figure 1). This increased responsiveness of individuals with higher IOP values has been reported for other antiglaucoma drugs.23
RNAi-based therapies use the endogenous RNAi machinery to exert its action, thus administering high doses of a siRNA could potentially saturate this endogenous machinery.24 This fact might explain why the low dose used in this study seems to have a greater effect than the high dose. This speculation might be a possibility, but other factors such as higher basal IOP in the low dose group should not be ruled out, as the design of this study is not optimal for analyzing efficacy of SYL040012. Furthermore, the study was performed in healthy subjects and the eventual efficacy of SYL040012 on IOP cannot be extrapolated to patients with elevated IOP. Further development of SYL040012 includes a double blind, placebo-controlled, dose-range finding study that is currently ongoing; results from this study will help selecting the optimal dose.
Another of the advantages of SYL040012 relies on its mechanism of action. siRNAs target mRNAs inhibiting the synthesis of specific proteins. Once treatment is stopped, the cellular machinery must resynthesize the mRNA and thereafter translate mRNA into protein. This characteristic of siRNAs is thought to extend the effect of siRNA-based compounds. SYL040012 has been shown to have an IOP-lowering effect that lasts 15 times longer than conventional antiglaucoma treatments in animal studies.17,25 These features are attractive since success with current medications requires frequent instillations. If the long-lasting effect of SYL040012 observed in animals is confirmed in humans, the compound might protect against eventual optical nerve damage caused by the reboot effect in IOP in patients with poor treatment compliance.
In conclusion, this was the first time that a siRNA compound was tested in humans via topical ocular route. In the current phase I clinical trial, SYL040012 eye drops were well tolerated and locally and systemically safe at the maximal dose used. Although no IOP-lowering effect was expected since the study was performed in healthy volunteers, SYL040012 reduced IOP in individuals with a higher baseline IOP.
Materials and Methods
The Ethics Committee of the Clínica Universidad de Navarra and the Spanish Regulatory Agency approved the study protocol. The study was conducted in accordance with the Declaration of Helsinki and with International Conference in Harmonization Guidelines on Good Clinical Practices CMP/ICH/135/95. Subjects signed a written consent form stating that they understood and agreed to participate in the clinical study. This study was registered on www.clinicaltrials.gov (NCT00990743) and EudraCT Number is 2008-008204-41.
SYL040012 synthesis. SYL040012, administered as eye drops, is a synthetic 21-base sodium salt, double-stranded RNA oligonucleotide duplex formulated in phosphate buffered saline (pH 7.2). The sequence of SYL040012 is as follows: sense strand (5′ to 3′) CAUUGUGCAUGUGAUCCAGdTdT; antisense strand (5′ to 3′) CUGGAUCACAUGCACAAUGdTdT. SYL040012 was prepared in preservative-free single-unit dose vials. The drug substance for the clinical batch was synthesized at Biospring (Frankfurt am Main, Germany) and aliquoted in sterile single-dose vials at GP Pharm (Barcelona, Spain).
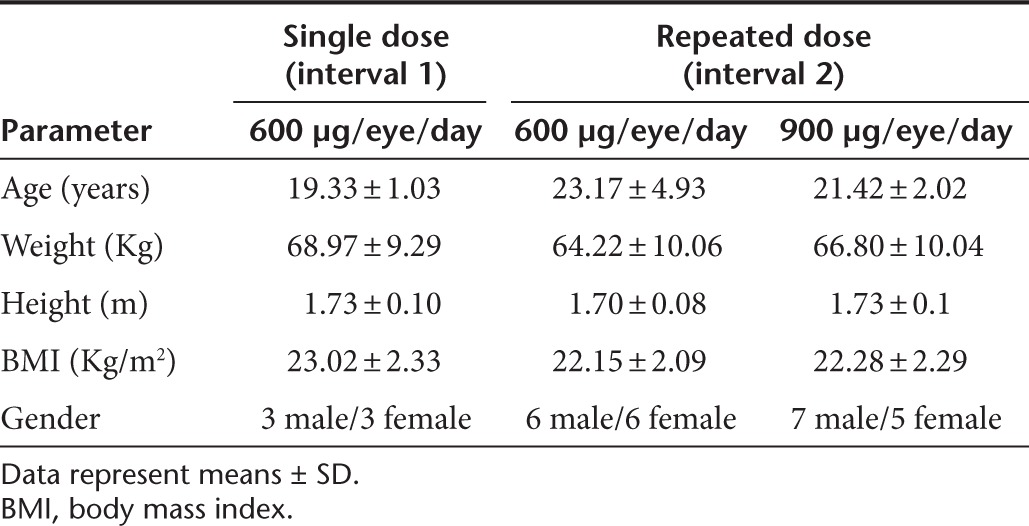
Subjects. Thirty healthy volunteers who had an IOP below 21 mmHg, Snellen visual acuity of 20/25 or better and who were at least 18 years of age were recruited. All subjects completed the study according to the protocol. The mean ± SDs of the subjects' demographic parameters are shown in Table 4. A comprehensive physical examination and an ocular examination were performed before admittance into the study to assure the suitability of the subjects for participation.
