Abstract
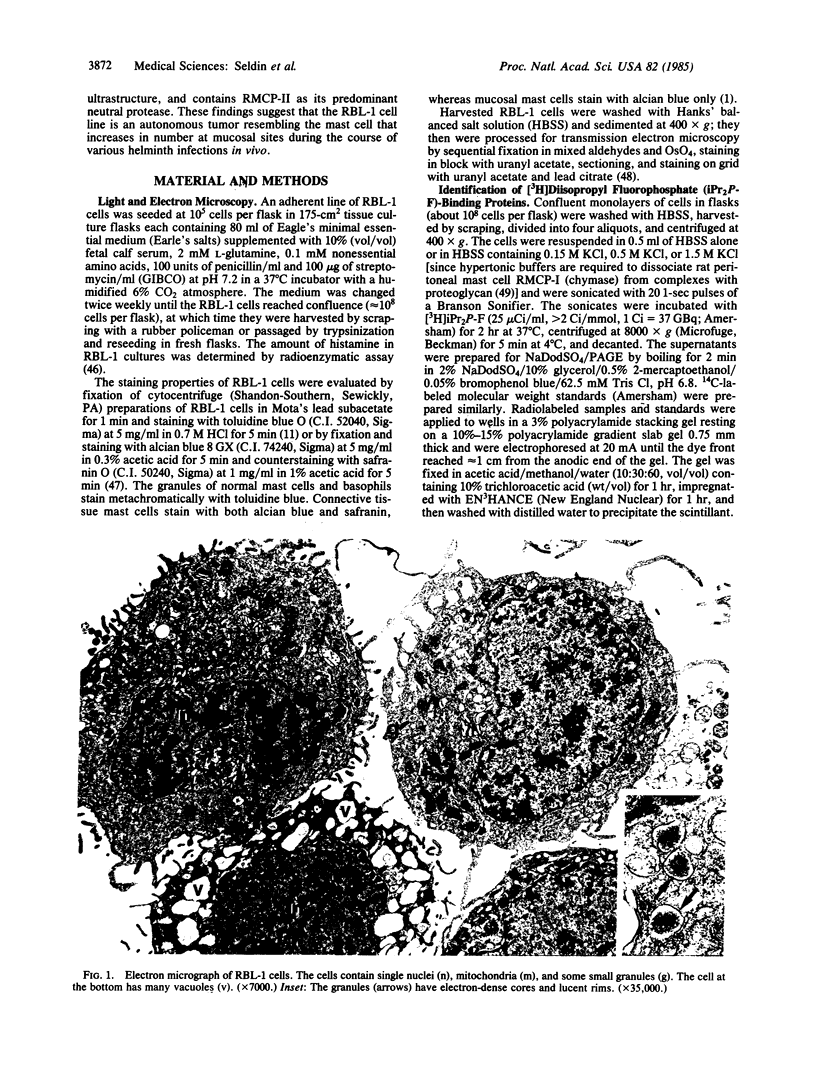
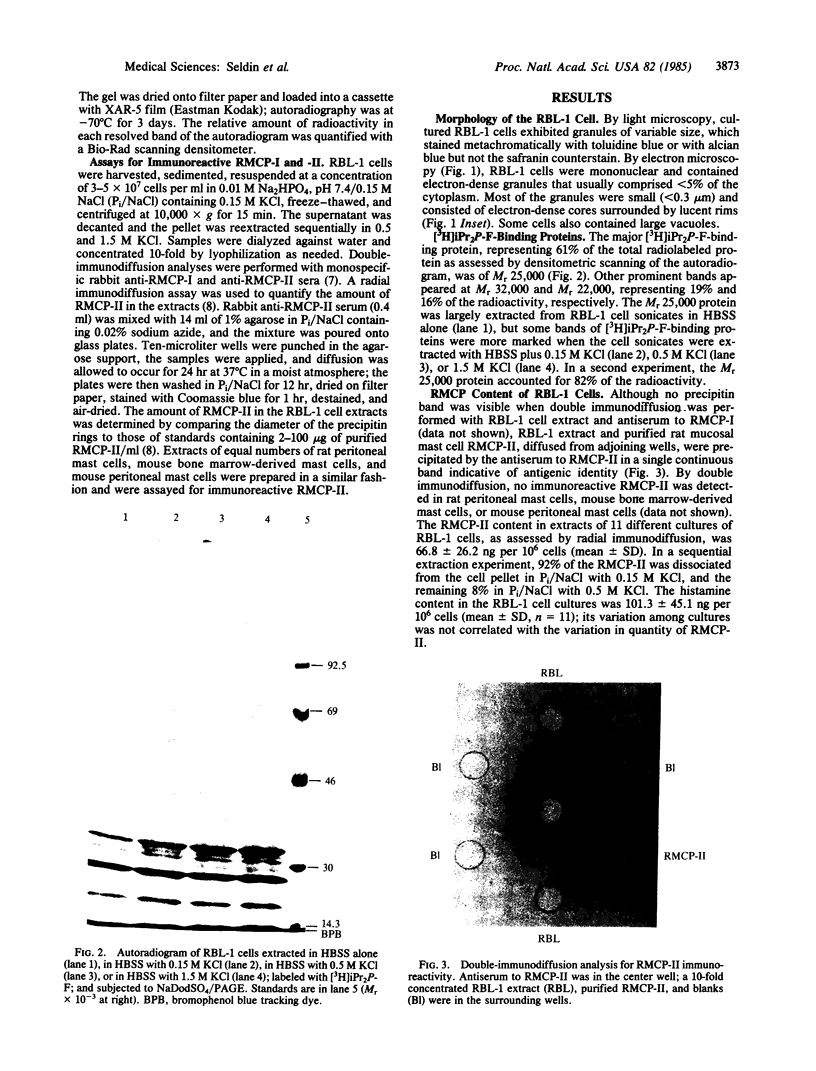
Secretory granules of the rat basophilic leukemia (RBL-1) cell, a chemically generated tumor cell line maintained in tissue culture, were shown to stain with alcian blue but not with safranin counterstain and to have sparse, small, electron-dense granules. A Mr 25,000 protein was the major [3H]diisopropyl fluorophosphate-binding protein in extracts of RBL-1 cells. Double-immunodiffusion analysis of extracts revealed immunoreactivity for rat mast cell protease (RMCP)-II, a Mr 25,000 neutral protease present in the secretory granules of rat mucosal mast cells and cultured rat bone marrow-derived mast cells, but no immunoreactivity for RMCP-I, the predominant neutral protease of rat connective tissue mast cells. By radial immunodiffusion, there was 66.8 ng of RMCP-II per 10(6) cells. Whereas rat connective tissue mast cells stain with alcian blue and safranin and contain heparin proteoglycan, rat mucosal and rat bone marrow-derived mast cells stain with alcian blue only and contain a non-heparin proteoglycan and lesser amounts of histamine. Proliferation of rat mucosal mast cells in vivo and rat bone marrow-derived mast cells in vitro requires T-cell factors, whereas no comparable requirement has been observed for connective tissue mast cells. The transformed RBL-1 tumor cells, whose growth is independent of factors other than those present in standard tissue culture medium, has previously been shown to contain predominantly chondroitin sulfate di-B proteoglycans and low amounts of histamine. The similar histology and secretory granule biochemistry of the rat mucosal mast cells, rat culture-derived mast cell, and RBL-1 cell suggest that they comprise a single mast cell subclass distinct from the rat connective tissue mast cell.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROWN H. E., DOUGHERTY T. F. The diurnal variation of blood leucocytes in normal and adrenalectomized mice. Endocrinology. 1956 Mar;58(3):365–375. doi: 10.1210/endo-58-3-365. [DOI] [PubMed] [Google Scholar]
- Beaven M. A., Rogers J., Moore J. P., Hesketh T. R., Smith G. A., Metcalfe J. C. The mechanism of the calcium signal and correlation with histamine release in 2H3 cells. J Biol Chem. 1984 Jun 10;259(11):7129–7136. [PubMed] [Google Scholar]
- Befus A. D., Pearce F. L., Gauldie J., Horsewood P., Bienenstock J. Mucosal mast cells. I. Isolation and functional characteristics of rat intestinal mast cells. J Immunol. 1982 Jun;128(6):2475–2480. [PubMed] [Google Scholar]
- Caulfield J. P., Lewis R. A., Hein A., Austen K. F. Secretion in dissociated human pulmonary mast cells. Evidence for solubilization of granule contents before discharge. J Cell Biol. 1980 May;85(2):299–312. doi: 10.1083/jcb.85.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman A. L. Leukocyte values of normal and adrenalectomized male and female C57 BL-cn mice. Lab Anim Care. 1968 Dec;18(6):616–622. [PubMed] [Google Scholar]
