Abstract
Background:
Granulosa cell tumors are rare neoplasms characterized by long natural history and favorable prognosis.
Aims:
The objective of this study was to determine the clinical presentation, treatment, outcome, and prognostic factors for patients of granulosa cell tumors.
Materials and Methods:
A retrospective analysis of 26 patients of granulosa cell tumor of ovary from 2002 to 2011 was carried out. The records of all patients were analyzed to determine clinical presentation, treatment, survival, and prognostic factors.
Results:
The median age of the patients was 50 years (range, 17-71 years). Abdominal pain was the most common presenting symptom. The median follow-up was 71.4 months (range, 21.6-149.9 months). The estimated 5 and 10 year overall survival (OS) was 84.6 and 72.5%, respectively. Event-free survival (EFS) was 76.5 and 52.9% at 5 and 10 years, respectively. Advanced stage was significant independent poor prognostic indicator for both OS and EFS.
Conclusion:
Majority of the patients with granulosa cell tumors of the ovary present in early stage. Surgery is the primary treatment modality for granulosa cell tumors. Advanced stage and presence of residual disease were associated with inferior survival, but only prospective studies can ascertain their definite role.
Keywords: Granulosa cell tumor, Ovary, Prognosis, Survival, Treatment
Introduction
Granulosa cell tumors are the most common type of sex cord stromal tumor accounting for about 2-5% of ovarian neoplasms.[1] The majority of patients are diagnosed at early stage and have a relatively favorable prognosis. Surgical management of granulosa tumors is based on the stage of the tumor as well as age of the patient. Premenarchal women or patients presenting in the reproductive years with early stage disease are often managed with unilateral salpingo-oophorectomy and appropriate surgical staging in an attempt to preserve fertility. In postmenopausal women and those who have completed childbearing, surgery consists of a total abdominal hysterectomy and bilateral salpingo-oophorectomy, along with standard surgical staging.
Stage is the most important prognostic factor, with 10-year survivals of 84-95% for stage I tumors, decreasing to 50-65% for stage II disease, and to 17-33% for stages III and IV.[1,2,3] Patients with low risk stage I tumor should be kept on observation.[1] Patients with high risk stage I disease associated with large tumor size (≥10-15 cm), stage IC, poorly differentiated tumor, high mitotic index, or tumor rupture might be considered for adjuvant chemotherapy in view of increased risk of relapse.[1,4] The prognostic significance of these factors and benefit of chemotherapy is still uncertain. For patients with stage II-IV granulosa cell tumors, postoperative treatment is recommended,[5,6] but the survival benefit is still not known due to rarity of these tumors and lack of randomized trials.
Data are based mainly on retrospective series including small number of patients treated over a long period of time. The aim of the present study was to evaluate the clinical presentation, pathological features, treatment, and outcome of patients with granulosa cell tumor at our institute.
Materials and Methods
A retrospective analysis of diagnosed cases of granulosa cell tumors of the ovary was carried out at our institute from 2002 to 2011. A total of 26 patients were retrieved from the records. The case records of all patients were reviewed to extract the following information: Age, clinical presentation, details of surgery, stage, pathological features, adjuvant therapy, recurrence, and follow-up.
Ethical clearance for the conduction of the study was obtained from the institutional ethics committee. Patients were staged according to International Federation of Gynecology and Obstetrics (FIGO) staging system for ovarian tumors.
The primary treatment consisted of surgery in all patients. Fertility sparing surgery comprised of unilateral salpingo-oophorectomy with surgical staging. Radical surgery consisted of total abdominal hysterectomy with bilateral salpingo-oophorectomy. Surgical staging included peritoneal washing, multiple peritoneal biopsies, omental biopsy, and biopsy of any suspicious area. All patients with stage II-IV received postoperative chemotherapy. The chemotherapy consisted of EP (etoposide 100 mg/m2/day on days 1-5 and cisplatin 20 mg/m2/ day on days 1-5), BEP (bleomycin 30 U on days 2, 9, and 16, etoposide 100 mg/m2/day on days 1-5, and cisplatin 20 mg/m2/day on days 1-5), or combination of paclitaxel (175 mg/m2) and carboplatin (AUC 5-7). EP and BEP regimen were administered every 3 weeks for four courses and combination of paclitaxel and carboplatin was administered every 3 weeks for six courses. If the absolute neutrophil count was less than 1,500/μL and/or the platelet count less than 100,000/μL, the chemotherapy was delayed by 1 week. In case of febrile neutropenia or chemotherapy delay in the previous cycle, granulocyte colony-stimulating factor (G-CSF) would be administered in the subsequent cycles. The adjuvant radiotherapy dose was 50 Gy in 25 fractions over 5 weeks.
