Introduction
Aggression is an innate, species-typical social behavior that is widespread in animal phylogeny. Expression of agonistic behavior is commonly observed between conspecific males in conflict over access to reproductively active females, food, territory or other resources (Siegel et al., 1997). In many animal species, aggression is often quantitatively higher in males than in females (Lorenz, 1966). In humans, violent aggression constitutes a major public health problem (Filley et al., 2001) and its incidence is overwhelmingly higher among males than females (Craig and Halton, 2009). In addition, the behavioral expression of aggression is often qualitatively different between males and females, and may differ in the contexts in which it is exhibited (Lorenz, 1966).
Despite recent progress (reviewed in (Manoli et al., 2013)), the neurobiological mechanisms underlying the evolutionarily conserved sexual dimorphism in aggressiveness remain poorly understood. Pheromones are known to play an important role in inter-male aggression ((Chamero et al., 2007; Fernandez et al., 2010; Liu et al., 2011; Wang and Anderson, 2010; Wang et al., 2011); reviewed in (Stowers and Logan, 2010)). However, in cases where the relevant receptors are known (Wang and Anderson, 2010), dimorphic expression of these molecules does not appear to explain sex differences in aggressiveness (Kurtovic et al., 2007; Ruta et al., 2010). Studies in numerous vertebrate species have identified sexual dimorphisms in the size of brain nuclei, or of their constituent neuronal subpopulations, that are controlled by gonadal steroid hormones in a manner that parallels the influence of these hormones on aggressive behavior (reviewed in (Wu and Shah, 2011)). Recent studies have shown that genetic ablation of hypothalamic neurons expressing the progesterone receptor decreases both aggression and mounting in males, and mating behavior in females (Yang et al., 2013). These neurons display sexual dimorphisms in their projections, but whether this dimorphism is causally responsible for sex differences in levels of aggressiveness is not yet clear.
Drosophila melanogaster is an attractive model system for studying aggression, because of its genetic tractability and the potential to identify individual neurons that control specific innate behaviors (Chen et al., 2002; Zwarts et al., 2012). As in other species, male flies are more aggressive than females and also exhibit qualitative differences in agonistic behavior (Nilsen et al., 2004; Vrontou et al., 2006). These sex differences in aggression are known to be under the control of fruitless (fru), a master regulator of sexual differentiation of the brain (Lee and Hall, 2000; Siwicki and Kravitz, 2009; Vrontou et al., 2006). While some efforts have been made to identify circuits through which fru exerts its influence on aggressive behavior (Certel et al., 2010; Certel et al., 2007; Chan and Kravitz, 2007; Mundiyanapurath et al., 2009), FruM+ neurons that are necessary, sufficient and specific for male-type aggression have not yet been identified.
Here we have identified a small group of sexually dimorphic, FruM+ neurons that promote aggressiveness in Drosophila males, but which have no influence on male-female courtship behavior. These neurons enhance aggression, at least in part, through the release of a neuropeptide, Drosophila tachykinin (DTK) (Nassel and Winther, 2010; Winther et al., 2003). Tachykinin/Substance P has been implicated in certain forms of aggression in several mammalian species(Katsouni et al., 2009). Thus the higher level of aggression characteristic of Drosophila males is promoted by sexually dimorphic neurons, which express a neuropeptide that regulates agonistic behavior across phylogeny.
Results
Tachykinin-GAL4 lines label aggression-promoting neurons
We reasoned that neural circuits controlling aggression, like those controlling other innate behaviors, are likely to be regulated by neuropeptides (Bargmann, 2012; Nassel and Winther, 2010; Taghert and Nitabach, 2012). To identify peptidergic neurons that control inter-male aggression in Drosophila, we used the thermosensitive cation channel Drosophila TRPA1 (dTRPA1) (Hamada et al., 2008) to activate neurons labeled by a set of ~40 GAL4 driver lines created from putative promoter regions of ~20 different Drosophila neuropeptide genes (Hergarden et al., 2012; Tayler et al., 2012). We screened these lines for increases in aggressive behavior, using a high-throughput modification of hardware and software that permit automated detection of fly aggressive behaviors (Dankert et al., 2009) (Figure 1A).
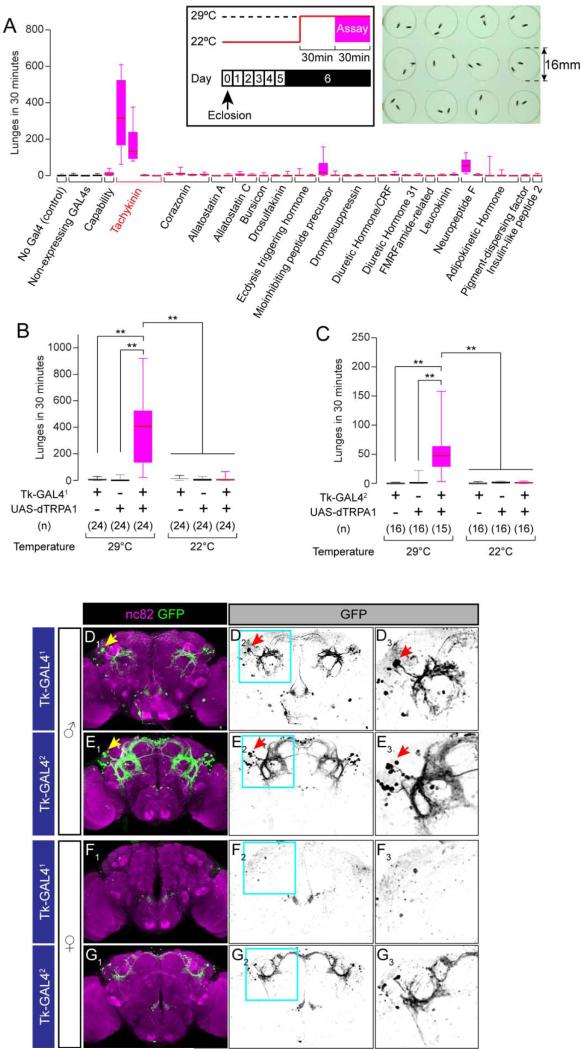
Figure 1. Tk-GAL4 Lines Label Aggression-promoting Neurons.
(A) Number of lunges (boxplot) during dTRPA1-mediated thermogenetic activation of neurons labeled by 42 neuropeptide-GAL4 drivers. A box indicates lower quartile, median and higher quartile, from bottom to top. Whiskers represent the range of the remaining data points. Names of the genes from which the promoter fragments were generated are listed below plots. n = 10-24. The inlet illustrates the experimental design (left) and the 12-well aggression chamber used in this assay (right).
(B, C) Number of lunges (boxplot) during thermogenetic activation of Tk-GAL41(B) and Tk-GAL42(C) neurons. Genotypes, number of pairs and temperature tested are indicated below the plot. ** p < 0.01 (Kruskal-Wallis and post-hoc Mann-Whitney U-tests). Horizontal bar above 22°C data indicates pooling for statistical analysis (Kruskal-Wallis test, p > 0.05).
(D-G) Brains of Tk-GAL41; UAS-mCD8:GFP male (D), female (F), Tk-GAL42; UAS-mCD8:GFP male (E) and female (G) immunostained with anti-GFP antibody (green) and the neuropil marker nc82 (magenta) (D1-G1). D2-G2: GFP only. D3-G3: region within a cyan square in D2-G2 (magnified). Arrows: the lateral cluster neurons (see text).
Also see Supplemental Figure S1 and Supplemental Movie S1.
This screen identified 2 Drosophila Tachykinin (Tk)-GAL4 lines that strongly increased aggression in combination with UAS-dTRPA1 at 29°C (Figure 1A). Activation of a Neuropeptide F-GAL4 line weakly enhanced aggression (Supplemental Figure S1A), an effect opposite to that described previously using a different NPF-GAL4 driver (Dierick and Greenspan, 2007). Secondary screens confirmed the genotype- and temperature-dependence of the enhanced aggression phenotype of the two Tk-GAL4 lines (which we henceforth refer to as Tk-GAL41 and Tk-GAL42, respectively) (Figure 1B, 1C). Aggression promoted by Tk-GAL41 neurons was particularly robust and intense (Supplemental Movie S1). The enhanced aggression phenotype was not due to an increase in locomotor activity (Supplemental Figure S1B, S1C). Two other Tk-GAL4 lines, Tk-GAL43 and Tk-GAL4GMR61H07, did not promote aggression (Supplemental Figure S1I, S1J). Activation of Tk-GAL41 neurons also increased aggression towards a wild type male (Supplemental Figure S1D). Thus, the aggression-promoting effect is likely due to a fly-autonomous influence, rather than for example to an increased release of aggression promoting pheromones (Fernandez et al., 2010; Wang and Anderson, 2010; Wang et al., 2011).
Expression analysis of Tk-GAL41 and Tk-GAL42, using a UAS-mCD8:GFP reporter, revealed a cluster of lateral protocerebral neurons that formed a ring-shaped arborization (Figure 1D, 1E), which resembled a previously characterized male-specific neuropil formed by fruitless-expressing neurons (Cachero et al., 2010; Yu et al., 2010). These cells and arborizations were absent in the corresponding area of the female brain (Figure 1F, 1G, D3-G3). A sexually dimorphic labeling pattern was also evident in the ventral nerve chord (VNC) (Supplemental Figure S1E-H).
Immunostaining against the male specific isoform of fruitless (FruM) revealed that the lateral cluster of Tk-GAL41 neurons indeed expresses FruM (5.3 ± 0.5 cells/hemibrain, n = 6, Figure 2A). We did not find other FruM-expressing Tk-GAL41 neurons in the central brain, except for a few inconsistently labeled cells located ventrally to the lateral cluster. The FruM+ lateral cluster neurons appeared to be labeled by Tk-GAL42 as well (Figure 2B; 7.6 ± 1.2 neurons/hemibrain, n = 6), although several other classes of FruM-expressing neurons were also labeled in that GAL4 line. The other 2 Tk-GAL4 lines that did not promote aggression did not contain the lateral cluster of FruM+ neurons associated with the ring-shaped arborization (Supplementary Figure S1K-N).