- Crews F. T., Morita Y., McGivney A., Hirata F., Siraganian R. P., Axelrod J. IgE-mediated histamine release in rat basophilic leukemia cells: receptor activation, phospholipid methylation, Ca2+ flux, and release of arachidonic acid. Arch Biochem Biophys. 1981 Dec;212(2):561–571. doi: 10.1016/0003-9861(81)90399-4. [DOI] [PubMed] [Google Scholar]
- Dieterich R. A. Hematologic values for five northern microtines. Lab Anim Sci. 1972 Jun;22(3):390–392. [PubMed] [Google Scholar]
- Dvorak A. M., Nabel G., Pyne K., Cantor H., Dvorak H. F., Galli S. J. Ultrastructural identification of the mouse basophil. Blood. 1982 Jun;59(6):1279–1285. [PubMed] [Google Scholar]
- Dy M., Lebel B., Kamoun P., Hamburger J. Histamine production during the anti-allograft response. Demonstration of a new lymphokine enhancing histamine synthesis. J Exp Med. 1981 Feb 1;153(2):293–309. doi: 10.1084/jem.153.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccleston E., Leonard B. J., Lowe J. S., Welford H. J. Basophilic leukaemia in the albino rat and a demonstration of the basopoietin. Nat New Biol. 1973 Jul 18;244(133):73–76. doi: 10.1038/newbio244073b0. [DOI] [PubMed] [Google Scholar]
- Enerbäck L. Mast cells in rat gastrointestinal mucosa. 2. Dye-binding and metachromatic properties. Acta Pathol Microbiol Scand. 1966;66(3):303–312. doi: 10.1111/apm.1966.66.3.303. [DOI] [PubMed] [Google Scholar]
- Enerbäck L. Mast cells in rat gastrointestinal mucosa. 3. Reactivity towards compound 48/80. Acta Pathol Microbiol Scand. 1966;66(3):313–322. doi: 10.1111/apm.1966.66.3.313. [DOI] [PubMed] [Google Scholar]
- Haig D. M., Jarrett E. E., Tas J. In vitro studies on mast cell proliferation in N. brasiliensis infection. Immunology. 1984 Apr;51(4):643–651. [PMC free article] [PubMed] [Google Scholar]
- Haig D. M., McKee T. A., Jarrett E. E., Woodbury R., Miller H. R. Generation of mucosal mast cells is stimulated in vitro by factors derived from T cells of helminth-infected rats. Nature. 1982 Nov 11;300(5888):188–190. doi: 10.1038/300188a0. [DOI] [PubMed] [Google Scholar]
- Haig D. M., McMenamin C., Gunneberg C., Woodbury R., Jarrett E. E. Stimulation of mucosal mast cell growth in normal and nude rat bone marrow cultures. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4499–4503. doi: 10.1073/pnas.80.14.4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasthorpe S. A hemopoietic cell line dependent upon a factor in pokeweed mitogen-stimulated spleen cell conditioning medium. J Cell Physiol. 1980 Nov;105(2):379–384. doi: 10.1002/jcp.1041050221. [DOI] [PubMed] [Google Scholar]
- Hatanaka K., Kitamura Y., Nishimune Y. Local development of mast cells from bone marrow-derived precursors in the skin of mice. Blood. 1979 Jan;53(1):142–147. [PubMed] [Google Scholar]
- Hurtado I., Urbina C. Ultrastructure of the mouse blood basophil. J Submicrosc Cytol. 1983 Oct;15(4):1041–1048. [PubMed] [Google Scholar]
