Abstract
Background
Right ventricular (RV) dysfunction is associated with poor prognosis in patients with heart failure (HF). Echocardiographic assessment of RV systolic function is challenging. The ability to visualize the right atrium (RA) allows a quantitative, highly reproducible assessment of RA volume.
Objective
The aim is to study the relationship between the right atrial volume index (RAVI) and prognosis in patients with chronic systolic HF.
Methods
120 patients with chronic systolic HF and left ventricular ejection fraction (LVEF) <40% were enrolled. The RA volume was calculated by Simpson’s method using single-plane RA area and indexed to body surface area (RAVI). RV systolic assessment was done using the RV fractional area change (RVFAC), and peak systolic velocity (Satri) using tissue Doppler imaging at the tricuspid annulus. The primary endpoint was death, urgent transplantation, or acute HF episode requiring hospital admission during a follow-up of 1 year.
Results
Follow up was complete for 117 of 120 patients. Fifty-two patients reached the primary endpoint. The mean RAVI was higher in patients with adverse events (45.5 ± 15 ml/m2 versus 25.2 ± 11 ml/m2, p < 0.001), and increased with worsening LVEF, RVFAC, Satri (Spearman’s r = −0.46, r = −0.45, r = −0.59, p < 0.001 for all). RAVI was not correlated with estimates of RV diastolic dysfunction. The cut-off threshold for RAVI to predict the primary endpoint using receiver-operating characteristic curve was 29 ml/m2 (area under the curve was 0.89%, 95% confidence interval: 0.82–0.95) with a sensitivity of 92%, and a specificity of 75%. NYHA > 2 (OR = 2.1, p < 0.01), and RAVI (OR = 1.6, p < 0.05) were found to be independent predictors of adverse outcome.
Conclusion
In patients with chronic systolic HF, RAVI is an independent predictor of adverse outcome with a threshold value of 29 ml/m2.
Keywords: Heart failure, Right atrium, Prognosis, Echocardiography
Abbreviations
- Aa
peak late diastolic TDI velocity
- ACE
angiotensin converting enzyme
- A-wave
peak late diastolic filling velocity
- CABG
coronary artery bypass grafting
- CI
95% confidence interval
- DT
early diastolic deceleration time
- Ea
peak early diastolic TDI velocity
- E-wave
peak early diastolic filling velocity
- HF
heart failure
- IVC
inferior vena cava
- LVEF
left ventricular ejection fraction
- NYHA
New York Heart Association
- PCI
percutaneous coronary intervention
- RA
right atrium
- RAVI
right atrial volume index
- ROC
receiver operator characteristic
- RV
right ventricular
- RVFAC
right ventricular fractional area change
- Sa
peak systolic TDI velocity
- Satri
peak tissue Doppler systolic velocity at the tricuspid annulus
- sPAP
systolic pulmonary artery systolic pressure
- TDI
tissue Doppler imaging
- Vmax
peak velocity
Introduction
Patients with congestive heart failure (HF) still have a poor prognosis, even after recent advances in therapy [1,2]. It is, therefore, important to establish a reliable means of identifying those patients at higher risk. Right ventricular (RV) systolic dysfunction is associated with poor long-term prognosis [3,4]. However, precise echocardiographic assessment of RV systolic function is challenging, primarily because the morphology of RV is complicated.
The ability to visualize the right atrium (RA) allows a quantitative, highly reproducible assessment of the RA volume that can be indexed to the body surface area [5,6]. However, there have been no many studies correlating RA volume and prognosis in patients with chronic HF [6].
Objective
Is to determine the relationship between the right atrial volume index and prognosis in patients with chronic systolic heart failure.
Methodology
Patient population
We enrolled consecutive patients with chronic heart failure evaluated at a tertiary cardiac center from 2009 to 2011. Inclusion criteria were age >18 years, symptomatic heart failure (New York Heart Association class II-IV), and left ventricular ejection fraction (LVEF) <40%. Exclusion criteria were mitral stenosis, mitral valve surgery, severe mitral regurgitation (>grade 3), severe aortic stenosis (peak velocity >4 m/s), malignancy, and severe renal failure requiring dialysis.