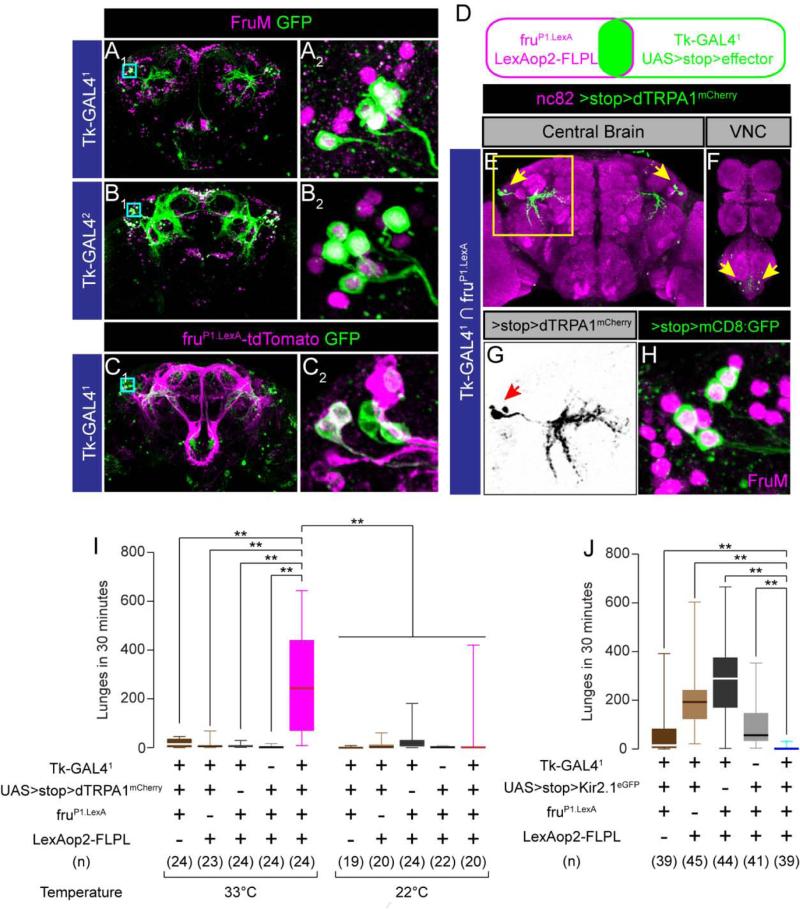
Figure 2. Tk-GAL4FruM neurons control male-male aggression.
(A, B) Tk-GAL41; UAS-mCD8:GFP (A) and Tk-GAL42; UAS-mCD8:GFP (B) male brains immunostained with anti-GFP antibody (green) and anti-FruM antisra (magenta). A2, B2: region within a cyan square in A1 and B1 (magnified).
(C) Tk-GAL41; fruP1.LexA/ UAS-mCD8:GFP, LexAop2-tdTomato male brain immunostained with anti-GFP antibody (green) and anti-DsRed antibody (magenta). C2: region within a cyan square in C1 (magnified).
(D) The schematic of the genetic intersectional strategy utilized in this study.
(E, F) Tk-GAL4FruM neurons in the brain (E) and VNC (F) immunostained with anti-DsRed antibody (green) and nc82 (magenta). Arrows: cell bodies stained with anti-DsRed antibody. (G) Region within a cyan square in (E) (magnified). Only the image of anti-DsRed is shown. Arrow: cell bodies stained with anti-DsRed antibody.
(H) Four Tk-GAL4FruM neurons in a male brain, immunostained with anti-GFP antibody (green) and anti-FruM antisera (magenta). The magnification compares to images A2, B2 and C2.
(I, J) Number of lunges during thermogenetic activation (I) and silencing (J) of Tk-GAL4FruM neurons.
For (I) and (J), ** p < 0.01 (Kruskal-Wallis and post-hoc Mann-Whitney U-tests).
Also see Supplemental Figure S2 and Supplemental Movie S2.
We confirmed the expression of FruM in the lateral cluster neurons using a genetic approach. Expression analysis of Tk-GAL41 and fruP1.LexA (Mellert et al., 2010) using dual reporters revealed that fruP1.LexA labels 3~4 of the ~6 Tk-GAL41 neurons in this cluster (Figure 2C; 3.1 ± 0.6 tdTomato+ cells/5.5 ± 0.5 GFP+ cells/hemibrain, n = 10). Similar results were obtained using fruP1.LexA to drive LexAop2-FLPL in combination with FLP recombinase-dependent reporters (Fig. 2D-H), including a UAS>stop>dTRPA1mCherry allele (Pan et al., 2012; Yu et al., 2010) used for functional manipulations (see below). Immunostaining with anti-FruM antibody confirmed that the Tk-GAL41 neurons identified by this intersectional strategy indeed express FruM protein (Figure 2H). The neurons revealed by dTRPA1mCherry expression (Figure 2G) appear similar to those in the aSPf/g FruM+ cluster, (Cachero et al., 2010; Yu et al., 2010), which was reported to contain 4-13 neurons in males. However intersectional expression of dTRPA1mCherry labeled only 4.2 ± 0.8 neurons/hemibrain (n = 8). We hereafter refer to these neurons as Tk-GAL4FruM neurons.
TK-GAL4FruM neurons control male-male aggression
To determine if Tk-GAL4FruM neurons are indeed responsible for the aggression phenotype, we carried out intersectional neuronal activation experiments (Pan et al., 2012; von Philipsborn et al., 2011) (Figure 2D). This approach yielded TRPA1mCherry expression exclusively in the lateral cluster (Figure 2E, arrows), and in 2 pairs of VNC neurons (Fig. 2F, arrows). No expression was detected when any one of the four genetic components used in the genetic intersection was omitted (Supplemental Figure S2A-S2D).
Thermogenetic activation of these Tk-GAL4FruM neurons induced robust male-male aggression, in a temperature-dependent manner (Figure 2I; Supplemental Movie S2). To determine whether the 2 pairs of abdominal ganglion neurons play a role in aggression, we generated a transgene, Otd-nls:FLPo, that expresses FLP specifically in the central brain (see Experimental Procedures). Tk-GAL41; Otd-nls:FLPo, UAS>stop>dTRPA1mCherry flies expressed dTRPA1mCherry selectively in the central brain, but not in the VNC (Supplemental Figure S2E-J). These flies showed robust male-male aggression at high temperature (Supplemental Figure S2K). Thus, activation of Tk-GAL4FruM neurons in the central brain is sufficient to promote inter-male aggression.
To determine whether Tk-GAL4FruM neurons are necessary for aggression, we genetically silenced them in single-housed flies, which display higher levels of baseline aggression than group-housed flies (Wang et al., 2008). To do this, we expressed the inwardly rectifying potassium channel Kir2.1 selectively in the Tk-GAL4FruM neurons by using UAS>stop>Kir2.1eGFP in combination with fruP1.LexA and LexAop2-FLPL. Such flies showed significantly reduced aggression compared to genetic controls, although the level of baseline aggression between different controls varied (Figure 2J). Thus, the activity of Tk-GAL4FruM neurons is necessary for aggression in single-housed flies. Together, these results identify a small subset of neurons that are necessary and sufficient for male-male aggression.
The function of Tk-GAL4FruM neurons is specific to aggression
FruM+ male neurons also control courtship behavior (Kimura et al., 2005; Manoli et al., 2005; Stockinger et al., 2005). Indirect evidence suggested that distinct populations of FruM+ neurons control inter-male aggression vs. male-female courtship (Chan and Kravitz, 2007), but FruM+ neurons that control male aggression but not male-female courtship have not previously been identified. For this reason, it was of interest to explore the influence of Tk-GAL4FruM neurons on male-female courtship.
We initially performed gain-of-function studies using intersectional expression of dTRPA1 in Tk-GAL4FruM neurons. Thermogenetic activation did not induce any aggressive behaviors toward females (0 lunges toward females by any of 24 males). Rather, the males copulated at a rate comparable to that of the control strains (Figure 3A). (An exception was a controlTk-GAL41; fruP1.LexA/ LexAop2-FLPL strain, which failed to copulate at 33°C, perhaps reflecting transcriptional “squelching” by the GAL4 activation domain in the absence of a UAS binding site (Gill and Ptashne, 1988).) We also measured copulation latency and the average duration of copulation, and found no significant difference between experimental and the other 3 control strains (Figure 3B, 3C).
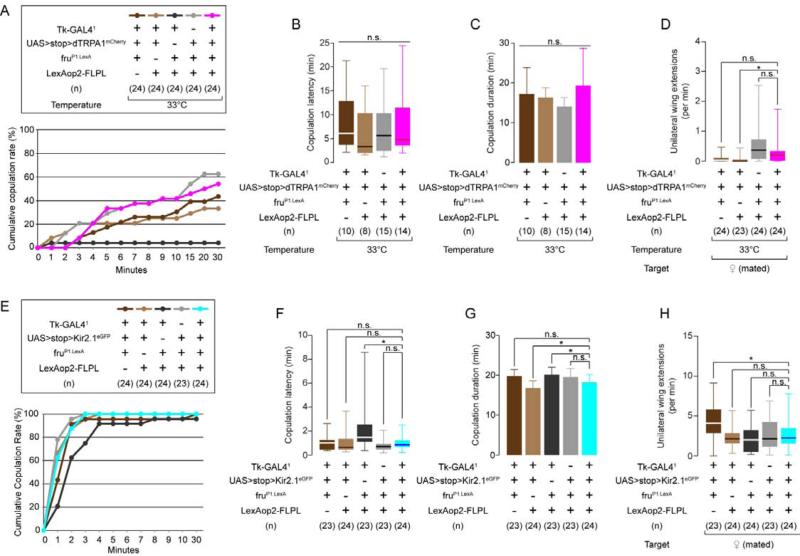
Figure 3. Tk-GAL4FruM neurons do not modulate courtship behavior.
