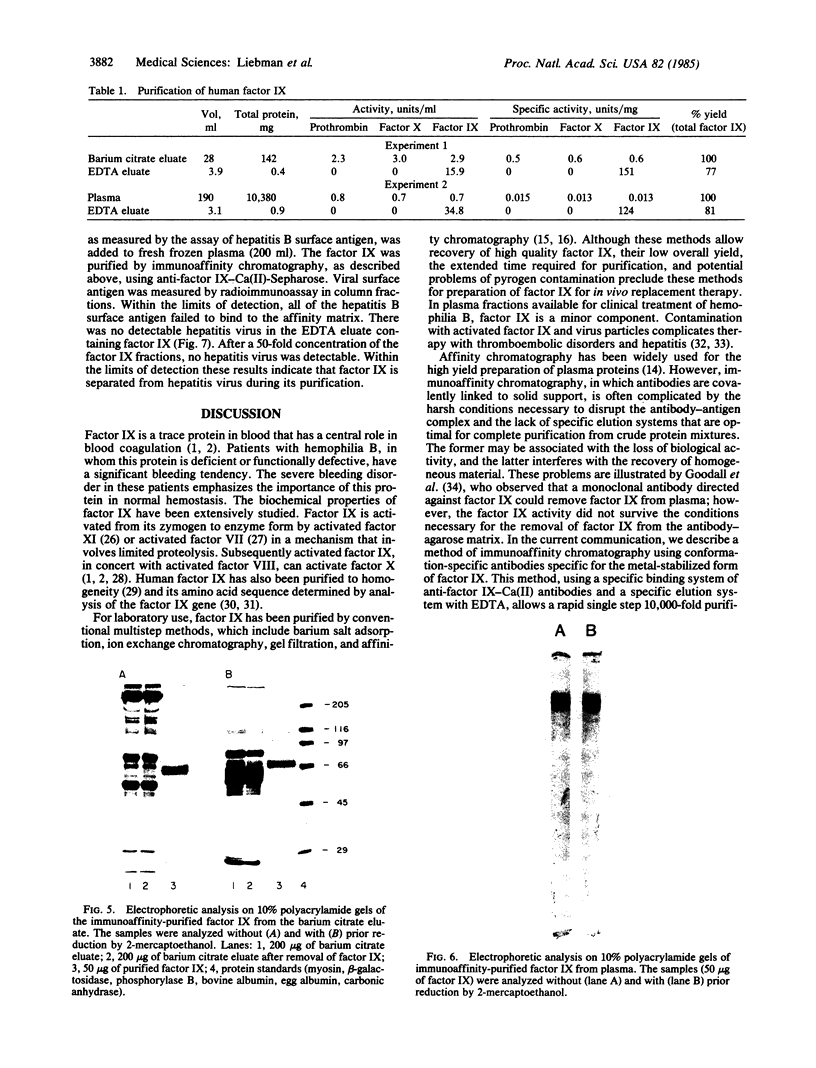
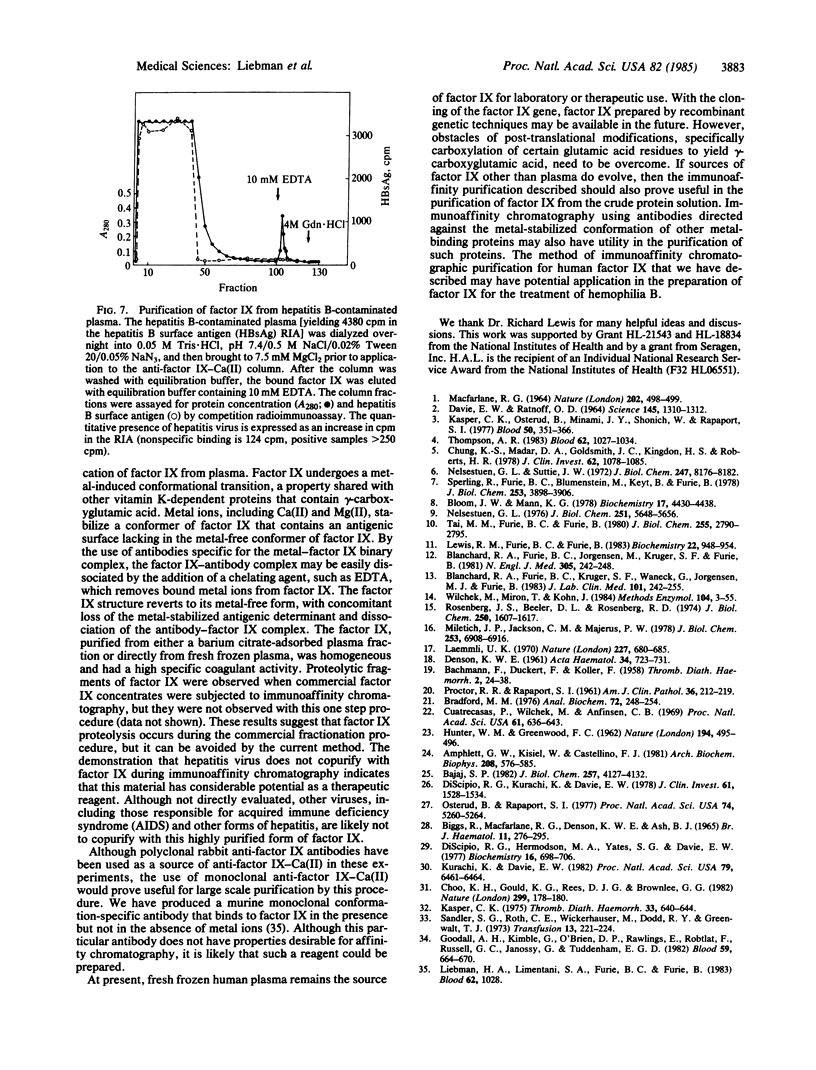
Abstract
Factor IX is a vitamin K-dependent blood clotting zymogen that is functionally defective or absent in patients with hemophilia B. A method of immunoaffinity chromatography has been developed for a one-step high yield purification of factor IX directly from plasma. The technique utilizes conformation-specific antibodies that bind solely to the metal-stabilized factor IX conformer, but not to the conformer of factor IX found in the absence of metal ions. Anti-factor IX-Ca(II) antibodies were immobilized on an agarose matrix. Human plasma in the presence of 7.5 mM MgCl2 was applied to the antibody-agarose column. The factor IX that binds to these antibodies was specifically eluted by metal chelation with EDTA. This immunopurification resulted in a 10,000-fold one-step purification of the fully functional zymogen. Purified factor IX yielded a single band upon gel electrophoresis in Na-DodSO4 and had a specific activity of 120-150 units/mg. The purified factor IX was separated from other vitamin K-dependent blood clotting proteins and hepatitis virus; no activated factor IX was detected. This method has application for the large scale purification of factor IX for the treatment of hemophilia B.