The study was approved by the medical ethics committee of our institution. The study protocol was designed in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. All patients gave informed consent before the procedure.
Echocardiographic examination
Comprehensive transthoracic echocardiography was performed using an Aloka alpha 5 echocardiography machine (Hitachi Aloka Medical, Ltd., manufactured in Tokyo, Japan) equipped with tissue Doppler imaging (TDI) technology. Two-dimensional, M-mode, Doppler echocardiography measurements and quantification were performed according to recommendations of the American Society of Echocardiography [7,8]. Right atrial volume was calculated using the Simpson’s method from the apical four-chamber view at end systole [5,6]. The right atrial volume index (RAVI) was derived by dividing the volume by body surface area, which was calculated using the Du Bois and Du Bois formula [9]. Continuous Doppler echocardiography was used to measure pulmonary artery and aortic velocities, tricuspid regurgitation velocity, and mitral regurgitation velocity. Pulsed Doppler echocardiography for the assessment of the standard diastolic filling velocities of both ventricles was performed using the apical four-chamber view. Thus, the peak early diastolic filling velocity (E-wave), early diastolic deceleration time (DT), and peak late diastolic filling velocity (A-wave) were recorded. For the right ventricle 2D and TDI measurements, care was taken to obtain an ultrasound beam parallel to the tricuspid annulus motion. The RV endocardium was traced manually in systole and diastole. The RV fractional area change (RVFAC) was calculated using the formula: (end-diastolic area – end-systolic area)/end-diastolic area. Tricuspid annular TDI was acquired in the apical 4-chamber view. Peak systolic (Sa), early diastolic (Ea), and late diastolic (Aa) velocities of the tricuspid annulus were measured as recommended previously (sample TDI volume less than 5 mL and an angle between the TDI sample volume and the longitudinal myocardial wall vector less than 20°) [10]. The systolic pulmonary artery systolic pressure (sPAP) was assessed by measuring the gradient between the right ventricle and the right atrium using the peak velocity (Vmax) of the tricuspid regurgitation (sPAP = 4(Vmax)2 + right atrial pressure). The right atrial pressure was based on both the size of the inferior vena cava (IVC) and the change in diameter of this vessel during respiration [11]. Briefly, IVC diameter ⩽2.1 cm that collapsed >50% with a sniff indicated normal RA pressure of 3 mmHg, whereas IVC diameter >2.1 cm that collapsed <50% with a sniff suggested high RA pressure of 15 mmHg. In scenarios in which IVC diameter and collapse did not fit this paradigm, an intermediate value of 8 mmHg was used. Pulsed-wave Doppler recording of the hepatic vein flow was done using a sample volume placed in the hepatic vein 1 cm proximal to junction of IVC and hepatic veins. Hepatic vein systolic/diastolic (S/D) ratio was calculated. Valvular regurgitation was graded according to guidelines of the American Society of Echocardiography [12]. All parameters were averaged over three heart cycles (five cycles for arrhythmia).
Clinical follow-up and endpoint definition
Clinical follow-up was for 1 year. The vital status of each patient was confirmed by a review of medical records or by telephone contact (family and/or patient). The primary endpoint was the combined risk of death or urgent heart transplantation or hospitalization for acute HF episode. Hospitalization for HF was defined as an admission for worsening HF in which intravenous therapy for HF was needed. The first event was considered for each patient.