(A) Cumulative copulation curve for males during thermogenetic activation of Tk-GAL4FruM neurons. Tk-GAL41; fruP1.LexA/ LexAop2-FLPL control males were eliminated from subsequent analyses.
(B-D) Copulation latency (B), duration (C, mean ± S.D.) and unilateral wing extension frequency (D) during thermogenetic activation of Tk-GAL4FruM neurons.
(E) Cumulative copulation curve for males during silencing of Tk-GAL4FruM neurons.
(F-H) Copulation latency (F), duration (G) and unilateral wing extension frequency (H) during silencing of Tk-GAL4FruM neurons.
For (B), (D), (F) and (H), * p < 0.05, n.s.: p > 0.05 (Kruskal-Wallis and post-hoc Mann-Whitney U-tests. For (C) and (G), * p < 0.05, n.s.: p > 0.05 (one-way ANOVA or one-way ANOVA and post-hoc Student's t-tests).
Also see Supplemental Figure S3.
To quantify the intensity of courtship behavior, we measured the frequency of unilateral wing extension or “singing” (Hall, 1994), using a new automated unilateral wing extension classifier (see Experimental Procedures and Supplemental Figure S3A, B). Thermogenetic activation of Tk-GAL4FruM neurons did not produce any consistent change, compared to controls, in the frequency of unilateral wing extension toward mated females (Figure 3D). Selective silencing of Tk-GAL4FruM neurons with Kir2.1 did not reduce male copulation efficiency (Figure 3E), copulation latency (Figure 3F), copulation duration (Figure 3G) or the frequency of unilateral wing extension (Figure 3H) compared to the controls. These results suggest that Tk-GAL4FruM neurons do not influence male courtship behavior toward females.
We next investigated whether genetic manipulations of Tk-GAL4FruM neuronal activity influenced male-male courtship, which normally occurs at low frequency. Silencing of Tk-GAL4FruM neurons did not consistently influence the frequency of unilateral wing extensions in comparison to control strains (Supplemental Figure S3C). Similarly, thermogenetic activation of Tk-GAL4FruM neurons using the UAS>stop>dTRPA1myc allele (von Philipsborn et al., 2011) did not increase male-male courtship (Supplemental Figure S3F), although it did increase aggression (Supplemental Figure S3E). Thus, thermogenetic activation of Tk-GAL4FruM neurons under the conditions of our assays produced consistent and significant effects on male-male aggressive behavior, but not on male-female or male-male courtship behavior.
The Drosophila Tachykinin gene is required for normal levels of inter-male aggression
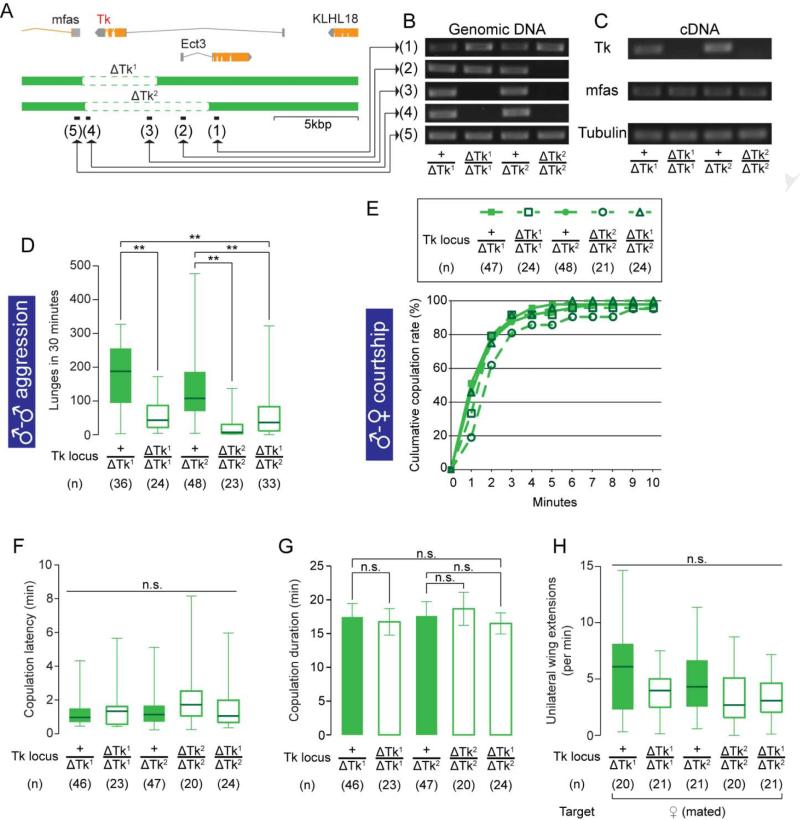
The foregoing observations raised the question of whether Tk itself plays a role in aggression, and if so whether it acts through Tk-GAL4 neurons. To address this question, we created two deletion alleles of Tk using FLP-mediated chromosome translocation (Parks et al., 2004), which we term ΔTk1 and ΔTk2, respectively (Figure 4A). We confirmed the deletion of Tk by PCR analysis at the Tk locus on genomic DNA samples (Figure 4B), and by RT-PCR (Figure 4C). The expression level of a neighboring gene, mfas, was not affected by the Tk deletion (Figure C, Supplemental Figure S4D). These deletion mutants were fully viable and fertile, and exhibited no obvious anatomical abnormalities or differences from controls in locomotor activity (Supplemental Figure S4B). Homozygotes of both ΔTk1 and ΔTk2, as well as ΔTk2/ΔTk1 transheterozygotes, showed a significant decrease in lunging compared to heterozygous controls (Figure 4D). This phenotype is fully recessive, as heterozygous males did not differ from wild type males of the same genetic background in lunging frequency (Supplemental Figure S4A), or in Tk mRNA expression (Supplemental Figure S4C).
Figure 4. Null mutations on Tk specifically affect male-male aggression.
(A) Schematic view of the Tk gene locus and deletions by ΔTk1 and ΔTk2. Black bars (1)-(5) represent the regions targeted in the PCR analysis in (B).
(B) PCR analysis against regions (1)-(5) in (A) from genomic DNA samples of Tk deletion mutants.
(C) RT-PCR targeted to the Tk, mfas and Tubulin (α-Tubulin at 84B) (positive control) gene transcripts from cDNA samples of Tk deletion mutants.
(D-H) Number of lunges (D), cumulative copulation curve (E), copulation latency (F), copulation duration (G) and unilateral wing extension frequency (H) performed by Tk deletion mutants. For (D), (F) and (H), ** p < 0.01, n.s.: p > 0.05 (Kruskal-Wallis or Kruskal-Wallis and post-hoc Mann-Whitney U-tests). For (G), n.s.: p > 0.05 (one-way ANOVA and post-hoc Student's t-test).
Also see Supplemental Figure S4.
We next examined the effect of the Tk deletions on male reproductive behaviors. Both homozygous and transheterozygous males copulated with virgin females at efficiencies comparable to heterozygous control males (Figure 4E). Copulation latency (Figure 4F) and copulation duration (Figure 4G) were also unaffected, as was the frequency of unilateral wing extension toward mated females (Figure 4H). The frequency of unilateral wing extension toward other males was also not consistently different from controls (data not shown). Thus, a null mutation in the Tk gene reduced male-male aggression without affecting male-female or male-male courtship behavior.
Tk exerts its aggression-promoting effect through Tk-GAL4FruM neurons
To investigate the relationship between the Tk gene and Tk-GAL4FruM neuronal function, we first asked whether Tk gene products (DTK peptides) are present in Tk-GAL4FruM neurons using immunostaining. We used two antisera against DTK peptides; one raised in rabbits (Winther and Nassel, 2001) and the other in guinea pigs (see Experimental Procedures). In double-labeling experiments, only the staining of cells labeled by both antibodies was eliminated in ΔTk2/ΔTk1 trans-heterozygous male brains, suggesting that single labeled cells reflected non-specific staining (Supplemental Figure S5A-C). Tk-expressing neurons defined by this criterion are distributed throughout the brain, with a pattern similar to that previously reported (Winther et al., 2003).