Statistical analysis
Statistical Package for Social Sciences (SPSS) version 15 was used for statistical analysis. Overall survival (OS) was defined as the time from date of diagnosis to the date of death for any reason or to the last follow-up. Event-free survival (EFS) was measured from the date of diagnosis to the date an event (recurrence or progression) occurred or date of last follow-up. Survival curves and rates were calculated using the Kaplan-Meier method. For univariate and multivariate analyses on EFS and OS, log-rank test and Cox regression analysis were applied, respectively. A P-value < 0.05 was taken as statistically significant. Variables with P < 0.05 on univariate analysis were selected for multivariate analysis.
Results
Clinicopathological profile
The median age of the patients was 50 years (range, 17-71 years). The median duration of symptoms was 5 months (range, 4-24). The common presenting symptoms were abdominal pain (57.7%) and postmenopausal bleeding (50%). Less common symptoms were secondary amenorrhea (23%) and abdominal distension (7.7%). Of 26 patients, 15 (57.7%) were postmenopausal. Four patients were nulliparous, while 22 patients were multiparous.
All patients underwent primary surgical management. Fertility preserving surgery was performed in three patients, while 23 patients underwent total abdominal hysterectomy with bilateral salpingo-oophorectomy. Lymph node sampling was done in 10 patients (38.5%). Ovarian tumor was bilateral in four patients (15.4%). The mean tumor size was 10.5 cm (range, 4-21). The pathological subtype of granulosa tumor was juvenile type in 3 patients and adult type in 23 patients. Endometrial status was assessed in all except three patients. Endometrial status was normal in 17 patients. Simple endometrial hyperplasia without atypia was found in three, simple endometrial hyperplasia with atypia in two, and complex hyperplasia without atypia in one patient. The mitotic index was < 5/10 HPF in 10 (38.5%) patients and ≥ 5/10 HPF in 16 patients (61.5%).
Eighteen patients (69.2%) had stage I disease, three (11.5%) had stage II, four (15.4%) had stage III, and one (3.8%) had stage IV. Residual tumor was present in five patients.
Treatment and outcome
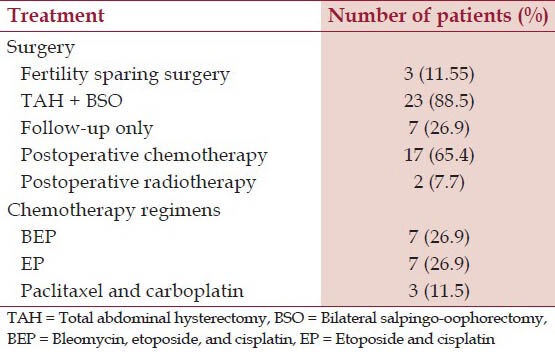
Seventeen patients received chemotherapy (10/18 in stage I, 2/3 in stage II, 4/4 in stage III, and 1/1 in stage IV). Ten patients of stage I received adjuvant chemotherapy due to capsular breech, tumor rupture, tumor size > 10 cm, or poorly-differentiated tumor. Adjuvant radiotherapy was given in two patients. Seven patients were kept on follow-up only. All patients completed treatment with no significant toxicity or treatment interruption except one patient who developed grade 3 neutropenia after third cycle. After treatment completion, patients were assessed both clinically and radiologically. A summary of treatment details is given in Table 1.
Table 1.