Table 4. Study subjects' demographic data.
Study design. A single-center, parallel, controlled, open-label phase I clinical study was designed to evaluate safety, tolerability, and bioavailability of SYL040012 administered as eye drops. An additional aim of the study was to determine the effect of different doses of SYL040012 on IOP. In all cases, the drug was instilled in one randomly chosen eye only; the fellow eye remained untreated and served as a control for ocular tolerance and safety. Both eyes were monitored in a blinded fashion.
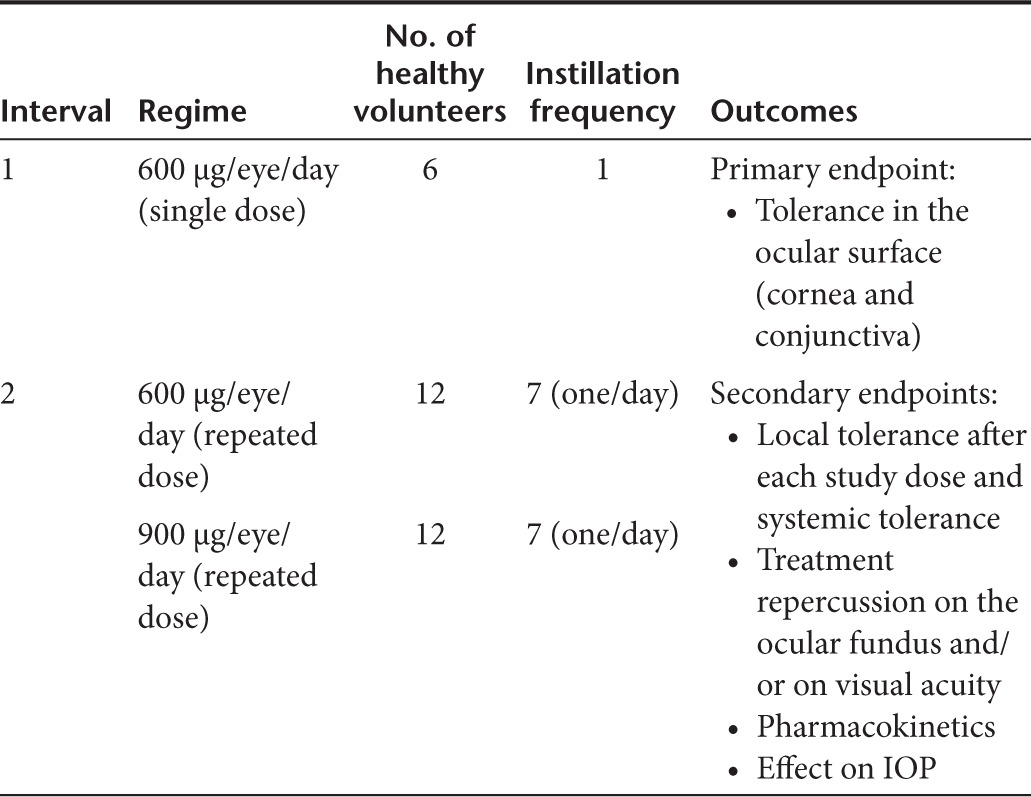
Treatment schedule. To minimize the risk of adverse effects and in accordance with the Guidelines on Strategies to Identify and Mitigate Risks for First-in-Human Clinical Trials with Investigational Medicinal Products (EMEA/CHMP/SWP/28367/07), the intervention phase was divided into two intervals. Interval 1 began with instillation of a single dose of 600 µg/eye/day SYL040012 in a dose volume of 26.6 µl to one subject who was observed for 72 hours. Tolerability was assessed at 24, 48, and 72 hours after instillation; when the tolerability criterion was met 72 hours after instillation, the next subject was dosed. The same procedure was followed for each new subject until six subjects had been administered. Good tolerance and thus the possibility of including the next volunteer was defined as an absence of grade 3 or higher toxicity on the Common Terminology Criteria for Adverse Events v3.0 scale.26 Safety and tolerability were assessed before interval 2 began.