Statistical analysis
The data were statistically analyzed using the Statistical Package for Social Sciences (SPSS) software version 17 (SPSS Inc., Chicago, IL, USA). Continuous variables were presented as mean ± standard deviation and categorical variables as absolute numbers (percentages). Categorical variables were compared by Chi-square test. Continuous normally distributed variables were compared by two-tailed t-test. Correlations between RAVI and echocardiographic variables were tested with Spearman’s r. The optimal cut-off for the prediction of primary event was determined by a receiver-operator characteristic (ROC) curve. ROC analysis was used to determine the sensitivity and specificity of RAVI in predicting the primary endpoint. Kaplan–Meier curves were constructed to show event-free survival according to time. Logistic regression backward likelihood ratio technique was used to find out the significant independent predictors of the primary endpoint. A p value ⩽ 0.05 (2-tailed) was considered significant and a p value ⩽ 0.01 was considered highly significant. Reproducibility of RAVI (intra-observer variability) was assessed by coefficient of variation for repeated measures in a random sample of 30 patients and was 6%.
Results
The study included 120 patients with chronic systolic HF and left ventricular ejection fraction (LVEF) <40%. Follow up was complete for 117 of 120 patients. Fifty-two (44.4%) of 117 patients reached the primary endpoint (7 deaths, and 45 hospital admission for HF). The baseline demographic and clinical characteristics of the studied patients are detailed in Table 1. Patients with the primary outcome events had higher incidence of advanced NYHA class, diabetes, and past heart failure admission. They also used more diuretics, spironolactone, and digitalis.
Table 1.
Baseline demographic and clinical characteristics of the patients according to the incidence of primary outcome events.
| Variables | Event |
P value | |
|---|---|---|---|
| Yes (n = 52) | No (n = 65) | ||
| Age (years) | 54.5 ± 14.8 | 54 ± 14 | NS |
| Body surface area (m2) | 1.81 ± 0.4 | 1.85 ± 0.2 | NS |
| Systolic BP (mmHg) | 120 ± 11 | 123 ± 12 | NS |
| Diastolic BP (mmHg) | 79 ± 4 | 80.3 ± 6 | NS |
| Gender | |||
| Male | 42(80.8%) | 45(69.2%) | NS |
| Female | 10(19.2%) | 20(30.8%) | |
| Etiology of HF | |||
| Ischemic | 24(46.2%) | 26(40%) | NS |
| Non-ischemic | 28(53.8%) | 39(60%) | |
| NYHA class III–IV | 50 (96.2%) | 39(60%) | <0.001 |
| Heart rate | 87 ± 5 | 93 ± 20 | NS |
| Hypertension | 10(19.2%) | 14(21.9%) | NS |
| Smoking | 14(26.9%) | 13(20%) | NS |
| Diabetes | 25(48.1%) | 18(27.7%) | <0.05 |
| Previous myocardial infarction | 19 (36.5%) | 19(29.2% | NS |
| Previous of HF admission | 36(69.2%) | 21(32.3%) | <0.001 |
| Previous PCI | 3(5.8%) | 2(3.1%) | NS |
| Previous CABG | 3(5.8%) | 5(7.7%) | NS |
| B-blockers | 36(69.2%) | 52(80%) | NS |
| ACE-inhibitors | 48(92.3%) | 65(100%) | NS |
| Diuretics | 51(98.1%) | 57(87.7%) | <0.05 |
| Digitalis | 31(59.6%) | 27(41.5%) | <0.05 |
| Spironolactone | 36(69.2%) | 32(49.2%) | <0.05 |
Data are expressed as a mean ± standard deviation or a number (percent). NS = non-significant, BP = blood pressure, HF = heart failure, NYHA = New York heart association, PCI = percutaneous coronary intervention, CABG = coronary artery bypass surgery, ACE = angiotensin converting enzyme.
Echocardiographic variables in patients with and without an event are listed in Table 2. For left sided parameters, the group with outcome events showed lower LVEF, mitral DT, mitral Sa, and mitral Aa, together with higher LA diameter, and mitral E/A ratio. For right-sided parameters, the group with primary endpoint events showed higher RAVI, higher RV area, higher sPAP, lower RVFAC, lower tricuspid Sa, and lower tricuspid Aa.
Table 2.
