Abstract
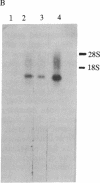
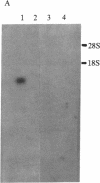
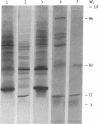
Rapid growth of human fetal tissues requires insulin or insulin-like growth factors. A high rate of human fetal growth occurs between implantation and about 14 weeks of gestation. Fetal pancreatic insulin secretion begins much later. Since maternal insulin does not cross the blood/placental barrier, other sources of insulin or insulin-like growth factors may be provided for fetal development. We report here that placental polyadenylylated RNAs from the first and third trimester of normal pregnancy as well as from term pregnancies of diabetic mothers hybridize to a 32P-labeled cloned cDNA of an insulin-related sequence expressed in fetal pancreas. Moreover, placentas from diabetic women express much more of these sequences. These results suggest that insulin-related genes are expressed in placental tissue during fetal development and may be a source of growth-promoting hormones for the human fetus. Fetuses developing in diabetic women receive a large influx of glucose. This in turn may stimulate the expression of insulin-related sequences, which may result in higher utilization of glucose, thus bringing about the macrosomia and high incidence of malformation and still-births known to result from pregnancies in diabetics.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam P. A., King K. C., Schwartz R., Teramo K. Human placental barrier to 125-I-glucagon early in gestation. J Clin Endocrinol Metab. 1972 May;34(5):772–782. doi: 10.1210/jcem-34-5-772. [DOI] [PubMed] [Google Scholar]
- Adam P. A., Teramo K., Raiha N., Gitlin D., Schwartz R. Human fetal insulin metabolismearly in gestation. Response to acutelevation of the fetal glucose concentration and placental tranfer of human insulin-I-131. Diabetes. 1969 Jun;18(6):409–416. doi: 10.2337/diab.18.6.409. [DOI] [PubMed] [Google Scholar]
- Adams S. O., Nissley S. P., Handwerger S., Rechler M. M. Developmental patterns of insulin-like growth factor-I and -II synthesis and regulation in rat fibroblasts. Nature. 1983 Mar 10;302(5904):150–153. doi: 10.1038/302150a0. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocek R. M., Beatty C. H. Effect of insulin on the carbohydrate metabolism of fetal rhesus monkey muscle. Endocrinology. 1969 Sep;85(3):615–618. doi: 10.1210/endo-85-3-615. [DOI] [PubMed] [Google Scholar]
- Bocek R. M., Young M. D., Beatty C. H. Effect of insulin and epinephrine on the carbohydrate metabolism and adenylate cyclase activity of rhesus fetal muscle. Pediatr Res. 1973 Oct;7(10):787–793. doi: 10.1203/00006450-197310000-00001. [DOI] [PubMed] [Google Scholar]
- Chez R. A., Mintz D. H., Epstein M. F., Fleischman A. R., Oakes G. K., Hutchinson D. L. Glucagon metabolism in nonhuman primate pregnancy. Am J Obstet Gynecol. 1974 Nov 1;120(5):690–696. doi: 10.1016/0002-9378(74)90612-7. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Clark C. M., Jr Carbohydrate metabolism in the isolated fetal rat heart. Am J Physiol. 1971 Mar;220(3):583–588. doi: 10.1152/ajplegacy.1971.220.3.583. [DOI] [PubMed] [Google Scholar]
- Clark C. M., Jr Characterization of glucose metabolism in the isolated rat heart during fetal and early neonatal development. Diabetes. 1973 Jan;22(1):41–49. doi: 10.2337/diab.22.1.41. [DOI] [PubMed] [Google Scholar]
- Cooke P. S., Nicoll C. S. Role of insulin in the growth of fetal rat tissues. Endocrinology. 1984 Feb;114(2):638–643. doi: 10.1210/endo-114-2-638. [DOI] [PubMed] [Google Scholar]
- D'Ercole A. J., Applewhite G. T., Underwood L. E. Evidence that somatomedin is synthesized by multiple tissues in the fetus. Dev Biol. 1980 Mar 15;75(2):315–328. doi: 10.1016/0012-1606(80)90166-9. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A., Posakony J. W., Maniatis T., Lawn R. M., O'Connell C., Spritz R. A., DeRiel J. K., Forget B. G., Weissman S. M., Slightom J. L. The structure and evolution of the human beta-globin gene family. Cell. 1980 Oct;21(3):653–668. doi: 10.1016/0092-8674(80)90429-8. [DOI] [PubMed] [Google Scholar]
- Eisen H. J., Glinsmann W. H., Sherline P. Effect of insulin on glycogen synthesis in fetal rat liver in organ culture. Endocrinology. 1973 Feb;92(2):584–588. doi: 10.1210/endo-92-2-584. [DOI] [PubMed] [Google Scholar]