- Isersky C., Rivera J., Triche T. J., Metzger H. Characterization of the receptors for IgE on membranes isolated from rats basophilic leukemia cells. Mol Immunol. 1982 Jul;19(7):925–941. doi: 10.1016/0161-5890(82)90359-5. [DOI] [PubMed] [Google Scholar]
- Ishizaka T., Okudaira H., Mauser L. E., Ishizaka K. Development of rat mast cells in vitro. I. Differentiation of mast cells from thymus cells. J Immunol. 1976 Mar;116(3):747–754. [PubMed] [Google Scholar]
- Jakschik B. A., Falkenhein S., Parker C. W. Precursor role of arachidonic acid in release of slow reacting substance from rat basophilic leukemia cells. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4577–4581. doi: 10.1073/pnas.74.10.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz H. R., LeBlanc P. A., Russell S. W. Two classes of mouse mast cells delineated by monoclonal antibodies. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5916–5918. doi: 10.1073/pnas.80.19.5916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura Y., Shimada M., Hatanaka K., Miyano Y. Development of mast cells from grafted bone marrow cells in irradiated mice. Nature. 1977 Aug 4;268(5619):442–443. doi: 10.1038/268442a0. [DOI] [PubMed] [Google Scholar]
- Kulczycki A., Jr, McNearney T. A., Parker C. W. The rat basophilic leukemia cell receptor for IgE. I. Characterization as a glycoprotein. J Immunol. 1976 Aug;117(2):661–665. [PubMed] [Google Scholar]
- Levine L. Inhibition of the A-23187-stimulated leukotriene and prostaglandin biosynthesis of rat basophil leukemia (RBL-1) cells by nonsteroidal anti-inflammatory drugs, antioxidants, and calcium channel blockers. Biochem Pharmacol. 1983 Oct 15;32(20):3023–3026. doi: 10.1016/0006-2952(83)90244-7. [DOI] [PubMed] [Google Scholar]
- MacGlashan D. W., Jr, Lichtenstein L. M. The purification of human basophils. J Immunol. 1980 May;124(5):2519–2521. [PubMed] [Google Scholar]
- Mayrhofer G., Fisher R. Mast cells in severely T-cell depleted rats and the response to infestation with Nippostrongylus brasiliensis. Immunology. 1979 May;37(1):145–155. [PMC free article] [PubMed] [Google Scholar]
- Miller H. R., Jarrett W. F. Immune reactions in mucous membranes. I. Intestinal mast cell response during helminth expulsion in the rat. Immunology. 1971 Mar;20(3):277–288. [PMC free article] [PubMed] [Google Scholar]
- Morita Y., Siraganian R. P. Inhibition of IgE-mediated histamine release from rat basophilic leukemia cells and rat mast cells by inhibitors of transmethylation. J Immunol. 1981 Oct;127(4):1339–1344. [PubMed] [Google Scholar]
- Nagao K., Yokoro K., Aaronson S. A. Continuous lines of basophil/mast cells derived from normal mouse bone marrow. Science. 1981 Apr 17;212(4492):333–335. doi: 10.1126/science.7209531. [DOI] [PubMed] [Google Scholar]
- Ogilvie B. M., Askenase P. W., Rose M. E. Basophils and eosinophils in three strains of rats and in athymic (nude) rats following infection with the nematodes Nippostrongylus brasiliensis or Trichinella spiralis. Immunology. 1980 Mar;39(3):385–389. [PMC free article] [PubMed] [Google Scholar]