During interval 2 SYL040012 was administered in daily instillations over 7 consecutive days. Two doses were assayed in this interval 600 and 900 µg/eye/day, each of which was administered to 12 subjects. For safety reasons, an initial group of three subjects received the low dose (600 µg/eye/day in a dose volume of 26.6 µl) of SYL040012; when the tolerability criterion previously described was met, the remaining subjects assigned to this dose were administered. The same procedure was performed for the high dose (900 µg/eye/day in a dose volume of 40 µl).
All subjects were treated in the Clinical Investigation Unit of the hospital, which guaranteed protocol compliance. Table 5 shows the design of the clinical trial.
Table 5. Clinical trial design.
Assessment of tolerability and outcome measures. The primary study endpoint was local ophthalmic tolerance. The occurrence and frequency of adverse events, ocular evaluation, pharmacokinetic analysis, and physical evaluations were secondary parameters evaluated to assess the drug's safety and systemic tolerability. The effect of SYL040012 on IOP was also evaluated.
The comprehensive clinical evaluations included a physical examination, monitoring of vital signs, clinical biochemistry and hematology, urine testing, and electrocardiography. Physical evaluations were performed during the screening phase and upon final examination at each interval.
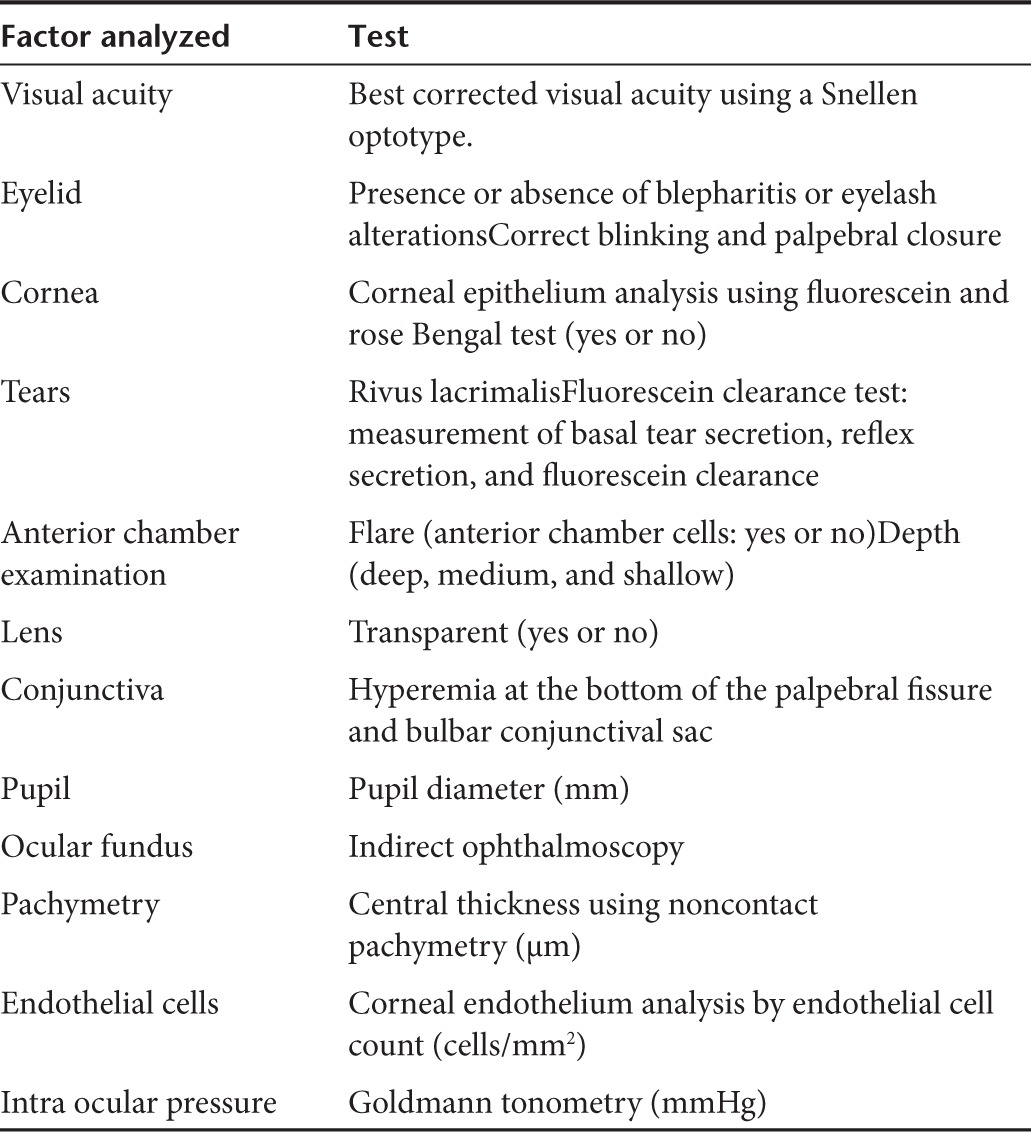
Two different groups of ophthalmic evaluations were performed; comprehensive ocular examinations and tolerance tests. Comprehensive bilateral ocular examinations were carried out during the screening phase, 72 hours after drug instillation at interval 1 and 1 hour after the last instillation at interval 2. Details of the ocular examination and tests are shown in Table 6. Tolerance tests consisting of ocular photographs and slit-lamp evaluation of the conjunctiva and cornea were carried out at the following time-points: before administration, and 1, 24, 48, and 72 