Treatment details
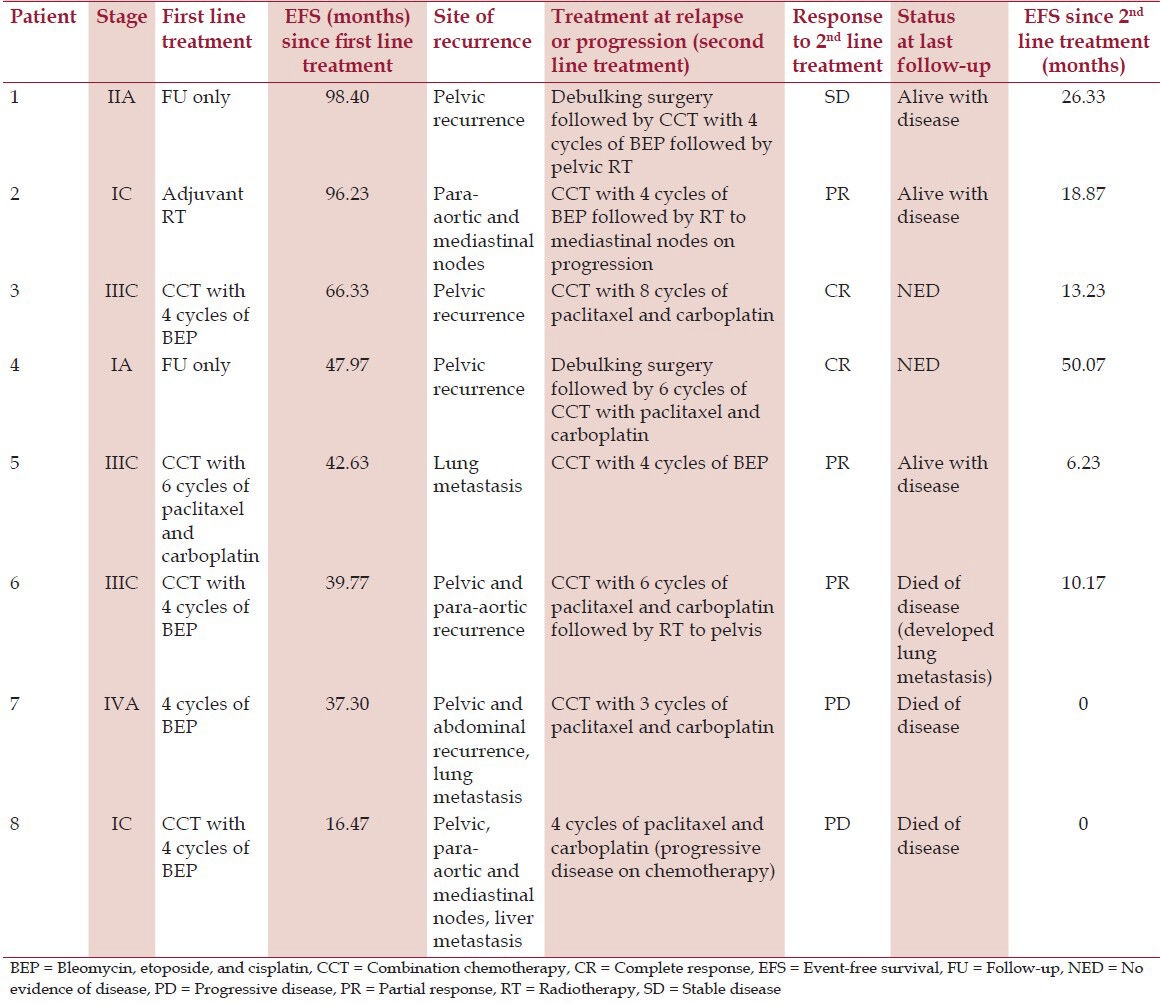
The median follow-up was 71.4 months (range, 21.6-149.9 months). Eight patients (30.8%) experienced a relapse or progression of disease and underwent second line treatment [Table 2]. Debulking surgery was done in two patients. All patients received second line chemotherapy. Two patients received radiotherapy to pelvis, while one patient received radiotherapy to mediastinum (due to relapse in mediastinal nodes) after chemotherapy. Out of 26, 20 patients are alive and disease free. Two patients are alive with stable disease. Three patients died due to disease and one due to myocardial infarction, but was disease free at last follow-up. The estimated 5 and 10 year OS was 84.6 and 72.5%, respectively. The 5 and 10 year EFS was 76.5 and 52.9%, respectively. The 5 year OS was 94.4, 67, 50, and 0% for stage I, II, III, and IV, respectively.