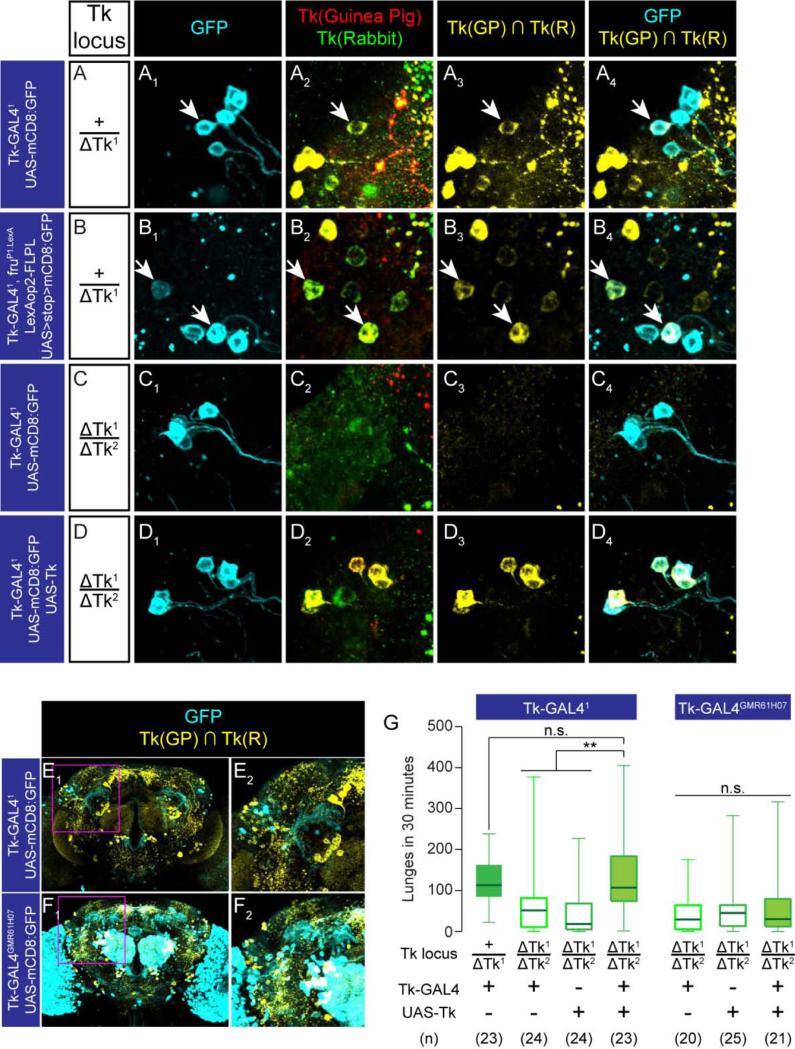
More detailed analysis indicated that a subset of Tk-GAL4FruM neurons expresses Tk (Figure 5A, 5B, 5E). This DTK immunoreactivity was undetectable in the ΔTk2/ΔTk1 transheterozygotes (Figure 5C3, C4), but was recovered when the mutation was rescued using the Tk-GAL41 driver and a UAS-Tk transgene (Figure 5D3, D4). The distribution of cell bodies and gross morphology of Tk-GAL41 neurons remained unchanged in the ΔTk2/ΔTk1 transheterozygotes (Supplemental Figure S5B, S5C) and in the rescue genotype (Supplemental Figure S5D). These genetic and immunohistochemical data suggest that at least a subset of Tk-GAL4FruM neurons expresses one or more DTK peptides.
Figure 5. Tk gene products in the Tk-GAL4FruM neurons are sufficient to maintain normal level of aggression.
(A-D) Tk-GAL41 lateral cluster neurons in +/ΔTk1 (A), ΔTk2/ΔTk1 (C), ΔTk2/ΔTk1 plus UAS-Tk (D) backgrounds, and Tk-GAL4FruM neurons in the +/ΔTk1 (B) background, immunostained with anti-GFP antibody (cyan; A1-D1, A4-D4), anti-DTK guinea pig antiserum (red, A2-D2) and anti-DTK rabbit antiserum (green, A2-D2). The overlap of the two antisera is shown in yellow in A3-D3 and A4-D4. Arrows: the GFP+, DTK+ neurons.
(E,F) Tk-GAL41 (E) and Tk-GAL4GMR61H07 (F) neurons in male brains immunostained with anti-GFP antibody (cyan), anti-Tk guinea pig antiserum and anti-Tk rabbit antiserum (shown as the overlap in yellow). (E2, F2): region within a magenta square in E1 and F1 (magnified).
(G) Number of lunges in ΔTk1/ΔTk2 rescued by Tk-GAL41 or Tk-GAL4GMR61H07 driving UAS-Tk. Left: ** p < 0.01, n.s. p > 0.05 (Kruskal-Wallis and post-hoc Mann-Whitney U-tests) Right: n.s.: Kruskal-Wallis test, p > 0.05.
Also see Supplemental Figure S5.
To determine whether the Tk gene exerts its influence on aggression in Tk-GAL4 neurons, we performed genetic rescue experiments. Expression of UAS-Tk under the control of Tk-GAL41 restored levels of aggression to that of +/ΔTk1 heterozygous controls (Figure 5G). Similar results were obtained using Tk-GAL42 (Supplemental Figure S5E, S5G) which also labels aggression-promoting Tk-GAL4FruM neurons. In contrast, Tk-GAL43 and Tk-GAL4GMR61H07, which label different subsets of Tk-expressing neurons (Figure 5F, Supplemental Figure S5F), but not Tk-GAL4FruM neurons, failed to rescue the reduced aggression of the transheterozygotes (Figure 5H, Supplemental Figure S5H). This result suggests that the rescue obtained using the Tk-GAL41 and Tk-GAL42 drivers is not due simply to extracellular diffusion of the DTK peptides. These data suggest that Tk is required specifically in Tk-GAL4FruM neurons to maintain normal levels of male-male aggression.
Tk expression levels are limiting for the aggression-promoting influence of Tk-GAL41 neuron activation
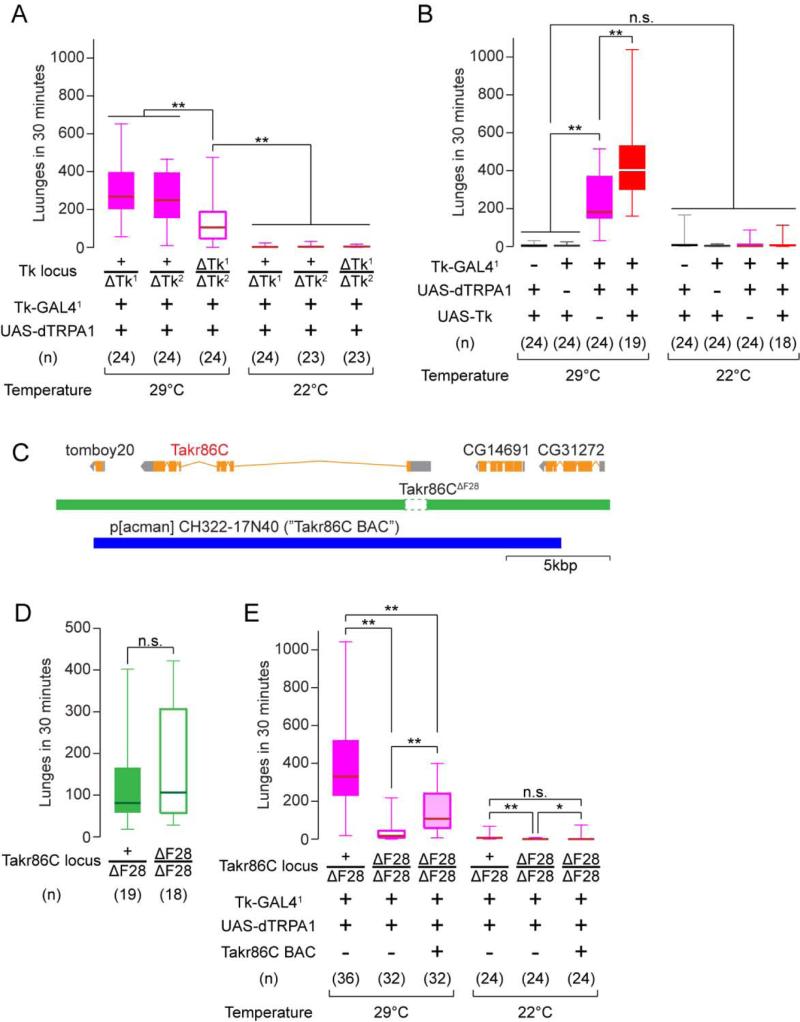
To determine whether release of DTK peptides from Tk-GAL4 neurons plays a role in aggression, we analyzed the effect of Tk gene dosage on the aggression-promoting phenotype of Tk-GAL41 neuron thermogenetic activation. In the transheterozygous Tk deletion mutant background, the aggression promoting effect of Tk-GAL41 neuron activation was significantly suppressed (by ~60%) in comparison to heterozygous controls (Figure 6A, 29°C). This incomplete suppression could reflect the presence of classical transmitters in Tk-GAL41 neurons that also contribute to aggression. Immunostaining experiments indicated that Tk-GAL4FruM neurons did not contain GABA (Supplemental Figure S5K), serotonin (5-HT; Supplemental Figure S5L) or dopamine (Supplemental Figure S5M), but a Cha-GAL80 transgene strongly suppressed GFP expression in these cells (Supplemental Figure S5I, J), suggesting that they are likely cholinergic.
Figure 6. The Tk system gates the aggression-promoting effect of Tk-GAL41 neurons.
(A, B) Number of lunges during thermogenetic activation of Tk-GAL41 neurons in the Tk null mutant background (A), or with Tk overexpression (B).
(C) Schematic of the Takr86C gene locus, its deletion in Takr86C F28 and the region covered by p[acman] CH322-17N40.
(D) Number of lunges performed by Takr86CΔF28 mutants.
(E) Number of lunges during thermogenetic activation of Tk-GAL41 neurons in the Takr86CΔF28 mutant background.
For (A), (B), (D) and (E), ** p < 0.01, * p < 0.05, n.s.: p > 0.05 (Kruskal-Wallis and/or post-hoc Mann-Whitney U-tests).
Also see Supplemental Figure S6.
We next examined the effect of increasing Tk gene dosage on aggressive behavior. Inclusion of the UAS-Tk transgene significantly potentiated the aggression-promoting effect of activating Tk-GAL41 neurons (Figure 6B). The supplemental Tk also increased the amount of tussling, a high-intensity agonistic behavior observed only infrequently in wild-type flies (Chen et al., 2002) (Supplemental Figure S6A). Importantly, Tk-GAL41; UAS-Tk flies lacking UAS-dTRPA1 did not show increased aggression at either 22°C or 29°C (Figure 6B), suggesting that increased DTK expression requires increased neuronal activity to enhance aggression. When Tk-GAL41; UAS-dTRPA1; UAS-Tk flies were tested at different temperatures, the inclusion of UAS-Tk had the effect of shifting the “dose-response” curve for behavior vs. temperature to the left (Supplemental Figure S6C). In contrast, Tk deletion reduced maximal lunge number in activated flies (Supplemental Figure S6C). These results demonstrate a strong interaction between thermogenetic activation of Tk-GAL41 neurons and levels of Tk expression in Tk-GAL41 neurons, supporting the idea that release of this peptide plays a role in the effect of these neurons to promote aggression.