Full text
PDF
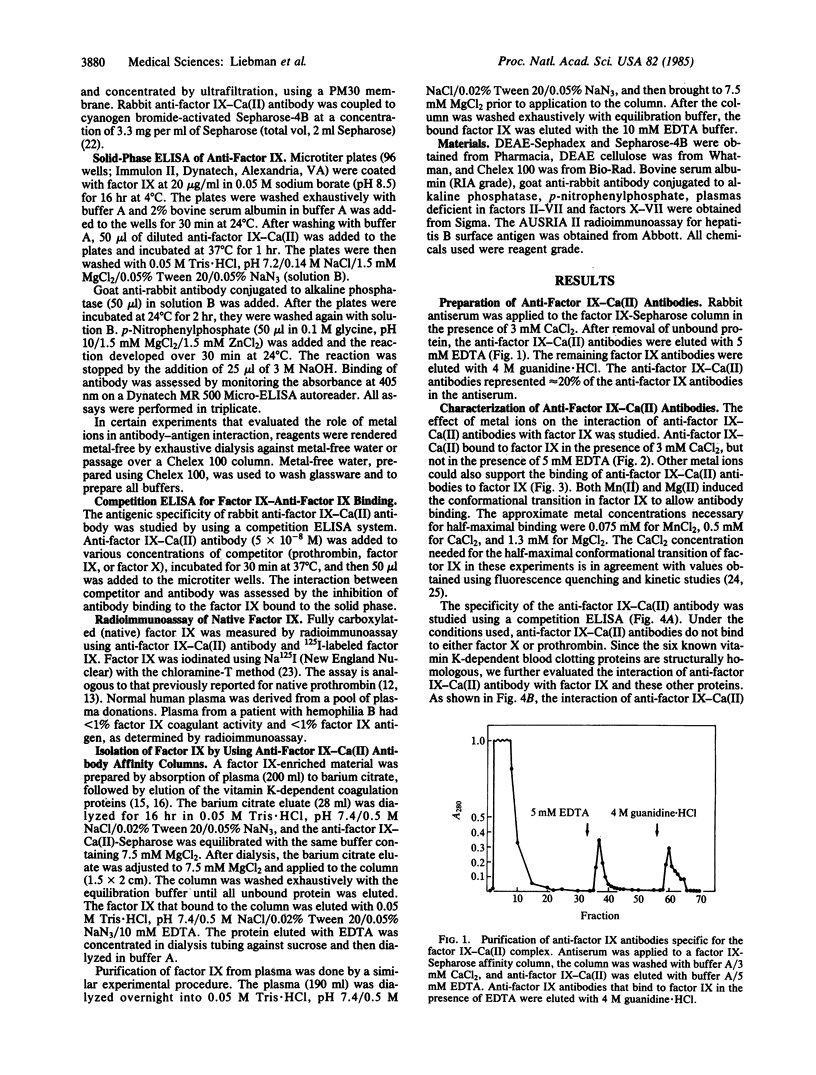
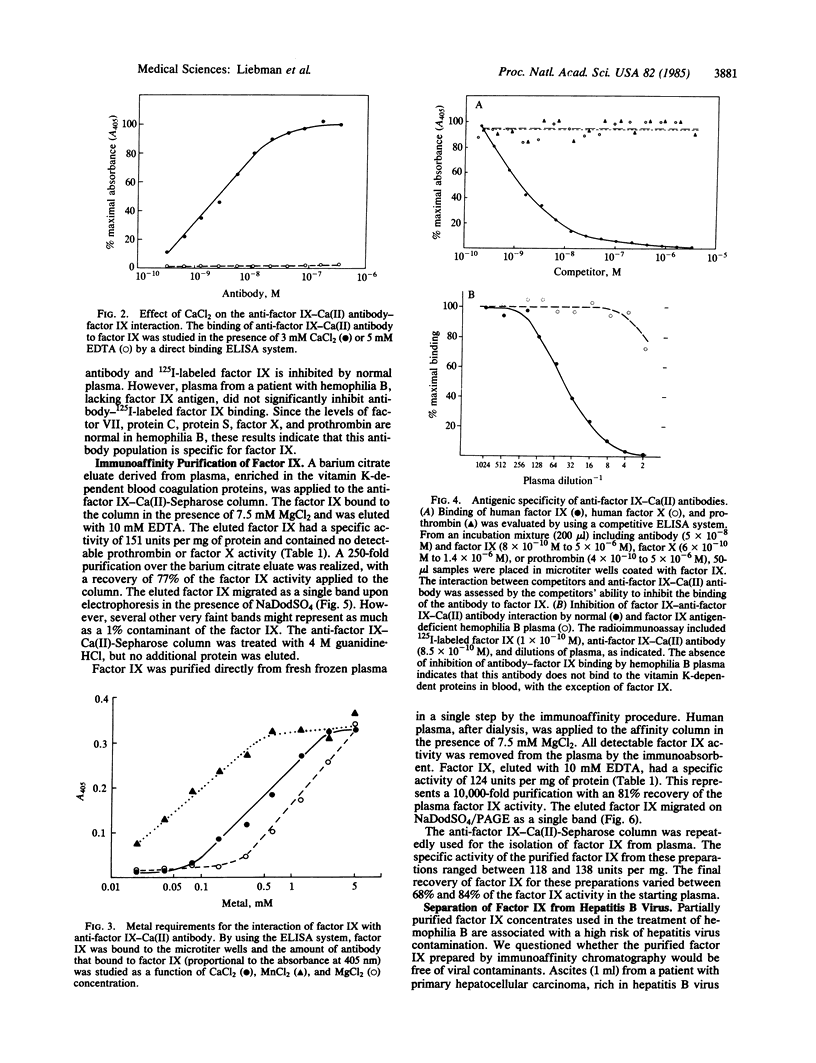
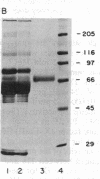
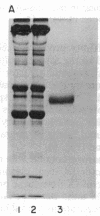
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amphlett G. W., Kisiel W., Castellino F. J. The interaction of Ca2+ with human Factor IX. Arch Biochem Biophys. 1981 May;208(2):576–585. doi: 10.1016/0003-9861(81)90546-4. [DOI] [PubMed] [Google Scholar]
- BACHMANN F., DUCKERT F., KOLLER F. The Stuart-Prower factor assay and its clinical significance. Thromb Diath Haemorrh. 1958 May 1;2(1-2):24–38. [PubMed] [Google Scholar]
- BIGGS R., MACFARLANE R. G., DENSON K. W., ASH B. J. THROMBIN AND THE INTERACTION OF FACTORS 8 AND 9. Br J Haematol. 1965 May;11:276–295. doi: 10.1111/j.1365-2141.1965.tb06588.x. [DOI] [PubMed] [Google Scholar]
- Bajaj S. P. Cooperative Ca2+ binding to human factor IX. Effects of Ca2+ on the kinetic parameters of the activation of factor IX by factor XIa. J Biol Chem. 1982 Apr 25;257(8):4127–4132. [PubMed] [Google Scholar]
- Blanchard R. A., Furie B. C., Jorgensen M., Kruger S. F., Furie B. Acquired vitamin K-dependent carboxylation deficiency in liver disease. N Engl J Med. 1981 Jul 30;305(5):242–248. doi: 10.1056/NEJM198107303050502. [DOI] [PubMed] [Google Scholar]
- Blanchard R. A., Furie B. C., Kruger S. F., Waneck G., Jorgensen M. J., Furie B. Immunoassays of human prothrombin species which correlate with functional coagulant activities. J Lab Clin Med. 1983 Feb;101(2):242–255. [PubMed] [Google Scholar]
- Bloom J. W., Mann K. G. Metal ion induced conformational transitions of prothrombin and prothrombin fragment 1. Biochemistry. 1978 Oct 17;17(21):4430–4438. doi: 10.1021/bi00614a012. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Choo K. H., Gould K. G., Rees D. J., Brownlee G. G. Molecular cloning of the gene for human anti-haemophilic factor IX. Nature. 1982 Sep 9;299(5879):178–180. doi: 10.1038/299178a0. [DOI] [PubMed] [Google Scholar]