Table 2.
Details of relapse and second line treatment
Survival was also compared in terms of stage (stage I vs stage II-IV), age (< 50 vs ≥ 50 years), menopausal status (premenopausal versus postmenopausal), tumor size (< 10 cm vs ≥ 10 cm), mitotic activity (< 5/10 HPF vs ≥ 5/10 HPF), and residual tumor (presence or absence) after surgery. There was significant difference in OS between patients with localized stage (94.4% at 5 and 10 years for stage I) and advanced stage (53.3 and 26.7% for stage II-IV) (P = 0.025) [Figure 1]. Patients with mitotic activity less than 5/10 HPF and absence of residual disease had better survival than those with mitotic activity more than 5/10 HPF (P = 0.04) and presence of residual disease (P = 0.02) [Figure 2], respectively. There was no significant difference in OS rates between age groups less than or equal to more than 50 years (P = 0.95), menopausal status (P = 0.57), and tumor size (P = 0.17), respectively. On multivariate analysis, only advanced stage was independent poor prognostic indicator for OS (P = 0.045, hazard ratio (HR) = 1.26, 95% confidence interval (CI) = 1.06-1.83). At univariate analysis, advanced stage (P = 0.04) and presence of residual tumor (P = 0.005) were associated with inferior disease-free survival (DFS) and both stage (P = 0.04, HR = 1.08, 95% CI 1.024-1.28) and residual tumor (P = 0.027, HR = 1.42, 95% CI 1.04-1.79) retained significant predictive value for recurrence at multivariate analysis.
Figure 1.
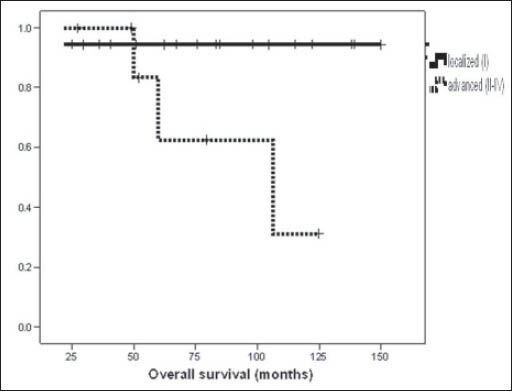
Kaplan-Meier curve showing comparison of overall survival between localized and advanced stage
Figure 2.
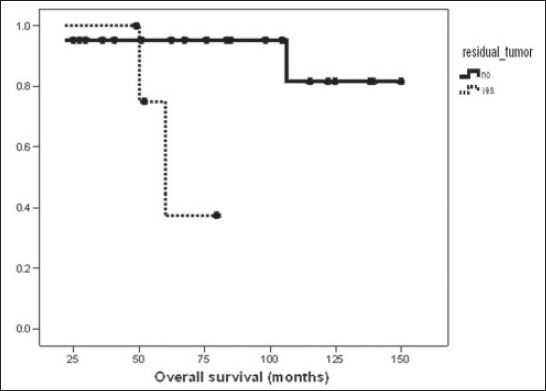
Kaplan-Meier curve comparing overall survival for patients with presence or absence of residual disease
Discussion
Granulosa cell tumors are rare neoplasms with indolent behavior. The optimal management of these tumors has never been determined by randomized trials. The present study highlights single institutional experience with these rare neoplasms.
Granulosa cell tumors have bimodal age distribution but the peak incidence is in postmenopausal period with median age of diagnosis around 50-55 years.[1] The median age was 50 years in our study. These patients may present with abdominal pain, distention, mass, and menstrual disturbances.[1,7] In this study, most of the patients presented with abdominal pain and postmenopausal bleeding.
Granulosa cell tumors are of two types based on clinical and histopathological characteristics: Juvenile and adult type. Juvenile type account for 90% of the granulosa cell tumors that occur in prepubertal girls and in women younger than 30 years of age.[8] In the current study, three patients were of juvenile type and all were below 30 years of age. Concomitant endometrial pathology in form of hyperplasia is a common finding in granulosa cell tumor, occurring in 25-50%, due to excess of estrogen produced by these tumors.[9,10] Endometrial adenocarcinoma can be found simultaneously in 5-10% of patients with granulosa cell tumor and are often well-differentiated and in an early stage with a favorable prognosis.[1,10,11] None of the patients in our study had endometrial adenocarcinoma.