The aggression-promoting effect of Tk-GAL41 neuron activation is suppressed by a mutation in Takr86C
To obtain further evidence that the effects of Tk-GAL4 neuron activation are mediated by DTK peptide release, we investigated whether these effects could be suppressed by loss-of-function mutations in DTK receptor genes. Two such genes have been identified in the fly genome: Takr86C (Poels et al., 2009) and Takr99D (Birse et al., 2006). We created a loss-of-function mutation in Takr86C by imprecise excision of a P-element insertion; we call this allele Takr86CΔF28. This deletion removes most of the first exon of Takr86C, including the start codon and the first 60 amino acids which contain the signal peptide and most of the N-teminal extracellular domain (Figure 6C).
In Takr86CΔF28 homozygotes, the aggression-promoting effect of activating Tk-GAL41 neurons was significantly suppressed (Figure 6E). A similar suppression was observed for Tk-GAL42 neuron activation (Supplemental Figure S6D). In contrast, a putative loss-of-function insertional mutation in Takr99D (Takr99DMB09356; (Metaxakis et al., 2005)) had no effect in either Tk-GAL4 line (Supplemental Figure S6E and data not shown). The suppressing effect of the Takr86CΔF28 mutation could be partially rescued by a bacterial artificial chromosome (BAC) (Venken et al., 2009; Venken et al., 2006) containing the complete Takr86C transcription unit (Figure 6C, 6E). These data provide further evidence that the effect of Tk-GAL4 neuron activation to promote aggression involves the release of DTK peptides. Surprisingly, the Takr86CΔF28 homozygous deletion on its own did not diminish baseline aggression in single housed flies (Figure 6D). This likely reflects compensation by Takr99D, since double-mutants showed a strong reduction in baseline as well as induced aggression (Supplemental Figure S6F, G). The reason why Takr99DMB09356 does not suppress TK-GAL41 neuron activation-induced aggression is not clear, but could reflect the circuitry where this receptor acts, or its relative affinity or specificity for different DTK peptides. Further studies are required to understand the respective roles of these two receptors in the control of aggression.
Activation of Tk-GAL4 neurons promotes a state of aggressive arousal
High levels of arousal or motivation have been proposed to diminish the requirement for an optimal specificity or salience of releasing signals that promote innate behaviors (Tinbergen, 1951). We therefore investigated whether activation of Tk-GAL4 neurons could overcome the requirement for conspecific sensory cues or environmental factors in Drosophila aggression.
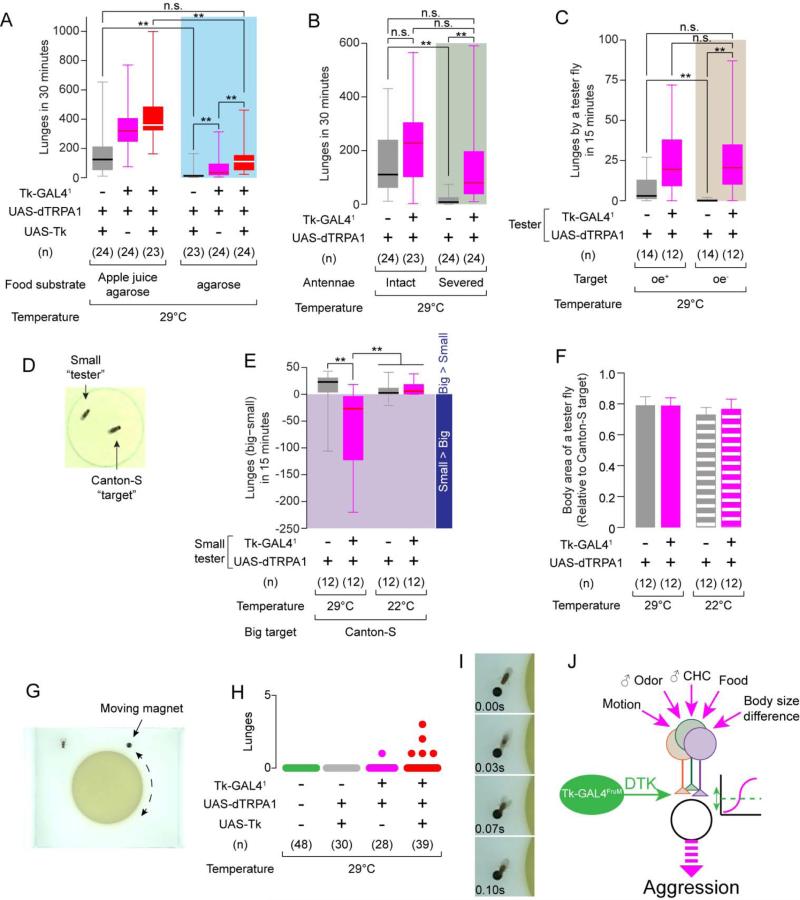
The presence of a resource such as food is essential for aggression in Drosophila (Chen et al., 2002; Svetec and Ferveur, 2005). We therefore tested whether activation of Tk-GAL41 neurons could overcome the effect of eliminating food from the aggression arena. On a pure agarose substrate, UAS-dTRPA1; UAS-Tk control flies showed almost no aggression, compared to the same genotype on an apple juice-agarose substrate (Figure 7A). Strikingly, activation of Tk-GAL41 neurons partially restored aggression on pure agarose (Figure 7A, blue shaded area, magenta box). Supplementation of DTK using UAS-Tk further increased the level of thermogenetically induced aggression on agarose, to levels not significantly different from those exhibited by control flies on apple juice-agarose (Figure 7A, gray box on left vs. red box in shaded area).
Figure 7. Activation of Tk-GAL41 neurons overrides the absence of aggression-promoting cues.
(A-C) Number of lunges during thermogenetic activation of Tk-GAL41 neurons, (A) on an apple juice agarose (left) or pure agarose (right) substrate; (B) with intact (left) or surgically removed (right) antennae; and (C) toward oe+ (left) or oe− (right) “target” males.
(D) Example image of a normal-sized “target” Canton-S male and a small “tester” male fly (70% the size of the target fly).
(E) Lunge number difference between smaller tester and larger target flies. Values in the shaded area indicate that the smaller testers performed more lunges than the larger targets.
(F) Relative body sizes (mean ± S.D.) of “tester”vs. target flies.
(G) Image of the “moving magnet” setup.
(H) Number of lunges (scatter plot) toward the magnet during thermogenetic activation of Tk-GAL41 neurons. Total recording time per session was 4 minutes (see Experimental Procedures).
(I) Example of a lunge performed by a male Tk-GAL41, UAS-dTRPA1; UAS-Tk fly toward a magnet.
(J) Schematic illustrating possible influence of Tk-GAL4FruM neurons in the male fly brain in relation to the processing of sensory cues regulating aggression.
For (A-C) and (E), ** p < 0.01, * p < 0.05 (Kruskal-Wallis and post-hoc Mann-Whitney U-tests). For (F), n.s.: p > 0.05 (one-way ANOVA and post hoc Student's t-test.
Also see Supplemental Movie S3.
We next investigated whether activation of Tk-GAL41 neurons could overcome the requirement for olfactory cues in aggression, one of which is the male-specific pheromone 11-cis-vaccenyl acetate (Wang and Anderson, 2010). To do this, we surgically removed the third antennal segment in Tk-GAL41; UAS-dTRPA1 and control males. While antennae-less +; UAS-dTRPA1 control flies showed a profound reduction in lunging (Figure 7B, gray boxes), activation of Tk-GAL41 neurons restored lunging to a level not significantly different from that of control flies with intact antennae (Figure 7B, gray box on left vs. magenta box in shaded area). Thus, the activation of Tk-GAL41 neurons can also compensate for loss of sensitivity to aggression-promoting olfactory cues.
Male-specific cuticular hydrocarbon (CH) pheromones, which are detected by the gustatory system (Lu et al., 2012; Thistle et al., 2012; Toda et al., 2012), are a second class of chemosensory cues required for male-male aggression in Drosophila (Fernandez et al., 2010; Wang et al., 2011). To test whether activation of Tk-GAL41 neurons could compensate for the absence of these cues, we paired thermogenetically activated tester flies with target “oe− flies”, which are depleted of most CHs via genetic ablation of oenocytes (Billeter et al., 2009). Such oe− target flies evoked significantly less aggression from +; UAS-dTRPA1 flies than did control “oe+ flies” (Figure 7C, gray boxes, and (Fernandez et al., 2010; Wang et al., 2011)). Remarkably, thermogenetic activation of Tk-GAL41 neurons in tester flies restored aggression towards oe− target flies to a level indistinguishable from that exhibited towards oe+ targets (Figure 7C, magenta boxes).
To explore further the idea that Tk-GAL4 neurons promote aggressive arousal, we investigated whether activation of these neurons could overcome the well-known size disadvantage in inter-male aggression (Briffa and Sneddon, 2007). In Drosophila, as in many other species, the smaller male of a pair is far less likely to attack its larger opponent (Hoffmann, 1987; Hoyer et al., 2008). We generated smaller males (21-27% body area size reduction compared to the wild type “target” males; Figure 7D, F) by larval caloric restriction (see Experimental Procedure), and paired them with normal-sized wild type males. At 22°C, the majority of lunges were performed by the normal-sized males towards the smaller fly, regardless of its genotype (Figure 7E). Thermogenetic activation of Tk-GAL41 neurons reversed this trend (Figure 7E, two left columns, Supplemental Movie S3). Thus, Tk-GAL41 neuron activation can overcome the decreased likelihood of attack towards goal objects lacking appropriate cues, or exhibiting unfavorable properties such as larger size.