Echocardiographic parameters of the patients according to the incidence of primary outcome events.
| Variables | Event |
P value | |
|---|---|---|---|
| Yes (n = 52) | No (n = 65) | ||
| Left atrial diameter (mm) | 48 ± 7 | 43.4 ± 3.9 | 0.0001 |
| LV parameters | |||
| LV end-diastolic dimension index (mm/m2) | 36.7 ± 5 | 35.8 ± 5 | NS |
| LV end-systolic dimension index (mm/m2) | 32 ± 5 | 30.2 ± 5 | NS |
| LV ejection fraction (%) | 24.5 ± 5.4 | 31.4 ± 4.9 | 0.0001 |
| Mitral early/late flow velocity | 3.5 ± 1.4 | 2.5 ± 1.7 | 0.001 |
| Mitral deceleration time (ms) | 130 ± 27 | 143.5 ± 39 | 0.04 |
| Mitral TD systolic velocity (cm/s) | 4.5 ± 1.4 | 5.2 ± 1.4 | 0.007 |
| Mitral TD early diastolic velocity (cm/s) | 7.1 ± 2.5 | 7.6 ± 3.3 | NS |
| Mitral TD late diastolic velocity (cm/s) | 3.4 ± 1.2 | 4.8 ± 2.2 | 0.001 |
| Right atrial volume index (ml/ m2) | 45.5 ± 15.7 | 25.2 ± 11.6 | 0.0001 |
| RV parameters | |||
| RV end-diastolic area (cm2) | 20.3 ± 6 | 15.9 ± 5 | 0.001 |
| RV end-systolic area (cm2) | 14.5 ± 5 | 9.9 ± 4 | 0.001 |
| RV fractional area change (%) | 30.1 ± 11 | 43.7 ± 6 | 0.025 |
| Tricuspid early/late flow velocity | 1.2 ± 0.6 | 0.90 ± 0.4 | 0.03 |
| Tricuspid TD systolic velocity (cm/s) | 10.3 ± 1.7 | 12.9 ± 1.7 | <0.001 |
| Tricuspid TD early diastolic velocity (cm/s) | 10.5 ± 5 | 10.7 ± 3 | NS |
| Tricuspid TD late diastolic velocity (cm/s) | 9.9 ± 4 | 12.6 ± 5 | <0.05 |
| Pulmonary artery systolic pressure (mmHg) | 51 ± 13 | 39 ± 10.5 | 0.001 |
| Hepatic vein systolic/diastolic ratio | 1.1 ± 0.5 | 1.4 ± 0.7 | 0.086 |
NS = non-significant, LV = left ventricle. TD = tissue Doppler. Data are expressed as a mean ± standard deviation or a number (percent).
In multivariate analysis, RAVI remained an independent predictor of adverse outcomes using logistic regression analysis (odd’s ratio of 1.6, 95% confidence interval: 0.4–7, p value <0.05, Table 3).
Table 3.
Relation of all predictors to the event by logistic regression analysis.
| Variables | Beta coefficient | Odd’s (95% CI) | P value |
|---|---|---|---|
| NYHA class > 2 | 0.98 | 2.1(0.9–8.5) | <0.001 |
| RAVI ⩾ 29 ml/m2 | 0.37 | 1.6(0.4–7) | <0.05 |
| Satri < 10 cm/s | 0.30 | 1.5(0.2–6.95) | <0.05 |
CI: confidence interval, NYHA:New York heart association, RAVI: right atrial volume index, Satri = Tricuspid TD systolic velocity.
The overall mean RAVI in the study population was 34.0 ± 16.7 ml/m2. The mean RAVI was higher in men than in women (35.8 ± 17.5 vs. 28.9 ± 13.1, p = 0.048). The mean RAVI was higher in patients with an event than in those without an event (45.5 ± 15.7 vs. 25.2 ± 11.6, p = 0.0001). The mean RAVI increased with worsening Satri, NYHA class, LVEF, RVFAC (Spearman’s r = −0.59, r = −0.55, r = −0.46, r = −0.45, p < 0.001 for all). RAVI was not correlated with estimates of RV diastolic dysfunction (tricuspid flow E/A ratio, or hepatic vein S/D ratio).