The majority of patients (80-90%) have stage I disease at diagnosis, and thus have a favorable outcome.[12] Nearly 70% of the patients in our study had stage I disease. The 5 and 10 year OS for stage I was 94.4%, which is in concordance with other series.[11,13,14] The mortality rate with extraovarian disease spread is at least 40%.[15] Tumor stage, age, tumor size, tumor rupture, mitotic index, presence of residual disease, and grade has been shown to be of prognostic significance in various studies.[14,16,17,18] The prognostic significance of stage was confirmed in our study. Patients with early stage (stage I) had better OS as compared to advanced stage (stage II-IV) which was statistically significant. The recurrence rates are also related to the stage.[7] In a study by Evans et al.,[11] only 9% of women with stage IA had tumor recurrence as compared to 30% or more for higher stage disease. In a study by Ayhan et al.,[16] recurrence rates were 5.4, 21, and 40% for stages I, II, and III, respectively. Mangili et al.,[18] found that 20% of patients with stage I disease recurred after 20 years from diagnosis, thus emphasizing the need of long-term follow-up in patients of granulosa cell tumors. Three patients of stage I disease had recurrence in our study.
Tumors greater than 10-15 cm in diameter have been associated with high recurrence rates and shorter progression-free survival, independent of stage.[11,19,20,21] However, in some series size was not found to have any prognostic significance when adjusted for stage.[22,23,24] In the current study, we could not find any significant difference in survival in patients with tumor size greater or less than 10 cm. High mitotic index has been found to be associated with high recurrence rates in few studies.[13,19,25] In the present study, patients with mitotic activity less than 5/10 HPF had better survival rate as compared to those with more than 5/10 HPF (100 vs 72% at 5 years, P = 0.04). In a study by Miller et al.,[23] 19 patients with recurrent disease were compared to 51 patients without disease. The mitotic rate was higher in the group with recurrence. In a study by Lauszus et al.,[14] there was no difference between women with relapse and women with no relapse with respect to mitotic count. Survival was significantly worse in patients with residual disease as compared to patients without residual disease (P = 0.02) in the current report. In various studies, presence of residual disease was associated with poor survival and outcome.[16,18,21,26]
Complete tumor resection should be considered as primary treatment. The optimal choice of combination platinum-based chemotherapy is still not well-defined. Chemotherapy typically includes combination of bleomycin, etoposide, cisplatin; etoposide/cisplatin; and paclitaxel and carboplatin.[1] The Gynecologic Oncology Group is conducting a randomized phase II trial of BEP versus the combination of paclitaxel and carboplatin for patients with newly diagnosed and recurrent chemonaive sex cord-stromal tumors of the ovary. The role of adjuvant radiation therapy in granulosa cell tumors remains controversial. The median follow-up was 71.4 months in our study. Nearly 31% of patients had relapse, which is in concordance to series by Mangili et al.[18] Recurrence occurred after a median of 45.3 months (range, 16.47-98.40 months) in our study. The most common site of recurrence was pelvis. There is no standard approach for management of relapsed disease. Surgery may provide long-term disease control in localized disease,[27] but diffuse intra-abdominal disease or metastases are difficult to treat effectively. Radiation therapy either pelvic or whole-abdomen radiotherapy can induce clinical responses in women with persistent or recurrent granulosa cell tumors, particularly if the disease is surgically cytoreduced or confined to pelvis and abdomen.[1,28] Platinum-based chemotherapy is the preferred option for treating more widespread disease or disease that is suboptimally cytoreduced at the time of relapse.[1] However, owing to their long natural history and potential for late relapse, sometimes occurring more than 10 years after diagnosis, long-term follow-up is warranted.