Classic experiments by Tinbergen using dummy fish suggested that increased levels of arousal in a male can promote aggression towards a goal object exhibiting sub-optimal “releasing stimuli” (Tinbergen, 1951). We therefore investigated whether activation of Tk-GAL41 neurons could promote attack towards a fly-sized inanimate object. To do this, we engineered a system similar to that described by (Zabala et al., 2012), in which a small, computer-controlled magnet circled a food patch in the presence of a single tester fly (Figure 7G). In close to 80 experiments, we did not observe a single lunge toward the magnet by either wild-type (CS) or genetic control males (Figure 7H, green and grey). Remarkably, however, thermogenetic activation of Tk-GAL41 neurons in the presence of supplemental Tk (UAS-Tk) elicited lunges towards the magnet from 5/39 tester males (Figure 7H, red; 7I); a single lunge was detected among 28 males activated without supplemental Tk (Fig. 7H, magenta). While the frequency of such attack towards a “dummy fly” was low, its occurrence was striking given that we never observed such behavior from control or wild-type flies.
Taken together, these data suggest that the activation of Tk-GAL41 neurons, particularly when supplemented with higher levels of Tk, can override the requirement for several categories of cues or conditions that are necessary for normal levels of male fly aggression, including social isolation, food, volatile and CH pheromones and fly-specific visual cues (Figure 7J).
Discussion
The interplay of genetic and neural circuit elements in the sexually dimorphic control of aggressive behavior is an important and poorly understood problem (Manoli et al., 2013). Here we have identified a sexually dimorphic neuron and a gene that play a critical and specific role in the expression of inter-male aggression in Drosophila. The gene encodes a neuropeptide homologous to mammalian Substance P, and its release from the identified neurons is important for aggression. Substance P has been implicated in aggression in several mammalian systems (Halasz et al., 2009; Katsouni et al., 2009; Siegel et al., 1997). Together, our data suggest that the higher level of aggressiveness in Drosophila males may be controlled by the expression in sexually dimorphic neurons of a neuropeptide that regulates forms of agonistic behavior across phylogeny.
Sexually dimorphic neurons that control higher levels of aggression in males
Previous studies have investigated the role of FruM+ neurons in aggression vs. courtship. Selective masculinization of certain groups of neurons in females masculinized courtship behavior but not aggression, suggesting that distinct subsets of FruM neurons may control these behaviors (Chan and Kravitz, 2007); however a selective masculinization of aggression but not courtship was not observed. Feminization of octopaminergic (OA) or cholinergic neurons, via expression of UAS-Tra, altered the balance between male-male courtship and aggression (Certel et al., 2007), or enhanced aggression (Mundiyanapurath et al., 2009), respectively. However the specific subsets of these neurons controlling aggression were not identified. Knockdown of fru mRNA in a small subset of OA neurons increased male-male courtship, but had no effect on agonistic behaviors (Certel et al., 2010). Functional manipulations of small numbers of OA and dopaminergic neurons can influence levels of aggression in flies (Alekseyenko et al., 2013; Zhou et al., 2008), but these neurons are not sexually dimorphic. The present results identify sexually dimorphic Tk-GAL4FruM neurons that are necessary, sufficient and specific for the quantitatively higher level of aggressiveness characteristic of Drosophila males. The neurons responsible for the qualitative sex-specific differences in the behavioral expression of aggression remain to be identified.
Studies in mice have localized aggression-promoting neurons to the ventrolateral subdivision of the ventromedial hypothalamus (VMHvl) (Lin et al., 2011). Genetic ablation of anatomically dimorphic neurons within VMHvl that express the progesterone receptor (PR) has been shown to partially reduce aggressive behavior (Yang et al., 2013). However, this effect of this ablation was not specific to aggression, since male mating behavior and female mating behavior were attenuated as well. In contrast the Tk-GAL4FruM neurons identified here control aggression but not mating behavior. Unlike PR+ neurons, moreover, these cells are not detectable in females.
The Tk-GAL4FruM neurons characterized in this study were identified using a GAL4 driver derived from the promoter of a neuropeptide gene, Tk, which is expressed in these cells. Our inability to visualize these neurons in females suggests that either the developmental generation of these neurons, and/or their expression of the neuropeptide is male-specific. Whatever the case, the absence of these neural elements from the female brain is likely to contribute to their lower level of aggressive behavior. Our data suggest that sex-typical features of some innate behaviors in Drosophila may be achieved, at least in part, by the sexually dimorphic expression in specific neurons of neuropeptides that coordinate male-specific behavioral subprograms (see also (Tayler et al., 2012)). Dimorphic populations of FruM-expressing neurons also regulate sexually dimorphic behaviors through the release of classical fast neurotransmitters that act on sexually dimorphic chemical synapses (Ruta et al., 2010).
Tk-GAL4FruM neurons as regulators of aggressive arousal
Several lines of evidence presented here argue that Tk-GAL4FruM neurons influence aggressive arousal or motivation, rather than simply acting as “command neurons” for aggressive actions. First, activation of these neurons did not trigger a single aggressive action, as would be expected for a command neuron (Bentley and Konishi, 1978), but rather increased the frequency of multiple agonistic behaviors including wing-threat, lunging and tussling. Second, thermogenetic activation of these neurons supervened the requirement for several aggression-permissive conditions and cues, some of which (such as male-specific pheromones) could be construed as “releasing signals” (Tinbergen, 1951). Surprisingly, the activation of Tk-GAL41 neurons was even able to promote lunging towards a moving dummy fly (albeit in a minority of trials). To the extent that increased arousal decreases the requirement for specific releasing signals to evoke innate behaviors (Tinbergen, 1951), activation of Tk-GAL4FruM neurons may generate an arousal-like state that is specific for aggression. Alternatively, Tk-GAL4FruM neurons may enhance behavioral sensitivity to multiple releasing signals that characterize an attackable object, either at the level of parallel sensory processing pathways, or at a locus downstream of the integration of these multisensory cues (Figure 7J). Similar conclusions have been drawn from studies of the neuropeptide regulation of feeding behavior in C. elegans (Macosko et al., 2009).
Tachykinins modulate agonistic behavior across phylogeny
Several lines of evidence presented here suggest that the release of DTK peptides indeed contributes to the aggression-promoting function of Tk-GAL4FruM neurons. First, DTK immunoreactivity was detected in a subset of these neurons,. Second, ΔTk mutations strongly suppressed the effect of Tk-GAL41 neuronal activation to promote aggression. Third, the decreased aggression of Tk mutants could be rescued by expression of UAS-Tk under the control of Tk-GAL41. Fourth, over-expression of DTK in Tk-GAL41 neurons increased their aggression-promoting influence, in a manner dependent upon the activation of these neurons. Finally, a mutation in the Takr86C gene suppressed the aggression-promoting effect of Tk-GAL41 neuron activation. Nevertheless, the release of a classical neurotransmitter, probably acetylcholine (Supplemental Figure 5I-M), likely contributes to the behavioral influence of Tk-GAL4FruM neurons as well. Furthermore, our data do not exclude additional sites of Tk action in the control of aggression, some of which may be mediated via Takr99D.
Among three species of vertebrate Tachykinin neuropeptides (Severini et al., 2002), Substance P has been implicated, directly or indirectly, in various forms of aggression including defensive rage and predatory attack in cats (reviewed in (Katsouni et al., 2009; Siegel et al., 1997)), and inter-male aggression in rats (Halasz et al., 2008; Halasz et al., 2009). Although not all functions of Substance P are necessarily conserved (such as nociception in mammals (Woolf et al., 1998) and olfactory modulation in the fly (Ignell et al., 2009; Winther et al., 2006)), these data suggest that this neuropeptide is broadly involved in the control of agonistic behavior in both vertebrates and invertebrates. They therefore add to the growing list of neuropeptide systems that show a remarkable evolutionary conservation of functions in the regulation of innate “survival behaviors” such as feeding and mating (reviewed in (Bargmann, 2012; Taghert and Nitabach, 2012)). Biogenic amines also control aggression across phylogeny (Alekseyenko et al., 2013; Alekseyenko et al., 2010 Certel, 2007 #37; Baier et al., 2002; Dierick and Greenspan, 2007; Hoyer et al., 2008; Zhou et al., 2008). However in the case of serotonin, the directionality of its influence is opposite in flies and humans (reviewed in (Zwarts et al., 2012)).
Our findings indicate that studies of agonistic behavior in Drosophila can identify aggression-regulating genes with direct relevance to vertebrates. Interestingly, in humans, the concentration of SP-like immunoreactivity in the CSF has been positively correlated with aggressive tendencies in patients with personality disorders (Coccaro et al., 2012). SP antagonists have been tested in humans as anxiolytic and antidepressant agents, although they failed to show efficacy (Keller et al., 2006; Steckler, 2009). The present findings, taken together with mammalian animal studies, suggest that it may be worthwhile to investigate the potential of these antagonists for reducing violent aggression in humans.
Experimental Procedures
Please see Extended Experimental Procedures for the complete methods, description of reagents and programs used.
Supplementary Material
Article Highlights.
A single class of neurons is identified that promotes aggression in male flies.
These neurons are FruM+ and sexually dimorphic, but do not control courtship.
These neurons promote aggressive arousal via release of a neuropeptide, DTK.
Tk is a neuropeptide gene that controls aggression in both flies and mammals.