hours after administration during interval 1; and 1 hour prior and 1 hour after administration on a daily basis, and 24 and 72 hours after the last administration during interval 2 of the study. Ophthalmologists blinded to the experimental condition performed all ocular evaluations. Subjective tolerance was only assessed if the subject reported ocular symptoms.
Table 6. Ocular tests performed at screening and final examination.
For the pharmacokinetic profile, during interval 1 blood samples were collected 1 hour before drug instillation and 5, 15, and 30 minutes and 4 hours after instillation and processed to obtain plasma. Results of the pharmacokinetic profile obtained during interval 1 determined the number of samples collected during interval 2. During interval 2, blood samples were collected approximately 1 hour before the first instillation and 5 minutes after the last instillation.
Analysis of samples. The bioanalytic method used to analyze the pharmacokinetic profile was validated at Harlan Laboratories (Barcelona, Spain). The lower limit of quantitation for this method was 44.2 ng/ml. Sample analysis was achieved in 15 minutes and SYL040012 was detected and quantified by tandem mass spectrometry. SYL040012 eluted at 11 minutes. SYL09000100 was used as the quantitative internal standard. The method was validated according to criteria from the US Food and Drug Administration entitled Bioanalytical Method Validation. The validated concentration interval of the bioanalytic procedure was 44.2 (lower limit of quantitation) to 441.8 ng/ml. Calibration curves obtained were characterized by a correlation coefficient higher than 0.99. Precision and accuracy were below 20% for the lower limit of quantitation level and below 15% for intermediate (110.5 ng/ml) and high (441.8 ng/ml) concentrations. The bioanalytic procedure was validated over 3 days and the results obtained for interassay accuracy and precision complied with acceptance criteria described in the Bioanalytical Method Validation.
