Abstract
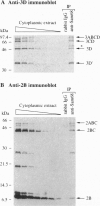
A HeLa cDNA expression library was screened for human polypeptides that interacted with the poliovirus RNA-dependent RNA polymerase, 3D, using the two-hybrid system in the yeast Saccharomyces cerevisiae. Sam68 (Src-associated in mitosis, 68 kDa) emerged as the human cDNA that, when fused to a transcriptional activation domain, gave the strongest 3D interaction signal with a LexA-3D hybrid protein. 3D polymerase and Sam68 coimmunoprecipitated from infected human cell lysates with antibodies that recognized either protein. Upon poliovirus infection, Sam68 relocalized from the nucleus to the cytoplasm, where poliovirus replication occurs. Sam68 was isolated from infected cell lysates with an antibody that recognizes poliovirus protein 2C, suggesting that it is found on poliovirus-induced membranes upon which viral RNA synthesis occurs. These data, in combination with the known RNA- and protein-binding properties of Sam68, make Sam68 a strong candidate for a host protein with a functional role in poliovirus replication.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andino R., Rieckhof G. E., Achacoso P. L., Baltimore D. Poliovirus RNA synthesis utilizes an RNP complex formed around the 5'-end of viral RNA. EMBO J. 1993 Sep;12(9):3587–3598. doi: 10.1002/j.1460-2075.1993.tb06032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andino R., Rieckhof G. E., Baltimore D. A functional ribonucleoprotein complex forms around the 5' end of poliovirus RNA. Cell. 1990 Oct 19;63(2):369–380. doi: 10.1016/0092-8674(90)90170-j. [DOI] [PubMed] [Google Scholar]
- Andrews N. C., Levin D., Baltimore D. Poliovirus replicase stimulation by terminal uridylyl transferase. J Biol Chem. 1985 Jun 25;260(12):7628–7635. [PubMed] [Google Scholar]
- Baker R. T., Board P. G. The human ubiquitin-52 amino acid fusion protein gene shares several structural features with mammalian ribosomal protein genes. Nucleic Acids Res. 1991 Mar 11;19(5):1035–1040. doi: 10.1093/nar/19.5.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienz K., Egger D., Pasamontes L. Association of polioviral proteins of the P2 genomic region with the viral replication complex and virus-induced membrane synthesis as visualized by electron microscopic immunocytochemistry and autoradiography. Virology. 1987 Sep;160(1):220–226. doi: 10.1016/0042-6822(87)90063-8. [DOI] [PubMed] [Google Scholar]
- Bienz K., Egger D., Rasser Y., Bossart W. Intracellular distribution of poliovirus proteins and the induction of virus-specific cytoplasmic structures. Virology. 1983 Nov;131(1):39–48. doi: 10.1016/0042-6822(83)90531-7. [DOI] [PubMed] [Google Scholar]
- Bienz K., Egger D., Troxler M., Pasamontes L. Structural organization of poliovirus RNA replication is mediated by viral proteins of the P2 genomic region. J Virol. 1990 Mar;64(3):1156–1163. doi: 10.1128/jvi.64.3.1156-1163.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal T., Carmichael G. G. RNA replication: function and structure of Qbeta-replicase. Annu Rev Biochem. 1979;48:525–548. doi: 10.1146/annurev.bi.48.070179.002521. [DOI] [PubMed] [Google Scholar]
- Chien C. T., Bartel P. L., Sternglanz R., Fields S. The two-hybrid system: a method to identify and clone genes for proteins that interact with a protein of interest. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9578–9582. doi: 10.1073/pnas.88.21.9578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S. A., Fumagalli S. A mitotic function for Src? Trends Cell Biol. 1994 Oct;4(10):345–347. doi: 10.1016/0962-8924(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Dasgupta A., Zabel P., Baltimore D. Dependence of the activity of the poliovirus replicase on the host cell protein. Cell. 1980 Feb;19(2):423–429. doi: 10.1016/0092-8674(80)90516-4. [DOI] [PubMed] [Google Scholar]