- Ogilvie B. M., Hesketh P. M., Rose M. E. Nippostrongylus brasiliensis: peripheral blood leucocyte response of rats, with special reference to basophils. Exp Parasitol. 1978 Nov;46(1):20–30. doi: 10.1016/0014-4894(78)90153-4. [DOI] [PubMed] [Google Scholar]
- Orning L., Hammarström S., Samuelsson B. Leukotriene D: a slow reacting substance from rat basophilic leukemia cells. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2014–2017. doi: 10.1073/pnas.77.4.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce F. L., Befus A. D., Gauldie J., Bienenstock J. Mucosal mast cells. II. Effects of anti-allergic compounds on histamine secretion by isolated intestinal mast cells. J Immunol. 1982 Jun;128(6):2481–2486. [PubMed] [Google Scholar]
- Razin E., Cordon-Cardo C., Good R. A. Growth of a pure population of mouse mast cells in vitro with conditioned medium derived from concanavalin A-stimulated splenocytes. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2559–2561. doi: 10.1073/pnas.78.4.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin E., Ihle J. N., Seldin D., Mencia-Huerta J. M., Katz H. R., LeBlanc P. A., Hein A., Caulfield J. P., Austen K. F., Stevens R. L. Interleukin 3: A differentiation and growth factor for the mouse mast cell that contains chondroitin sulfate E proteoglycan. J Immunol. 1984 Mar;132(3):1479–1486. [PubMed] [Google Scholar]
- Razin E., Stevens R. L., Akiyama F., Schmid K., Austen K. F. Culture from mouse bone marrow of a subclass of mast cells possessing a distinct chondroitin sulfate proteoglycan with glycosaminoglycans rich in N-acetylgalactosamine-4,6-disulfate. J Biol Chem. 1982 Jun 25;257(12):7229–7236. [PubMed] [Google Scholar]
- Razin E., Stevens R. L., Austen K. F., Caulfield J. P., Hein A., Liu F. T., Clabby M., Nabel G., Cantor H., Friedman S. Cloned mouse mast cells derived from immunized lymph node cells and from foetal liver cells exhibit characteristics of bone marrow-derived mast cells containing chondroitin sulphate E proteoglycan. Immunology. 1984 Jul;52(3):563–575. [PMC free article] [PubMed] [Google Scholar]
- Roth R. L., Levy D. A. Nippostrongylus brasiliensis: peripheral leukocyte responses and correlation of basophils with blood histamine concentration during infection in rats. Exp Parasitol. 1980 Dec;50(3):331–341. doi: 10.1016/0014-4894(80)90036-3. [DOI] [PubMed] [Google Scholar]
- Ruitenberg E. J., Elgersma A. Absence of intestinal mast cell response in congenitally athymic mice during Trichinella spiralis infection. Nature. 1976 Nov 18;264(5583):258–260. doi: 10.1038/264258a0. [DOI] [PubMed] [Google Scholar]