Acknowledgements
We thank B.J. Dickson, B. Pfeiffer, G.M. Rubin and D. Nässel for sharing fly strains and antibodies; S. Jeeda and C. Khanbijian for maintenance of fly stocks; G. Mancuso for administrative support and C. Chiu for laboratory management; B. Pfeiffer, A. M. Wong and W. Hong for helpful comments on the manuscript. K.A. was a JSPS Postdoctoral Fellow for Research Abroad. K.W. was a Human Frontier Science Program Postdoctoral Fellow. B.J.D. is an Ellison Medical Foundation Fellow of the Life Science Research Foundation. E.H. is supported by an NRSA postdoctoral fellowship. This research was supported in part by NIH grants R01-DA031389 to D.J.A. and a Moore Foundation Grant to D.J.A. and P.P. D.J.A is a Howard Hughes Medical Institute investigator.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alekseyenko OV, Chan YB, Li R, Kravitz EA. Single dopaminergic neurons that modulate aggression in Drosophila. Proc Natl Acad Sci U S A. 2013;110:6151–6156. doi: 10.1073/pnas.1303446110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alekseyenko OV, Lee C, Kravitz EA. Targeted manipulation of serotonergic neurotransmission affects the escalation of aggression in adult male Drosophila melanogaster. PLoS One. 2010;5:e10806. doi: 10.1371/journal.pone.0010806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baier A, Wittek B, Brembs B. Drosophila as a new model organism for the neurobiology of aggression? J Exp Biol. 2002;205:1233–1240. doi: 10.1242/jeb.205.9.1233. [DOI] [PubMed] [Google Scholar]
- Bargmann CI. Beyond the connectome: how neuromodulators shape neural circuits. Bioessays. 2012;34:458–465. doi: 10.1002/bies.201100185. [DOI] [PubMed] [Google Scholar]
- Bentley D, Konishi M. Neural control of behavior. Annu Rev Neurosci. 1978;1:35–59. doi: 10.1146/annurev.ne.01.030178.000343. [DOI] [PubMed] [Google Scholar]
- Billeter JC, Atallah J, Krupp JJ, Millar JG, Levine JD. Specialized cells tag sexual and species identity in Drosophila melanogaster. Nature. 2009;461:987–991. doi: 10.1038/nature08495. [DOI] [PubMed] [Google Scholar]
- Birse RT, Johnson EC, Taghert PH, Nassel DR. Widely distributed Drosophila G-protein-coupled receptor (CG7887) is activated by endogenous tachykinin-related peptides. J Neurobiol. 2006;66:33–46. doi: 10.1002/neu.20189. [DOI] [PubMed] [Google Scholar]
- Briffa M, Sneddon LU. Physiological constraints on contest behaviour. Functional Ecology. 2007;21:627–637. [Google Scholar]
- Cachero S, Ostrovsky AD, Yu JY, Dickson BJ, Jefferis GS. Sexual dimorphism in the fly brain. Curr Biol. 2010;20:1589–1601. doi: 10.1016/j.cub.2010.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Certel SJ, Leung A, Lin CY, Perez P, Chiang AS, Kravitz EA. Octopamine neuromodulatory effects on a social behavior decision-making network in Drosophila males. PLoS One. 2010;5:e13248. doi: 10.1371/journal.pone.0013248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Certel SJ, Savella MG, Schlegel DC, Kravitz EA. Modulation of Drosophila male behavioral choice. Proc Natl Acad Sci U S A. 2007;104:4706–4711. doi: 10.1073/pnas.0700328104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamero P, Marton TF, Logan DW, Flanagan K, Cruz JR, Saghatelian A, Cravatt BF, Stowers L. Identification of protein pheromones that promote aggressive behaviour. Nature. 2007;450:899–902. doi: 10.1038/nature05997. [DOI] [PubMed] [Google Scholar]
- Chan YB, Kravitz EA. Specific subgroups of FruM neurons control sexually dimorphic patterns of aggression in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2007;104:19577–19582. doi: 10.1073/pnas.0709803104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Lee AY, Bowens NM, Huber R, Kravitz EA. Fighting fruit flies: a model system for the study of aggression. Proc Natl Acad Sci U S A. 2002;99:5664–5668. doi: 10.1073/pnas.082102599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccaro EF, Lee R, Owens MJ, Kinkead B, Nemeroff CB. Cerebrospinal fluid substance P-like immunoreactivity correlates with aggression in personality disordered subjects. Biol Psychiatry. 2012;72:238–243. doi: 10.1016/j.biopsych.2012.02.023. [DOI] [PubMed] [Google Scholar]
- Craig IW, Halton KE. Genetics of human aggressive behaviour. Hum Genet. 2009;126:101–113. doi: 10.1007/s00439-009-0695-9. [DOI] [PubMed] [Google Scholar]
- Dankert H, Wang L, Hoopfer ED, Anderson DJ, Perona P. Automated monitoring and analysis of social behavior in Drosophila. Nat Methods. 2009;6:297–303. doi: 10.1038/nmeth.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierick HA, Greenspan RJ. Serotonin and neuropeptide F have opposite modulatory effects on fly aggression. Nat Genet. 2007;39:678–682. doi: 10.1038/ng2029. [DOI] [PubMed] [Google Scholar]
- Fernandez MP, Chan YB, Yew JY, Billeter JC, Dreisewerd K, Levine JD, Kravitz EA. Pheromonal and behavioral cues trigger male-to-female aggression in Drosophila. PLoS Biol. 2010;8:e1000541. doi: 10.1371/journal.pbio.1000541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filley CM, Price BH, Nell V, Antoinette T, Morgan AS, Bresnahan JF, Pincus JH, Gelbort MM, Weissberg M, Kelly JP. Toward an understanding of violence: neurobehavioral aspects of unwarranted physical aggression: Aspen Neurobehavioral Conference consensus statement. Neuropsychiatry Neuropsychol Behav Neurol. 2001;14:1–14. [PubMed] [Google Scholar]
- Gill G, Ptashne M. Negative effect of the transcriptional activator GAL4. Nature. 1988;334:721–724. doi: 10.1038/334721a0. [DOI] [PubMed] [Google Scholar]
- Halasz J, Toth M, Mikics E, Hrabovszky E, Barsy B, Barsvari B, Haller J. The effect of neurokinin1 receptor blockade on territorial aggression and in a model of violent aggression. Biol Psychiatry. 2008;63:271–278. doi: 10.1016/j.biopsych.2007.04.022. [DOI] [PubMed] [Google Scholar]
- Halasz J, Zelena D, Toth M, Tulogdi A, Mikics E, Haller J. Substance P neurotransmission and violent aggression: the role of tachykinin NK(1) receptors in the hypothalamic attack area. Eur J Pharmacol. 2009;611:35–43. doi: 10.1016/j.ejphar.2009.03.050. [DOI] [PubMed] [Google Scholar]
- Hall JC. The mating of a fly. Science. 1994;264:1702–1714. doi: 10.1126/science.8209251. [DOI] [PubMed] [Google Scholar]
- Hamada FN, Rosenzweig M, Kang K, Pulver SR, Ghezzi A, Jegla TJ, Garrity PA. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454:217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hergarden AC, Tayler TD, Anderson DJ. Allatostatin-A neurons inhibit feeding behavior in adult Drosophila. Proc Natl Acad Sci U S A. 2012;109:3967–3972. doi: 10.1073/pnas.1200778109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann AA. Territorial encounters between Drosophila males of different sizes. Animal Behaviour. 1987;35:1899–1901. [Google Scholar]
- Hoyer SC, Eckart A, Herrel A, Zars T, Fischer SA, Hardie SL, Heisenberg M. Octopamine in male aggression of Drosophila. Curr Biol. 2008;18:159–167. doi: 10.1016/j.cub.2007.12.052. [DOI] [PubMed] [Google Scholar]
- Ignell R, Root CM, Birse RT, Wang JW, Nassel DR, Winther AM. Presynaptic peptidergic modulation of olfactory receptor neurons in Drosophila. Proc Natl Acad Sci U S A. 2009;106:13070–13075. doi: 10.1073/pnas.0813004106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsouni E, Sakkas P, Zarros A, Skandali N, Liapi C. The involvement of substance P in the induction of aggressive behavior. Peptides. 2009;30:1586–1591. doi: 10.1016/j.peptides.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Keller M, Montgomery S, Ball W, Morrison M, Snavely D, Liu G, Hargreaves R, Hietala J, Lines C, Beebe K, et al. Lack of efficacy of the substance p (neurokinin1 receptor) antagonist aprepitant in the treatment of major depressive disorder. Biol Psychiatry. 2006;59:216–223. doi: 10.1016/j.biopsych.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Kimura K, Ote M, Tazawa T, Yamamoto D. Fruitless specifies sexually dimorphic neural circuitry in the Drosophila brain. Nature. 2005;438:229–233. doi: 10.1038/nature04229. [DOI] [PubMed] [Google Scholar]
- Kurtovic A, Widmer A, Dickson BJ. A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature. 2007;446:542–546. doi: 10.1038/nature05672. [DOI] [PubMed] [Google Scholar]
- Lee G, Hall JC. A newly uncovered phenotype associated with the fruitless gene of Drosophila melanogaster: aggression-like head interactions between mutant males. Behav Genet. 2000;30:263–275. doi: 10.1023/a:1026541215546. [DOI] [PubMed] [Google Scholar]
- Lin D, Boyle MP, Dollar P, Lee H, Lein ES, Perona P, Anderson DJ. Functional identification of an aggression locus in the mouse hypothalamus. Nature. 2011;470:221–226. doi: 10.1038/nature09736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Liang X, Gong J, Yang Z, Zhang YH, Zhang JX, Rao Y. Social regulation of aggression by pheromonal activation of Or65a olfactory neurons in Drosophila. Nat Neurosci. 2011;14:896–902. doi: 10.1038/nn.2836. [DOI] [PubMed] [Google Scholar]
- Lorenz K. On aggression. 1st edn Harcourt; New York: 1966. [Google Scholar]