In conclusion, majority of the patients with granulosa cell tumors of the ovary present in early stage and have favorable prognosis. Advanced stage, high mitotic activity, and presence of residual disease were associated with poor prognosis. The limitations of our study are its retrospective nature and small number of patients. Prospective multicentric trial is needed to address the role of adjuvant therapies for optimal management of these rare neoplasms.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Schumer ST, Cannistra SA. Granulosa cell tumor of the ovary. J Clin Oncol. 2003;21:1180–9. doi: 10.1200/JCO.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 2.Pankratz E, Boyes DA, White GW, Galliford BW, Fairey RN, Benedet JL. Granulosa cell tumors. A clinical review of 61 cases. Obstet Gynecol. 1978;52:718–23. [PubMed] [Google Scholar]
- 3.Schwartz PE, Smith JP. Treatment of ovarian stromal tumors. Am J Obstet Gynecol. 1976;125:402–11. doi: 10.1016/0002-9378(76)90577-9. [DOI] [PubMed] [Google Scholar]
- 4.Schneider DT, Calaminus G, Wessalowski R, Pathmanathan R, Selle B, Sternschulte W, et al. Ovarian sex cord-stromal tumors in children and adolescents. J Clin Oncol. 2003;21:2357–63. doi: 10.1200/JCO.2003.05.038. [DOI] [PubMed] [Google Scholar]
- 5.Homesley HD, Bundy BN, Hurteau JA, Roth LM. Bleomycin, etoposide, and cisplatin combination therapy of ovarian granulosa cell tumors and other stromal malignancies: A Gynecologic Oncology Group study. Gynecol Oncol. 1999;72:131–7. doi: 10.1006/gyno.1998.5304. [DOI] [PubMed] [Google Scholar]
- 6.Pautier P, Gutierrez-Bonnaire M, Rey A, Sillet-Bach I, Chevreau C, Kerbrat P, et al. Combination of bleomycin, etoposide, and cisplatin for the treatment of advanced ovarian granulosa cell tumors. Int J Gynecol Cancer. 2008;18:446–52. doi: 10.1111/j.1525-1438.2007.01049.x. [DOI] [PubMed] [Google Scholar]
- 7.Uygun K, Aydiner A, Saip P, Basaran M, Tas F, Kocak Z, et al. Granulosa cell tumor of the ovary: retrospective analysis of 45 cases. Am J Clin Oncol. 2003;26:517–21. doi: 10.1097/01.coc.0000037918.88451.6A. [DOI] [PubMed] [Google Scholar]
- 8.Young RH, Dickersin GR, Scully RE. Juvenile granulosa cell tumor of the ovary. A clinicopathological analysis of 125 cases. Am J Surg Pathol. 1984;8:575–96. doi: 10.1097/00000478-198408000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Pectasides D, Pectasides E, Psyrri A. Granulosa cell tumor of the ovary. Cancer Treat Rev. 2008;34:1–12. doi: 10.1016/j.ctrv.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Kanthan R, Senger JL, Kanthan S. The multifaceted granulosa cell tumours-myths and realities: A review. ISRN Obstet Gynecol 2012. 2012 doi: 10.5402/2012/878635. 878635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans AT, 3rd, Gaffey TA, Malkasian GD, Jr, Annegers JF. Clinicopathologic review of 118 granulosa and 82 theca cell tumors. Obstet Gynecol. 1980;55:231–8. [PubMed] [Google Scholar]
- 12.Stuart GC, Dawson LM. Update on granulosa cell tumours of the ovary. Curr Opin Obstet Gynecol. 2003;15:33–7. doi: 10.1097/00001703-200302000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Björkholm E, Silfverswärd C. Prognostic factors in granulosa-cell tumors. Gynecol Oncol. 1981;11:261–74. doi: 10.1016/0090-8258(81)90040-8. [DOI] [PubMed] [Google Scholar]
- 14.Lauszus FF, Petersen AC, Greisen J, Jakobsen A. Granulosa cell tumor of the ovary: A population-based study of 37 women with stage I disease. Gynecol Oncol. 2001;81:456–60. doi: 10.1006/gyno.2001.6183. [DOI] [PubMed] [Google Scholar]