- Schrader J. W., Lewis S. J., Clark-Lewis I., Culvenor J. G. The persisting (P) cell: histamine content, regulation by a T cell-derived factor, origin from a bone marrow precursor, and relationship to mast cells. Proc Natl Acad Sci U S A. 1981 Jan;78(1):323–327. doi: 10.1073/pnas.78.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz L. B., Riedel C., Caulfield J. P., Wasserman S. I., Austen K. F. Cell association of complexes of chymase, heparin proteoglycan, and protein after degranulation by rat mast cells. J Immunol. 1981 Jun;126(6):2071–2078. [PubMed] [Google Scholar]
- Shaff R. E., Beaven M. A. Increased sensitivity of the enzymatic isotopic assay of histamine: measurement of histamine in plasma and serum. Anal Biochem. 1979 Apr 15;94(2):425–430. doi: 10.1016/0003-2697(79)90385-3. [DOI] [PubMed] [Google Scholar]
- Shanahan F., Denburg J. A., Bienenstock J., Befus A. D. Mast cell heterogeneity. Can J Physiol Pharmacol. 1984 Jun;62(6):734–737. doi: 10.1139/y84-121. [DOI] [PubMed] [Google Scholar]
- Stevens R. L., Razin E., Austen K. F., Hein A., Caulfield J. P., Seno N., Schmid K., Akiyama F. Synthesis of chondroitin sulfate E glycosaminoglycan onto p-nitrophenyl-beta-D-xyloside and its localization to the secretory granules of rat serosal mast cells and mouse bone marrow-derived mast cells. J Biol Chem. 1983 May 10;258(9):5977–5984. [PubMed] [Google Scholar]
- Tas J., Berndsen R. G. Does heparin occur in mucosal mast cells of the rat small intestine? J Histochem Cytochem. 1977 Sep;25(9):1058–1062. doi: 10.1177/25.9.71326. [DOI] [PubMed] [Google Scholar]
- Tertian G., Yung Y. P., Guy-Grand D., Moore M. A. Long-term in vitro culture of murine mast cells. I. Description of a growth factor-dependent culture technique. J Immunol. 1981 Aug;127(2):788–794. [PubMed] [Google Scholar]
- Urbina C., Ortiz C., Hurtado I. A new look at basophils in mice. Int Arch Allergy Appl Immunol. 1981;66(2):158–160. doi: 10.1159/000232814. [DOI] [PubMed] [Google Scholar]
- Woodbury R. G., Everitt M., Sanada Y., Katunuma N., Lagunoff D., Neurath H. A major serine protease in rat skeletal muscle: evidence for its mast cell origin. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5311–5313. doi: 10.1073/pnas.75.11.5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury R. G., Gruzenski G. M., Lagunoff D. Immunofluorescent localization of a serine protease in rat small intestine. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2785–2789. doi: 10.1073/pnas.75.6.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury R. G., Miller H. R., Huntley J. F., Newlands G. F., Palliser A. C., Wakelin D. Mucosal mast cells are functionally active during spontaneous expulsion of intestinal nematode infections in rat. 1984 Nov 29-Dec 5Nature. 312(5993):450–452. doi: 10.1038/312450a0. [DOI] [PubMed] [Google Scholar]
- Woodbury R. G., Miller H. R. Quantitative analysis of mucosal mast cell protease in the intestines of Nippostrongylus-infected rats. Immunology. 1982 Jul;46(3):487–495. [PMC free article] [PubMed] [Google Scholar]
- Woodbury R. G., Neurath H. Purification of an atypical mast cell protease and its levels in developing rats. Biochemistry. 1978 Oct 3;17(20):4298–4304. doi: 10.1021/bi00613a029. [DOI] [PubMed] [Google Scholar]