The cut-off threshold for RAVI to predict the primary endpoint using receiver-operating characteristic curve was 29 ml/m2 (area under the curve was 0.89, 95% confidence interval: 0.82–0.95) with a sensitivity of 92%, and a specificity of 75% (Fig. 1). Kaplan–Meier event-free survival curves for RAVI ⩾ 29 ml/m2 (optimal cut-off value determined using receiver-operating characteristic analysis) are shown in Fig. 2. In the subgroup with RAVI ⩾ 29 ml/m2, 46 out of 64 patients had an event (71.9%). In the subgroup with RAVI < 29 ml/m2, only 6 out of 53 patients had an event (11.3%).
Figure 1.
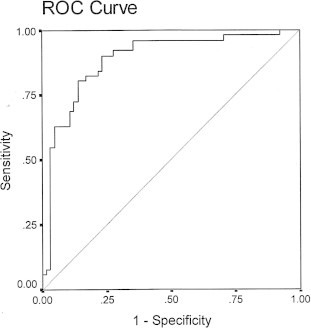
The cut-off value for RAVI using ROC curve was 29 ml/m2, AUC (95% CI) = 0.89 (0.82–0.95).
Figure 2.
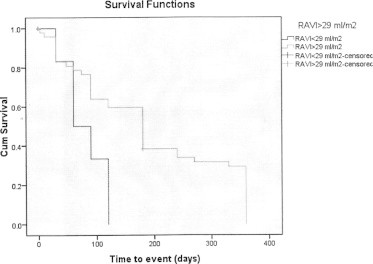
Kaplan–Meier curves for RAVI ⩾ 29 ml/m2 and event-free survival. Log Rrank (Mantel–Cox) between both lines is 0.009.
Discussion
The present study shows that RAVI is an independent predictor of outcome (death, urgent heart transplantation, or hospitalization for acute HF episode) in patients with chronic systolic HF with reduced LVEF, when other important clinical and echocardiographic markers such as NYHA, LVEF, and Satri are considered. This finding is important as it might help to identify the HF patient with poor prognosis.
Right atrial volume index and prognosis in heart failure
Right ventricular (RV) systolic dysfunction is associated with poor long-term prognosis in patients with HF [3,4]. Pulmonary artery pressure and thermodilution-derived RV ejection fraction were found to be independent predictors of adverse outcome on Cox multivariate survival analysis [3]. However, precise echocardiographic assessment of RV systolic function is challenging, primarily because the morphology of RV is complicated. The ability to visualize the RA allows a quantitative, highly reproducible assessment of the RAVI [5,6]. In the present study, RAVI remained an independent predictor of outcome even when RV echocardiographic markers such as RVFAC, Satri, tricuspid E/A ratio, and hepatic vein S/D ratio are considered.
Sallach et al. showed that RAVI is an independent predictor of cardiovascular events in patients with chronic stable heart failure [6]. RAVI remained an independent predictor of poor outcomes after adjusting for age, brain naturetic peptide, LVEF, RV systolic dysfunction stage, and tricuspid E/Ea ratio (p = 0.005) [6]. In ROC analysis, RAVI ⩾ 30.6 ml/m2 (optimal ROC cutoff) had a 78% sensitivity and a 77% specificity (p < 0.0001) for predicting RV systolic dysfunction stage ⩾3; and RAVI ⩾ 41.6 ml/m2 (optimal ROC cutoff) had a 68% sensitivity and a 92% specificity (p < 0.0004) for predicting NYHA functional class ⩾3 [6]. D’Andrea et al. demonstrated that a right atrial area >16 cm2 had 87.1% sensitivity and 95.4% specificity in predicting a negative response to cardiac resynchronization therapy in patients with dilated cardiomyopathy [13].