- 15.Uygun K, Aydiner A, Saip P, Kocak Z, Basaran M, Dincer M, et al. Clinical parameters and treatment results in recurrent granulosa cell tumor of the ovary. Gynecol Oncol. 2003;88:400–3. doi: 10.1016/s0090-8258(02)00141-5. [DOI] [PubMed] [Google Scholar]
- 16.Ayhan A, Salman MC, Velipasaoglu M, Sakinci M, Yuce K. Prognostic factors in adult granulose cell tumors of the ovary: A retrospective analysis of 80 cases. J Gynecol Oncol. 2009;20:158–63. doi: 10.3802/jgo.2009.20.3.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iyibozkurt AC, Topuz S, Gungor F, Akhan SE, Demirci F, Salihoglu Y, et al. Factors affecting recurrence and disease-free survival in granulosa cell tumors of the ovary. Eur J Gynaecol Oncol. 2010;31:667–71. [PubMed] [Google Scholar]
- 18.Mangili G, Ottolina J, Gadducci A, Giorda G, Breda E, Savarese A, et al. Long-term follow-up is crucial after treatment for granulosa cell tumours of the ovary. Br J Cancer. 2013;109:29–34. doi: 10.1038/bjc.2013.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stenwig JT, Hazekamp JT, Beecham JB. Granulosa cell tumors of the ovary. A clinicopathological study of 118 cases with long-term follow-up. Gynecol Oncol. 1979;7:136–52. doi: 10.1016/0090-8258(79)90090-8. [DOI] [PubMed] [Google Scholar]
- 20.Fox H, Agrawal K, Langley FA. A clinicopathologic study of 92 cases of granulosa cell tumor of the ovary with special reference to the factors influencing prognosis. Cancer. 1975;35:231–41. doi: 10.1002/1097-0142(197501)35:1<231::aid-cncr2820350128>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 21.Chan JK, Zhang M, Kaleb V, Loizzi V, Benjamin J, Vasilev S, et al. Prognostic factors responsible for survival in sex cord stromal tumors of the ovary–a multivariate analysis. Gynecol Oncol. 2005;96:204–9. doi: 10.1016/j.ygyno.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 22.Malmstrom H, Hogberg T, Risberg B, Simonsen E. Granulosa cell tumors of the ovary: Prognostic factors and outcome. Gynecol Oncol. 1994;52:50–5. doi: 10.1006/gyno.1994.1010. [DOI] [PubMed] [Google Scholar]
- 23.Miller BE, Barron BA, Wan JY, Delmore JE, Silva EG, Gershenson DM. Prognostic factors in adult granulosa cell tumor of the ovary. Cancer. 1997;79:1951–5. doi: 10.1002/(sici)1097-0142(19970515)79:10<1951::aid-cncr16>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 24.Zhang M, Cheung MK, Shin JY, Kapp DS, Husain A, Teng NN, et al. Prognostic factors responsible for survival in sex cord stromal tumors of the ovary–an analysis of 376 women. Gynecol Oncol. 2007;104:396–400. doi: 10.1016/j.ygyno.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 25.King LA, Okagaki T, Gallup DG, Twiggs LB, Messing MJ, Carson LF. Mitotic count, nuclear atypia, and immunohistochemical determination of Ki-67, c-myc, p21- ras, c-erbB2, and p53 expression in granulosa cell tumors of the ovary: mitotic count and Ki-67 are indicators of poor prognosis. Gynecol Oncol. 1996;61:227–32. doi: 10.1006/gyno.1996.0130. [DOI] [PubMed] [Google Scholar]
- 26.Al-Badawi IA, Brasher PM, Ghatage P, Nation JG, Schepansky A, Stuart GC. Postoperative chemotherapy in advanced ovarian granulosa cell tumors. Int J Gynecol Cancer. 2002;12:119–23. doi: 10.1046/j.1525-1438.2002.01067.x. [DOI] [PubMed] [Google Scholar]
- 27.Sehouli J, Drescher FS, Mustea A, Elling D, Friedmann W, Kühn W, et al. Granulosa cell tumor of the ovary: 10 years follow-up data of 65 patients. Anticancer Res. 2004;24:1223–9. [PubMed] [Google Scholar]
- 28.Wolf JK, Mullen J, Eifel PJ, Burke TW, Levenback C, Gershenson DM. Radiation treatment of advanced or recurrent granulosa cell tumor of the ovary. Gynecol Oncol. 1999;73:35–41. doi: 10.1006/gyno.1998.5287. [DOI] [PubMed] [Google Scholar]