IOP measurements. During interval 1, IOP was measured 1, 2, 4, 48, and 72 hours after instillation using Goldmann tonometry with the subjects sitting. During interval 2, the IOP curves were determined before the first instillation (screening) and after 4 days of treatment. In both cases, IOP was measured at 9:00, 12:00, 15:00, 18:00, and 21:00 hours. IOP was also measured every time ocular tolerance was assessed during intervals 1 and 2, 1 hour before and after instillation. Measurements performed outside of an IOP curve were taken in the morning between 9:00 and 12:00.
Statistical analysis. Ocular and conjunctival local tolerance after SYL040012 treatment was assessed by analyzing occurrence and frequency of ocular adverse effects 72 hours after instillation for interval 1 and 24 hours after the last instillation during interval 2. Comparisons were made between eyes (administered versus nonadministered) using the χ2 test.
Analysis of single daily IOP values after one instillation was performed by comparing values obtained after SYL040012 instillation to the basal value at screening. Statistical significance was assessed by paired Student's t-test. The effect of SYL040012 on IOP during interval 2 was assessed by comparing the IOP curve obtained at day 4 to the one obtained at screening. Statistical significance was assessed by repeated measures two-way analysis of variance, using treatment and time of day as variables and IOP as the repeated measure followed by a Bonferroni post hoc test to assess the significance at each time point. Other parameters (clinical analysis, visual acuity, and symptom duration) were analyzed using paired Student's t-tests or Wilcoxon test depending on compliance of the conditions required for using each of these statistical tests. P < 0.05 was considered significant.
Acknowledgments
The authors are grateful to Natalia Wright, Tamara Martínez and Beatriz Vargas (Sylentis) for assistance with the preparation of the manuscript and discussion of the results. Grant information: IDI-20090417 from Centro para el Desarrollo Tecnológico Industrial. V.R., M.V.G., C.P., and A.I.J. are employed by Sylentis. The other authors declare no conflict of interest.
References
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- Zamore PD, Tuschl T, Sharp PA, Bartel DP. RNAi: double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell. 2000;101:25–33. doi: 10.1016/S0092-8674(00)80620-0. [DOI] [PubMed] [Google Scholar]
- Lu PY, Xie F, Woodle MC. In vivo application of RNA interference: from functional genomics to therapeutics. Adv Genet. 2005;54:117–142. doi: 10.1016/S0065-2660(05)54006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behlke MA. Progress towards in vivo use of siRNAs. Mol Ther. 2006;13:644–670. doi: 10.1016/j.ymthe.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooddell CI, Rozema DB, Hossbach M, John M, Hamilton HL, Chu Q, et al. Hepatocyte-targeted RNAi therapeutics for the treatment of chronic hepatitis B virus infection. Mol Ther. 2013;21:973–985. doi: 10.1038/mt.2013.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed Z, Kalinski H, Berry M, Almasieh M, Ashush H, Slager N, et al. Ocular neuroprotection by siRNA targeting caspase-2. Cell Death Dis. 2011;2:e173. doi: 10.1038/cddis.2011.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen QD, Schachar RA, Nduaka CI, Sperling M, Basile AS, Klamerus KJ, et al. PF-04523655 Study Group Phase 1 dose-escalation study of a siRNA targeting the RTP801 gene in age-related macular degeneration patients. Eye (Lond) 2012;26:1099–1105. doi: 10.1038/eye.2012.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVincenzo J, Lambkin-Williams R, Wilkinson T, Cehelsky J, Nochur S, Walsh E, et al. A randomized, double-blind, placebo-controlled study of an RNAi-based therapy directed against respiratory syncytial virus. Proc Natl Acad Sci USA. 2010;107:8800–8805. doi: 10.1073/pnas.0912186107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokofyeva E, Zrenner E. Epidemiology of major eye diseases leading to blindness in Europe: a literature review. Ophthalmic Res. 2012;47:171–188. doi: 10.1159/000329603. [DOI] [PubMed] [Google Scholar]