- Chung K. S., Madar D. A., Goldsmith J. C., Kingdon H. S., Roberts H. R. Purification and characterization of an abnormal factor IX (Christmas factor) molecule. Factor IX Chapel Hill. J Clin Invest. 1978 Nov;62(5):1078–1085. doi: 10.1172/JCI109213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P., Wilchek M., Anfinsen C. B. Selective enzyme purification by affinity chromatography. Proc Natl Acad Sci U S A. 1968 Oct;61(2):636–643. doi: 10.1073/pnas.61.2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIE E. W., RATNOFF O. D. WATERFALL SEQUENCE FOR INTRINSIC BLOOD CLOTTING. Science. 1964 Sep 18;145(3638):1310–1312. doi: 10.1126/science.145.3638.1310. [DOI] [PubMed] [Google Scholar]
- Di Scipio R. G., Hermodson M. A., Yates S. G., Davie E. W. A comparison of human prothrombin, factor IX (Christmas factor), factor X (Stuart factor), and protein S. Biochemistry. 1977 Feb 22;16(4):698–706. doi: 10.1021/bi00623a022. [DOI] [PubMed] [Google Scholar]
- Di Scipio R. G., Kurachi K., Davie E. W. Activation of human factor IX (Christmas factor). J Clin Invest. 1978 Jun;61(6):1528–1538. doi: 10.1172/JCI109073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Kasper C. K., Osterud B., Minami J. Y., Shonick W., Rapaport S. I. Hemophilia B: characterization of genetic variants and detection of carriers. Blood. 1977 Sep;50(3):351–366. [PubMed] [Google Scholar]
- Kasper C. K. Thromboembolic complications. Thromb Diath Haemorrh. 1975 Jun 30;33(3):640–644. [PubMed] [Google Scholar]
- Kurachi K., Davie E. W. Isolation and characterization of a cDNA coding for human factor IX. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6461–6464. doi: 10.1073/pnas.79.21.6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewis R. M., Furie B. C., Furie B. Conformation-specific monoclonal antibodies directed against the calcium-stabilized structure of human prothrombin. Biochemistry. 1983 Feb 15;22(4):948–954. doi: 10.1021/bi00273a037. [DOI] [PubMed] [Google Scholar]
- MACFARLANE R. G. AN ENZYME CASCADE IN THE BLOOD CLOTTING MECHANISM, AND ITS FUNCTION AS A BIOCHEMICAL AMPLIFIER. Nature. 1964 May 2;202:498–499. doi: 10.1038/202498a0. [DOI] [PubMed] [Google Scholar]
- Miletich J. P., Jackson C. M., Majerus P. W. Properties of the factor Xa binding site on human platelets. J Biol Chem. 1978 Oct 10;253(19):6908–6916. [PubMed] [Google Scholar]