Right atrial volume index and right ventricular functions
The present study showed a good correlation between RAVI and worsening systolic right ventricular parameters (Satri, and RVFAC). Similar findings were reported in previous studies [6,14]. RAVI showed good correlation with RV systolic dysfunction stage, and tricuspid annular Sa (Spearman’s r = 0.61, and −0.34, respectively, p = 0.0001 for both) [6]. Another report demonstrated that RAVI showed good correlation with RV end-systolic diameter (r = −0.484, p = 0.002), and tricuspid annular plane systolic excursion (r = −0.47, p = 0.001) [14].
In the present study, RAVI was not correlated with estimates of RV diastolic dysfunction (tricuspid flow E/A ratio, or hepatic vein S/D ratio). This finding was consistent with findings of Sallach et al. [6]. They demonstrated that RAVI showed only a modest correlation with tricuspid flow E/A ratio, or hepatic vein S/D ratio, and no correlation with the tricuspid E/Ea ratio [6]. This suggests that RAVI may be reflective of the chronicity of RV diastolic function over time (similar to left atrial volume index) rather than a measure of instantaneous diastolic function [6]. This is also emphasizes the complexity of measuring right-sided diastolic function and the poor correlation of right atrial volume to RV filling pressure due to the additional effect of RA stiffness [15].
RV impairment secondary to LV failure usually initiates due to pressure overload, which is translated to tricuspid regurgitation, and evolves to right ventricular and right atrial dilatation [16]. Right atrial dilatation is usually observed at an advanced stage of pressure and volume overload. Thus, it may reflect a more severe clinical presentation in patients with heart failure and reduced LVEF.
Limitations of the study
The population studied was relatively small, but despite the sample size, there was a high event rate (44%). Therefore, we were able to reach several significant conclusions. The RA volumes were calculated using Simpson’s method from only one view (the apical 4-chamber). This view places the right atrium in the far field; therefore, diminishing lateral resolution and adversely affecting visualization of right atrial endocardium. Finally the present study did not include biological markers for adverse outcome in heart failure such as brain naturetic peptide, but this would have increased the cost of the present study.
Conclusion and recommendations
In patients with chronic systolic heart failure and reduced LV ejection fraction, RAVI is an independent predictor of adverse outcome with a threshold value of 29 ml/m2.
In view of the small sample size included in this report, larger clinical studies are needed to confirm these observations.
Funding
The study was supported by our institution.
Footnotes
Peer review under responsibility of King Saud University.
Appendix A. Supplementary data
Supplementary Figure 2.
Measurement of right atrial volume using Simpson’s method, case 85.
Supplementary Figure 3.
Measurement of right ventricular fractional area change, case 88.
Supplementary Figure 4.
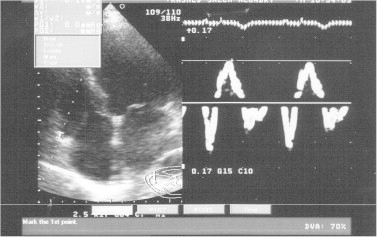
Measurement of tricuspid annular systolic wave (Satri) with pulsed-wave tissue Doppler imaging, case 88.