- Doedens J. R., Kirkegaard K. Inhibition of cellular protein secretion by poliovirus proteins 2B and 3A. EMBO J. 1995 Mar 1;14(5):894–907. doi: 10.1002/j.1460-2075.1995.tb07071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elble R. A simple and efficient procedure for transformation of yeasts. Biotechniques. 1992 Jul;13(1):18–20. [PubMed] [Google Scholar]
- Evan G. I., Lewis G. K., Ramsay G., Bishop J. M. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985 Dec;5(12):3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanegan J. B., Baltimore D. Poliovirus polyuridylic acid polymerase and RNA replicase have the same viral polypeptide. J Virol. 1979 Jan;29(1):352–360. doi: 10.1128/jvi.29.1.352-360.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli S., Totty N. F., Hsuan J. J., Courtneidge S. A. A target for Src in mitosis. Nature. 1994 Apr 28;368(6474):871–874. doi: 10.1038/368871a0. [DOI] [PubMed] [Google Scholar]
- Gyuris J., Golemis E., Chertkov H., Brent R. Cdi1, a human G1 and S phase protein phosphatase that associates with Cdk2. Cell. 1993 Nov 19;75(4):791–803. doi: 10.1016/0092-8674(93)90498-f. [DOI] [PubMed] [Google Scholar]
- Hoffman C. S., Winston F. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene. 1987;57(2-3):267–272. doi: 10.1016/0378-1119(87)90131-4. [DOI] [PubMed] [Google Scholar]
- Jore J., De Geus B., Jackson R. J., Pouwels P. H., Enger-Valk B. E. Poliovirus protein 3CD is the active protease for processing of the precursor protein P1 in vitro. J Gen Virol. 1988 Jul;69(Pt 7):1627–1636. doi: 10.1099/0022-1317-69-7-1627. [DOI] [PubMed] [Google Scholar]
- Kalpana G. V., Marmon S., Wang W., Crabtree G. R., Goff S. P. Binding and stimulation of HIV-1 integrase by a human homolog of yeast transcription factor SNF5. Science. 1994 Dec 23;266(5193):2002–2006. doi: 10.1126/science.7801128. [DOI] [PubMed] [Google Scholar]
- Kirkegaard K., Nelsen B. Conditional poliovirus mutants made by random deletion mutagenesis of infectious cDNA. J Virol. 1990 Jan;64(1):185–194. doi: 10.1128/jvi.64.1.185-194.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee C. K., Wimmer E. Proteolytic processing of poliovirus polyprotein: elimination of 2Apro-mediated, alternative cleavage of polypeptide 3CD by in vitro mutagenesis. Virology. 1988 Oct;166(2):405–414. doi: 10.1016/0042-6822(88)90511-9. [DOI] [PubMed] [Google Scholar]
- Legrain P., Rosbash M. Some cis- and trans-acting mutants for splicing target pre-mRNA to the cytoplasm. Cell. 1989 May 19;57(4):573–583. doi: 10.1016/0092-8674(89)90127-x. [DOI] [PubMed] [Google Scholar]
- Luban J., Bossolt K. L., Franke E. K., Kalpana G. V., Goff S. P. Human immunodeficiency virus type 1 Gag protein binds to cyclophilins A and B. Cell. 1993 Jun 18;73(6):1067–1078. doi: 10.1016/0092-8674(93)90637-6. [DOI] [PubMed] [Google Scholar]
- Lund P. K., Moats-Staats B. M., Simmons J. G., Hoyt E., D'Ercole A. J., Martin F., Van Wyk J. J. Nucleotide sequence analysis of a cDNA encoding human ubiquitin reveals that ubiquitin is synthesized as a precursor. J Biol Chem. 1985 Jun 25;260(12):7609–7613. [PubMed] [Google Scholar]
- Maynell L. A., Kirkegaard K., Klymkowsky M. W. Inhibition of poliovirus RNA synthesis by brefeldin A. J Virol. 1992 Apr;66(4):1985–1994. doi: 10.1128/jvi.66.4.1985-1994.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerovitch K., Svitkin Y. V., Lee H. S., Lejbkowicz F., Kenan D. J., Chan E. K., Agol V. I., Keene J. D., Sonenberg N. La autoantigen enhances and corrects aberrant translation of poliovirus RNA in reticulocyte lysate. J Virol. 1993 Jul;67(7):3798–3807. doi: 10.1128/jvi.67.7.3798-3807.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow C. D., Gibbons G. F., Dasgupta A. The host protein required for in vitro replication of poliovirus is a protein kinase that phosphorylates eukaryotic initiation factor-2. Cell. 1985 Apr;40(4):913–921. doi: 10.1016/0092-8674(85)90351-4. [DOI] [PubMed] [Google Scholar]