- Nelsestuen G. L. Role of gamma-carboxyglutamic acid. An unusual protein transition required for the calcium-dependent binding of prothrombin to phospholipid. J Biol Chem. 1976 Sep 25;251(18):5648–5656. [PubMed] [Google Scholar]
- Nelsestuen G. L., Suttie J. W. The purification and properties of an abnormal prothrombin protein produced by dicumarol-treated cows. A comparison to normal prothrombin. J Biol Chem. 1972 Dec 25;247(24):8176–8182. [PubMed] [Google Scholar]
- Osterud B., Rapaport S. I. Activation of factor IX by the reaction product of tissue factor and factor VII: additional pathway for initiating blood coagulation. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5260–5264. doi: 10.1073/pnas.74.12.5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg J. S., Beeler D. L., Rosenberg R. D. Activation of human prothrombin by highly purified human factors V and X-a in presence of human antithrombin. J Biol Chem. 1975 Mar 10;250(5):1607–1617. [PubMed] [Google Scholar]
- Sandler S. G., Rath C. E., Wickerhauser M., Dodd R. Y., Greenwalt T. J. Post-Konyne hepatitis: the ineffectiveness of screening for the hepatitis B antigen (HBAg). Transfusion. 1973 Jul-Aug;13(4):221–224. doi: 10.1111/j.1537-2995.1973.tb05478.x. [DOI] [PubMed] [Google Scholar]
- Sperling R., Furie B. C., Blumenstein M., Keyt B., Furie B. Metal binding properties of gamma-carboxyglutamic acid. Implications for the vitamin K-dependent blood coagulation proteins. J Biol Chem. 1978 Jun 10;253(11):3898–3906. [PubMed] [Google Scholar]
- Tai M. M., Furie B. C., Furie B. Conformation-specific antibodies directed against the bovine prothrombin . calcium complex. J Biol Chem. 1980 Apr 10;255(7):2790–2795. [PubMed] [Google Scholar]
- Thompson A. R. Monoclonal antibody to an epitope on the heavy chain of factor IX missing in three hemophilia-B patients. Blood. 1983 Nov;62(5):1027–1034. [PubMed] [Google Scholar]
- Wilchek M., Miron T., Kohn J. Affinity chromatography. Methods Enzymol. 1984;104:3–55. doi: 10.1016/s0076-6879(84)04082-9. [DOI] [PubMed] [Google Scholar]