- Lu B, LaMora A, Sun Y, Welsh MJ, Ben-Shahar Y. ppk23-Dependent chemosensory functions contribute to courtship behavior in Drosophila melanogaster. PLoS Genet. 2012;8:e1002587. doi: 10.1371/journal.pgen.1002587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macosko EZ, Pokala N, Feinberg EH, Chalasani SH, Butcher RA, Clardy J, Bargmann CI. A hub-and-spoke circuit drives pheromone attraction and social behaviour in C. elegans. Nature. 2009;458:1171–1175. doi: 10.1038/nature07886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoli DS, Fan P, Fraser EJ, Shah NM. Neural control of sexually dimorphic behaviors. Curr Opin Neurobiol. 2013;23:330–338. doi: 10.1016/j.conb.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoli DS, Foss M, Villella A, Taylor BJ, Hall JC, Baker BS. Male-specific fruitless specifies the neural substrates of Drosophila courtship behaviour. Nature. 2005;436:395–400. doi: 10.1038/nature03859. [DOI] [PubMed] [Google Scholar]
- Mellert DJ, Knapp JM, Manoli DS, Meissner GW, Baker BS. Midline crossing by gustatory receptor neuron axons is regulated by fruitless, doublesex and the Roundabout receptors. Development. 2010;137:323–332. doi: 10.1242/dev.045047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metaxakis A, Oehler S, Klinakis A, Savakis C. Minos as a genetic and genomic tool in Drosophila melanogaster. Genetics. 2005;171:571–581. doi: 10.1534/genetics.105.041848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundiyanapurath S, Chan YB, Leung AK, Kravitz EA. Feminizing cholinergic neurons in a male Drosophila nervous system enhances aggression. Fly (Austin) 2009;3:179–184. doi: 10.4161/fly.3.3.8989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassel DR, Winther AM. Drosophila neuropeptides in regulation of physiology and behavior. Prog Neurobiol. 2010;92:42–104. doi: 10.1016/j.pneurobio.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Nilsen SP, Chan YB, Huber R, Kravitz EA. Gender-selective patterns of aggressive behavior in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2004;101:12342–12347. doi: 10.1073/pnas.0404693101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Meissner GW, Baker BS. Joint control of Drosophila male courtship behavior by motion cues and activation of male-specific P1 neurons. Proc Natl Acad Sci U S A. 2012;109:10065–10070. doi: 10.1073/pnas.1207107109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks AL, Cook KR, Belvin M, Dompe NA, Fawcett R, Huppert K, Tan LR, Winter CG, Bogart KP, Deal JE, et al. Systematic generation of high-resolution deletion coverage of the Drosophila melanogaster genome. Nat Genet. 2004;36:288–292. doi: 10.1038/ng1312. [DOI] [PubMed] [Google Scholar]
- Poels J, Birse RT, Nachman RJ, Fichna J, Janecka A, Vanden Broeck J, Nassel DR. Characterization and distribution of NKD, a receptor for Drosophila tachykinin-related peptide 6. Peptides. 2009;30:545–556. doi: 10.1016/j.peptides.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Ruta V, Datta SR, Vasconcelos ML, Freeland J, Looger LL, Axel R. A dimorphic pheromone circuit in Drosophila from sensory input to descending output. Nature. 2010;468:686–690. doi: 10.1038/nature09554. [DOI] [PubMed] [Google Scholar]
- Severini C, Improta G, Falconieri-Erspamer G, Salvadori S, Erspamer V. The tachykinin peptide family. Pharmacol Rev. 2002;54:285–322. doi: 10.1124/pr.54.2.285. [DOI] [PubMed] [Google Scholar]
- Siegel A, Schubert KL, Shaikh MB. Neurotransmitters regulating defensive rage behavior in the cat. Neurosci Biobehav Rev. 1997;21:733–742. doi: 10.1016/s0149-7634(96)00056-5. [DOI] [PubMed] [Google Scholar]
- Siwicki KK, Kravitz EA. Fruitless, doublesex and the genetics of social behavior in Drosophila melanogaster. Curr Opin Neurobiol. 2009;19:200–206. doi: 10.1016/j.conb.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steckler T. Developing Small Molecule Nonpeptidergic Drugs for the Treatment of Anxiety Disorders: Is the Challenge Still Ahead? In: Stein MB, Steckler T, editors. Current Topics in Behavioral Neurosciences. Springer-Verlag Berlin Heidelberg; 2009. pp. 415–428. [DOI] [PubMed] [Google Scholar]
- Stockinger P, Kvitsiani D, Rotkopf S, Tirian L, Dickson BJ. Neural circuitry that governs Drosophila male courtship behavior. Cell. 2005;121:795–807. doi: 10.1016/j.cell.2005.04.026. [DOI] [PubMed] [Google Scholar]
- Stowers L, Logan DW. Olfactory mechanisms of stereotyped behavior: on the scent of specialized circuits. Curr Opin Neurobiol. 2010;20:274–280. doi: 10.1016/j.conb.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svetec N, Ferveur JF. Social experience and pheromonal perception can change male-male interactions in Drosophila melanogaster. J Exp Biol. 2005;208:891–898. doi: 10.1242/jeb.01454. [DOI] [PubMed] [Google Scholar]
- Taghert PH, Nitabach MN. Peptide neuromodulation in invertebrate model systems. Neuron. 2012;76:82–97. doi: 10.1016/j.neuron.2012.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayler TD, Pacheco DA, Hergarden AC, Murthy M, Anderson DJ. A neuropeptide circuit that coordinates sperm transfer and copulation duration in Drosophila. Proc Natl Acad Sci U S A. 2012;109:20697–20702. doi: 10.1073/pnas.1218246109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thistle R, Cameron P, Ghorayshi A, Dennison L, Scott K. Contact chemoreceptors mediate male-male repulsion and male-female attraction during Drosophila courtship. Cell. 2012;149:1140–1151. doi: 10.1016/j.cell.2012.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinbergen N. The study of instinct. Clarendon Press; Oxford Eng: 1951. [Google Scholar]
- Toda H, Zhao X, Dickson BJ. The Drosophila female aphrodisiac pheromone activates ppk23(+) sensory neurons to elicit male courtship behavior. Cell Rep. 2012;1:599–607. doi: 10.1016/j.celrep.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Venken KJ, Carlson JW, Schulze KL, Pan H, He Y, Spokony R, Wan KH, Koriabine M, de Jong PJ, White KP, et al. Versatile P[acman] BAC libraries for transgenesis studies in Drosophila melanogaster. Nat Methods. 2009;6:431–434. doi: 10.1038/nmeth.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venken KJ, He Y, Hoskins RA, Bellen HJ. P[acman]: a BAC transgenic platform for targeted insertion of large DNA fragments in D. melanogaster. Science. 2006;314:1747–1751. doi: 10.1126/science.1134426. [DOI] [PubMed] [Google Scholar]
- von Philipsborn AC, Liu T, Yu JY, Masser C, Bidaye SS, Dickson BJ. Neuronal control of Drosophila courtship song. Neuron. 2011;69:509–522. doi: 10.1016/j.neuron.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Vrontou E, Nilsen SP, Demir E, Kravitz EA, Dickson BJ. fruitless regulates aggression and dominance in Drosophila. Nat Neurosci. 2006;9:1469–1471. doi: 10.1038/nn1809. [DOI] [PubMed] [Google Scholar]
- Wang L, Anderson DJ. Identification of an aggression-promoting pheromone and its receptor neurons in Drosophila. Nature. 2010;463:227–231. doi: 10.1038/nature08678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Dankert H, Perona P, Anderson DJ. A common genetic target for environmental and heritable influences on aggressiveness in Drosophila. Proc Natl Acad Sci U S A. 2008;105:5657–5663. doi: 10.1073/pnas.0801327105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Han X, Mehren J, Hiroi M, Billeter JC, Miyamoto T, Amrein H, Levine JD, Anderson DJ. Hierarchical chemosensory regulation of male-male social interactions in Drosophila. Nat Neurosci. 2011;14:757–762. doi: 10.1038/nn.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winther AM, Acebes A, Ferrus A. Tachykinin-related peptides modulate odor perception and locomotor activity in Drosophila. Mol Cell Neurosci. 2006;31:399–406. doi: 10.1016/j.mcn.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Winther AM, Nassel DR. Intestinal peptides as circulating hormones: release of tachykinin-related peptide from the locust and cockroach midgut. J Exp Biol. 2001;204:1269–1280. doi: 10.1242/jeb.204.7.1269. [DOI] [PubMed] [Google Scholar]
- Winther AM, Siviter RJ, Isaac RE, Predel R, Nassel DR. Neuronal expression of tachykinin-related peptides and gene transcript during postembryonic development of Drosophila. J Comp Neurol. 2003;464:180–196. doi: 10.1002/cne.10790. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Mannion RJ, Neumann S. Null mutations lacking substance: elucidating pain mechanisms by genetic pharmacology. Neuron. 1998;20:1063–1066. doi: 10.1016/s0896-6273(00)80487-0. [DOI] [PubMed] [Google Scholar]
- Wu MV, Shah NM. Control of masculinization of the brain and behavior. Curr Opin Neurobiol. 2011;21:116–123. doi: 10.1016/j.conb.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CF, Chiang MC, Gray DC, Prabhakaran M, Alvarado M, Juntti SA, Unger EK, Wells JA, Shah NM. Sexually dimorphic neurons in the ventromedial hypothalamus govern mating in both sexes and aggression in males. Cell. 2013;153:896–909. doi: 10.1016/j.cell.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JY, Kanai MI, Demir E, Jefferis GS, Dickson BJ. Cellular organization of the neural circuit that drives Drosophila courtship behavior. Curr Biol. 2010;20:1602–1614. doi: 10.1016/j.cub.2010.08.025. [DOI] [PubMed] [Google Scholar]
- Zabala F, Polidoro P, Robie A, Branson K, Perona P, Dickinson MH. A simple strategy for detecting moving objects during locomotion revealed by animal-robot interactions. Curr Biol. 2012;22:1344–1350. doi: 10.1016/j.cub.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Rao Y, Rao Y. A subset of octopaminergic neurons are important for Drosophila aggression. Nat Neurosci. 2008 doi: 10.1038/nn.2164. [DOI] [PubMed] [Google Scholar]
- Zwarts L, Versteven M, Callaerts P. Genetics and neurobiology of aggression in Drosophila. Fly (Austin) 2012;6:35–48. doi: 10.4161/fly.19249. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.