- Coleman AL, Miglior S. Risk factors for glaucoma onset and progression. Surv Ophthalmol. 2008;53 suppl. 1:S3–10. doi: 10.1016/j.survophthal.2008.08.006. [DOI] [PubMed] [Google Scholar]
- De Natale R, Le Pen C, Berdeaux G. Efficiency of glaucoma drug regulation in 5 European countries: a 1995-2006 longitudinal prescription analysis. J Glaucoma. 2011;20:234–239. doi: 10.1097/ijg.0b013e3181e0791c. [DOI] [PubMed] [Google Scholar]
- Singh K, Shrivastava A. Medical management of glaucoma: principles and practice. Indian J Ophthalmol. 2011;59 suppl.:S88–S92. doi: 10.4103/0301-4738.73691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SK, Agarwal R, Galpalli ND, Srivastava S, Agrawal SS, Saxena R. Comparative efficacy of pilocarpine, timolol and latanoprost in experimental models of glaucoma. Methods Find Exp Clin Pharmacol. 2007;29:665–671. doi: 10.1358/mf.2007.29.10.1147765. [DOI] [PubMed] [Google Scholar]
- Servat JJ, Bernardino CR. Effects of common topical antiglaucoma medications on the ocular surface, eyelids and periorbital tissue. Drugs Aging. 2011;28:267–282. doi: 10.2165/11588830-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Han JA, Frishman WH, Wu Sun S, Palmiero PM, Petrillo R. Cardiovascular and respiratory considerations with pharmacotherapy of glaucoma and ocular hypertension. Cardiol Rev. 2008;16:95–108. doi: 10.1097/CRD.0b013e318156ec64. [DOI] [PubMed] [Google Scholar]
- Jimenez A, González V, Panizo G, Martínez T. SYL040012 a new treatment for glaucoma: Pharmacokinetics and mechanism of action. ARVO Meeting Abstracts. 2010;51:176. [Google Scholar]
- Bucolo C, Salomone S, Drago F, Reibaldi M, Longo A, Uva MG. Pharmacological management of ocular hypertension: current approaches and future prospective. Curr Opin Pharmacol. 2013;13:50–55. doi: 10.1016/j.coph.2012.09.012. [DOI] [PubMed] [Google Scholar]
- Haupenthal J, Baehr C, Kiermayer S, Zeuzem S, Piiper A. Inhibition of RNAse A family enzymes prevents degradation and loss of silencing activity of siRNAs in serum. Biochem Pharmacol. 2006;71:702–710. doi: 10.1016/j.bcp.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Kain H, Goldblum D, Geudelin B, Thorin E, Beglinger C. Tolerability and safety of GS-101 eye drops, an antisense oligonucleotide to insulin receptor substrate-1: a ‘first in man' phase I investigation. Br J Clin Pharmacol. 2009;68:169–173. doi: 10.1111/j.1365-2125.2009.03450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieminen T, Lehtimäki T, Mäenpää J, Ropo A, Uusitalo H, Kähönen M. Ophthalmic timolol: plasma concentration and systemic cardiopulmonary effects. Scand J Clin Lab Invest. 2007;67:237–245. doi: 10.1080/00365510601034736. [DOI] [PubMed] [Google Scholar]
- Renard P, Kovalski JL, Cochereau I, Jaulerry S, Williamson W, Elena PP, et al. Comparison of carteolol plasmatic levels after repeated instillations of long-acting and regular formulations of carteolol 2% in glaucoma patients. Graefes Arch Clin Exp Ophthalmol. 2005;243:1221–1227. doi: 10.1007/s00417-005-0024-5. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Tanihara H, Hiroi K, Honda Y. Prognostic factors for hypotensive effects of isopropyl unoprostone in eyes with primary open-angle glaucoma. Jpn J Ophthalmol. 1998;42:417–423. doi: 10.1016/s0021-5155(98)00036-7. [DOI] [PubMed] [Google Scholar]
- Bian Y, Zhou W, Zhao Y, Li X, Geng W, Hao R, et al. High-dose siRNAs upregulate mouse Eri-1 at both transcription and posttranscription levels. PLoS ONE. 2011;6:e26466. doi: 10.1371/journal.pone.0026466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez A, Mediero A, Loma P, Pintor J, Peral A, Gonzalez V. Efficacy of topically administered siRNAs in glaucoma treatment: In vivo results in hypertensive model. ARVO Meeting Abstracts. 2009;50:4054. [Google Scholar]
- Common Terminology Criteria for Adverse Events v3.0. 2006.