- O'Neill R. E., Palese P. NPI-1, the human homolog of SRP-1, interacts with influenza virus nucleoprotein. Virology. 1995 Jan 10;206(1):116–125. doi: 10.1016/s0042-6822(95)80026-3. [DOI] [PubMed] [Google Scholar]
- Pasamontes L., Egger D., Bienz K. Production of monoclonal and monospecific antibodies against non-capsid proteins of poliovirus. J Gen Virol. 1986 Nov;67(Pt 11):2415–2422. doi: 10.1099/0022-1317-67-11-2415. [DOI] [PubMed] [Google Scholar]
- Pata J. D., Schultz S. C., Kirkegaard K. Functional oligomerization of poliovirus RNA-dependent RNA polymerase. RNA. 1995 Jul;1(5):466–477. [PMC free article] [PubMed] [Google Scholar]
- Quadt R., Kao C. C., Browning K. S., Hershberger R. P., Ahlquist P. Characterization of a host protein associated with brome mosaic virus RNA-dependent RNA polymerase. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1498–1502. doi: 10.1073/pnas.90.4.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard S., Yu D., Blumer K. J., Hausladen D., Olszowy M. W., Connelly P. A., Shaw A. S. Association of p62, a multifunctional SH2- and SH3-domain-binding protein, with src family tyrosine kinases, Grb2, and phospholipase C gamma-1. Mol Cell Biol. 1995 Jan;15(1):186–197. doi: 10.1128/mcb.15.1.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehl H. H., Semler B. L. Poliovirus infection enhances the formation of two ribonucleoprotein complexes at the 3' end of viral negative-strand RNA. J Virol. 1995 May;69(5):2954–2961. doi: 10.1128/jvi.69.5.2954-2961.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruden D. M., Ma J., Li Y., Wood K., Ptashne M. Generating yeast transcriptional activators containing no yeast protein sequences. Nature. 1991 Mar 21;350(6315):250–252. doi: 10.1038/350250a0. [DOI] [PubMed] [Google Scholar]
- Semler B. L., Hanecak R., Dorner L. F., Anderson C. W., Wimmer E. Poliovirus RNA synthesis in vitro: structural elements and antibody inhibition. Virology. 1983 Apr 30;126(2):624–635. doi: 10.1016/s0042-6822(83)80018-x. [DOI] [PubMed] [Google Scholar]
- Taylor S. J., Anafi M., Pawson T., Shalloway D. Functional interaction between c-Src and its mitotic target, Sam 68. J Biol Chem. 1995 Apr 28;270(17):10120–10124. doi: 10.1074/jbc.270.17.10120. [DOI] [PubMed] [Google Scholar]
- Taylor S. J., Shalloway D. An RNA-binding protein associated with Src through its SH2 and SH3 domains in mitosis. Nature. 1994 Apr 28;368(6474):867–871. doi: 10.1038/368867a0. [DOI] [PubMed] [Google Scholar]
- Troxler M., Egger D., Pfister T., Bienz K. Intracellular localization of poliovirus RNA by in situ hybridization at the ultrastructural level using single-stranded riboprobes. Virology. 1992 Dec;191(2):687–697. doi: 10.1016/0042-6822(92)90244-j. [DOI] [PubMed] [Google Scholar]
- Wimmer E., Hellen C. U., Cao X. Genetics of poliovirus. Annu Rev Genet. 1993;27:353–436. doi: 10.1146/annurev.ge.27.120193.002033. [DOI] [PubMed] [Google Scholar]
- Wong G., Müller O., Clark R., Conroy L., Moran M. F., Polakis P., McCormick F. Molecular cloning and nucleic acid binding properties of the GAP-associated tyrosine phosphoprotein p62. Cell. 1992 May 1;69(3):551–558. doi: 10.1016/0092-8674(92)90455-l. [DOI] [PubMed] [Google Scholar]
- Ypma-Wong M. F., Dewalt P. G., Johnson V. H., Lamb J. G., Semler B. L. Protein 3CD is the major poliovirus proteinase responsible for cleavage of the P1 capsid precursor. Virology. 1988 Sep;166(1):265–270. doi: 10.1016/0042-6822(88)90172-9. [DOI] [PubMed] [Google Scholar]