- Espinosa de los Monteros, Driscoll S. G., Steinke J. Insulin release from isolated human fetal pancreatic islets. Science. 1970 May 29;168(3935):1111–1112. doi: 10.1126/science.168.3935.1111. [DOI] [PubMed] [Google Scholar]
- Gabbe S. G., Quilligan E. J. Fetal carbohydrate metabolism: its clinical importance. Am J Obstet Gynecol. 1977 Jan 1;127(1):92–103. doi: 10.1016/0002-9378(77)90321-0. [DOI] [PubMed] [Google Scholar]
- Hill D. E. Insulin and fetal growth. Prog Clin Biol Res. 1976;10:127–139. [PubMed] [Google Scholar]
- Kalhan S. C., Schwartz R., Adam P. A. Placental barrier to human insulin-I125 in insulin-dependent diabetic mothers. J Clin Endocrinol Metab. 1975 Jan;40(1):139–142. doi: 10.1210/jcem-40-1-139. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mills N. C., Gyves M. T., Ilan J. Comparisons of human placental lactogen mRNA levels from placentas of diabetics and normal term. Mol Cell Endocrinol. 1985 Jan;39(1):61–69. doi: 10.1016/0303-7207(85)90092-9. [DOI] [PubMed] [Google Scholar]
- Paxson C. L., Jr, Morriss F. H., Jr, Adcock E. W., 3rd Effect of uterine artery insulin infusions on umbilical glucose uptake in sheep. Pediatr Res. 1978 Aug;12(8):864–867. doi: 10.1203/00006450-197808000-00012. [DOI] [PubMed] [Google Scholar]
- Poggi C., Le Marchand-Brustel Y., Zapf J., Froesch E. R., Freychet P. Effects and binding of insulin-like growth factor I in the isolated soleus muscle of lean and obese mice: comparison with insulin. Endocrinology. 1979 Sep;105(3):723–730. doi: 10.1210/endo-105-3-723. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Maniatis T. The structure of a human alpha-globin pseudogene and its relationship to alpha-globin gene duplication. Cell. 1980 Sep;21(2):537–544. doi: 10.1016/0092-8674(80)90491-2. [DOI] [PubMed] [Google Scholar]
- Rabain F., Picon L. Effect of insulin on the materno-fetal transfer of glucose in the rat. Horm Metab Res. 1974 Sep;6(5):376–380. doi: 10.1055/s-0028-1093828. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sara V. R., Hall K., Misaki M., Fryklund L., Christensen N., Wetterberg L. Ontogenesis of somatomedin and insulin receptors in the human fetus. J Clin Invest. 1983 May;71(5):1084–1094. doi: 10.1172/JCI110858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. Metabolic fuels in the foetus. Proc R Soc Med. 1968 Nov;61(11 Pt 2):1231–1236. doi: 10.1177/003591576806111P208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelley H. J., Bassett J. M., Milner R. D. Control of carbohydrate metabolism in the fetus and newborn. Br Med Bull. 1975 Jan;31(1):37–43. doi: 10.1093/oxfordjournals.bmb.a071239. [DOI] [PubMed] [Google Scholar]
- Simmons M. A., Jones M. D., Jr, Battaglia F. C., Meschia G. Insulin effect on fetal glucose utilization. Pediatr Res. 1978 Feb;12(2):90–92. doi: 10.1203/00006450-197802000-00004. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE P. Pregnancy complicating diabetes. Am J Med. 1949 Nov;7(5):609–616. doi: 10.1016/0002-9343(49)90382-4. [DOI] [PubMed] [Google Scholar]
- Wolf H., Sabata V., Frerichs H., Stubbe P. Evidence for the impermeability of the human placenta for insulin. Horm Metab Res. 1969 Nov;1(6):274–275. doi: 10.1055/s-0028-1095127. [DOI] [PubMed] [Google Scholar]
- Zapf J., Rinderknecht E., Humbel R. E., Froesch E. R. Nonsuppressible insulin-like activity (NSILA) from human serum: recent accomplishments and their physiologic implications. Metabolism. 1978 Dec;27(12):1803–1828. doi: 10.1016/0026-0495(78)90267-6. [DOI] [PubMed] [Google Scholar]
- Zapf J., Schoenle E., Froesch E. R. Insulin-like growth factors I and II: some biological actions and receptor binding characteristics of two purified constituents of nonsuppressible insulin-like activity of human serum. Eur J Biochem. 1978 Jun 15;87(2):285–296. doi: 10.1111/j.1432-1033.1978.tb12377.x. [DOI] [PubMed] [Google Scholar]
- Zapf J., Schoenle E., Waldvogel M., Sand I., Froesch E. R. Effect of trypsin treatment of rat adipocytes on biological effects and binding of insulin and insulin-like growth factors: further evidence for the action of insulin-like growth factors through the insulin receptor. Eur J Biochem. 1981 Jan;113(3):605–609. doi: 10.1111/j.1432-1033.1981.tb05105.x. [DOI] [PubMed] [Google Scholar]