References
- 1.Solomon S.D., Anavekar N., Skali H., McMurray J.J., Swedberg K., Yusuf S. Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation. 2005;112:3738–3744. doi: 10.1161/CIRCULATIONAHA.105.561423. [DOI] [PubMed] [Google Scholar]
- 2.Elasfar A.A., Alhabib K.F., Qasim A., Bahamaid R.A., Youssef M.A. Clinical characteristics, management, and outcomes of patients with high risk chronic heart failure referred to a Heart Failure Clinic in Saudi Arabia. J Saudi Heart Assoc. 2013;25:113. [abs] [Google Scholar]
- 3.Ghio S., Gavazzi A., Campana C., Inserra C., Klersy C., Sebastiani R. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol. 2001;37(1):183–188. doi: 10.1016/s0735-1097(00)01102-5. [DOI] [PubMed] [Google Scholar]
- 4.Di Salvo T.G., Mathier M., Semigran M.J., Dec G.W. Preserved right ventricular ejection fraction predicts exercise capacity and survival in advanced heart failure. J Am Coll Cardiol. 1995;25:1143–1153. doi: 10.1016/0735-1097(94)00511-n. [DOI] [PubMed] [Google Scholar]
- 5.Cioffi G., de Simone G., Mureddu G., Tarantini L., Stefenelli C. Right atrial size and function in patients with pulmonary hypertension associated with disorders of respiratory system or hypoxemia. Eur J Echocardiogr. 2007;8(5):322–331. doi: 10.1016/j.euje.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Sallach J.A., Tang W.H., Borowski A.G., Tong W., Porter T., Martin M.G. Right atrial volume index in chronic systolic heart failure and prognosis. JACC Cardiovasc Imaging. 2009;2(5):527–534. doi: 10.1016/j.jcmg.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 7.Lang R.M., Bierig M., Devereux R.B., Flachskampf F.A., Foster E., Pellikka P.A. Recommendations for chamber quantification: a report from the American society of echocardiography’s guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the European association of echocardiography, a branch of the European society of cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Quinones M.A., Otto C.M., Stoddard M., Waggoner A., Zoghbi W.A. Recommendations for quantification of Doppler echocardiography: a report from the Doppler quantification task force of the nomenclature and standards committee of the American society of echocardiography. J Am Soc Echocardiogr. 2002;15:167–184. doi: 10.1067/mje.2002.120202. [DOI] [PubMed] [Google Scholar]
- 9.Du Bois D., Du Bois E. Measurement of surface area in man. Arch Int Med. 1915;15:868–881. [Google Scholar]
- 10.Meluzin J., Spinarova L., Bakala J., Toman J., Krejci J., Hude P. Pulsed Doppler tissue imaging of the velocity of tricuspid annular systolic motion; a new, rapid, and non-invasive method of evaluating right ventricular systolic function. Eur Heart J. 2001;22:340–348. doi: 10.1053/euhj.2000.2296. [DOI] [PubMed] [Google Scholar]
- 11.Brennan J.M., Blair J.E., Goonewardena S., Ronan A., Shah D., Vasaiwala S. Reappraisal of the use of inferior vena cava for estimating right atrial pressure. J Am Soc Echocardiogr. 2007;20:857–861. doi: 10.1016/j.echo.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Zoghbi W.A., Enriquez-Sarano M., Foster E., Grayburn P.A., Kraft C.D., Levine R.A. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. doi: 10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 13.D’Andrea A., Scarafile R., Riegler L. Right atrial size and deformation in patients with dilated cardiomyopathy undergoing cardiac resynchronization therapy. Eur J Heart Fail. 2009;11:1169–1177. doi: 10.1093/eurjhf/hfp158. [DOI] [PubMed] [Google Scholar]
- 14.Mantziari L., Kamperidis V., Ventoulis I., Damvopoulou E., Giannakoulas G., Efthimiadis G. Increased right atrial volume index predicts low Duke activity status index in patients with chronic heart failure. Hellenic J Cardiol. 2013;54(1):32–38. [PubMed] [Google Scholar]
- 15.Nagueh S.F., Zoghbi W.A. Evaluation of right ventricular diastolic function. In: Klein A.L., Garcia M.J., editors. Diastology: clinical approach to diastolic heart failure. Elsevier; New York, NY: 2008. pp. 171–180. [Google Scholar]
- 16.Bogaard H.J., Abe K., Vonk Noordegraaf A., Voelkel N.F. The right ventricle under pressure: cellular and molecular mechanisms of right-heart failure in pulmonary hypertension. Chest. 2009;135:794–804. doi: 10.1378/chest.08-0492. [DOI] [PubMed] [Google Scholar]



