Abstract
Background:
Periodontal disease in diabetic patients can compromise a patient's ability to maintain a proper metabolic control and may be associated with diabetic complication.
Aims:
This study was designed to evaluate the frequency of periodontal disease in patients with type 2 diabetes mellitus (DM) and how this was related with the presence of diabetic retinopathy (DR).
Materials and Methods:
A comparison was made of periodontal parameters (plaque index (PI), community periodontal index of treatment needs (CPITN), periodontal disease severity measured in quartiles of probing depth (PD), and clinical attachment loss (CAL)) in a group of diabetic patients with retinopathy (n = 84) versus a group of diabetic patients without retinopathy (n = 129). In addition, 73 age- and sex-matched individuals were selected to serve as the control group. Analysis was performed to evaluate the relationships between periodontal disease and DR.
Results:
In terms of PI, no statistically significant differences were observed, so, oral hygiene was similar in both groups. Diabetic patients with retinopathy had greater CPITN (P < 0.001) and more severe periodontal disease (P < 0.001) than no retinopathy. Also, our results indicated a relationship between type 2 DM and periodontal disease.
Conclusions:
The patients with diabetes retinopathy appear to show increased periodontal disease susceptibility.
Keywords: Diabetes mellitus, Diabetic retinopathy, Periodontitis
Introduction
Diabetes mellitus (DM) is affecting over 217 million people worldwide, and its prevalence is expected to increase over the next few years.[1] According to the World Health Organization (WHO), million people worldwide suffer from DM with at least 366 predicted to have the condition by the year 2030.[2] Diabetic retinopathy (DR) is a vascular disorder affecting the microvasculature of the retina. DR is estimated to be the most frequent cause of new cases of blindness among adults aged 20-74 years. It is estimated that DM affects 4% of the world's population, almost half of whom have some degree of DR at any time of life.[3] The population-based Wisconsin Epidemiologic Study of DR showed that from 1980 to 2007, the estimated annual incidence of proliferative DR (PDR) decreased by 77% and vision impairment decreased by 57% among persons with type 1 diabetes.[4] The incidence and the risk of progression of DR have both declined over the past 30 years, from up to 90% to less than 50% due to improvement of medical care.[5]
Periodontal disease, which is one of the most common dental problems and a major cause of tooth loss, is caused by a variety of oral plaque bacteria. Periodontal disease defined as an inflammatory condition of the soft tissues surrounding the teeth (i.e., gingivitis) and the destruction of the supporting structures of the teeth, including the periodontal ligament, bone, cementum, and soft tissues.[6] There is increasing evidence indicating that periodontitis as risk factor of various of systemic diseases such as cardiovascular diseases and DM.[7,8] Periodontal disease is one of the most common chronic infectious disorders known in humans, with a reported prevalence varying between 10 and 60% in adults, depending on the diagnostic criteria.[9,10] There is an abundance of recent evidence of the epidemiological links between periodontal disease and DM complications. Several studies establishing the relationship among DM, periodontal health have been widely reported.[11,12,13,14,15]
Various reports identified, however, no significant differences in the levels of periodontal disease between those subjects with and without DM.[16,17] Therefore, commentary of research findings should be made with caution because of the differences in the types and number of subjects, research design, definition of DM, periodontal parameters, and other clinical conditions. The objective of this cross-sectional study was to analyze the dental health status (i.e., level of periodontal disease, plaque index (PI), and community periodontal index of treatment needs (CPITN)) among Iranian residents with and without DM to emphasize the relationship between DM and periodontal disease and to analyze how diabetes retinal complications are related with periodontal parameters.
Materials and Methods
Subjects
The subjects were type 2 diabetic patients of referral Diabetes Research Center of Imam Hospital, Sari City, to the clinic of dentistry to receive intensive dental health care after a trained interviewer explained the program to them and obtained informed consent between December 2010 and August 2012.
This study was approved by the Molecular Biology Research Center of Mazandaran University of Medical Sciences.
The patients were selected based on the following two criteria: Aged between 30 and 65 years and medically diagnosed type 2 DM. They were excluded if they had less than eight functional teeth, any ocular or systemic inflammatory diseases, intraocular surgery or laser photocoagulation, renal impairment (blood creatinine: 130 mol/L), chronic liver disease, or were receiving medical treatments that could influence the studied parameters such as antibiotics, antiepileptic, or immunosuppressive drugs. As a control group, we selected healthy individuals matched to the cases by both age and sex. These individuals were nondiabetic patients receiving treatment in the dental clinic of the same hospital. According to the data from previous study, we estimated that a total of 183 cases as well as 73 controls would be sufficient to detect an odds ratio (OR) of 3.00 with 80% power and alpha of 0.05, assuming a frequency of periodontal disease of at least 20% in the control group.[18]
Medical examination
The subjects assumed a routine clinical and laboratory evaluation, including information regarding blood glucose level and HbA1c, diabetes duration, and the type of diabetic treatment obtained from the medical records transferred from the department of internal medicine supervised by endocrinologist.
Ocular examination
Fundus examination was done by a trained ophthalmologist, and in case of any abnormality, this was documented by fundus fluorescein angiography. Retinopathy was defined by the presence of characteristic changes, including hemorrhages, exudates, laser marks, and fibrous proliferation, detected by ophthalmoscopy through dilated pupils. DR is classified either as nonproliferative DR (NPDR) or PDR.[19]
Dental examination
The diagnosis and assessment of periodontal disease is based on clinical criteria. Clinical examinations were performed by dentists from the local dental association, which included the assessment of PI, CPITN, and periodontal disease severity of the subjects.
The PI was specified according to the hygiene index of O’Leary et al.[20] The presence or absence of bacterial plaque was visually assessed at four points of each tooth, and the results were displayed as a percentage. Bacterial plaque was sampled by displacing a periodontal probe along the gum margins.
The CPITN is an epidemiologic tool developed by the WHO for the evaluation of periodontal disease in population surveys. It can be used to recommend the kind of treatment needed to prevent periodontal diseases according to standardized clinical criteria.[21] The highest community periodontal index code was recorded in each segment (code 0: No signs of periodontal disease, code 1: Gingival bleeding after gentle probing, code 2: Supragingival or subgingival calculus, code 3: 4-5 mm deep pathologic pockets, code 4: 6 mm or deeper pathologic pockets, and code X: Missing index teeth). Periodontal status was divided into three categories according to this code: 0, 1 and 2, 3 and 4, and X. Maximum punctuation for the whole mouth was used as a treatment recommendation. Treatment needs:
No need for treatment;
Need for oral hygiene instruction;
Need for oral hygiene instruction + supragingival and subgingival scaling + root planning.
There is no universally accepted standard for periodontal disease diagnosis. For this study, periodontitis was defined as presence of any sites exhibiting probing depth (PD) ≥ 4 mm or clinical attachment loss (CAL) ≥4 mm. PD was defined as the distance in millimeters from the gingival margin to the apical part of the pocket. The CAL was calculated from recession and PD measurements and represents the distance from the cementoenamel junction to the most apical portion of the sulcus/pocket in millimeters. The severity of periodontal disease was measured in quartiles of the mean levels of PD and CAL. In addition, we also used the criteria from the study of Offenbacher et al., on periodontal disease, which defined periodontal disease as any site with PD ≥ 4 mm and CAL ≥3 mm and severe periodontal disease as at least four sites with PD ≥ 5 mm and four sites with CAL ≥ 3 mm.[22]
Statistical analysis
Univariable analysis was performed to compare the different periodontal disease measurements, as well as the characteristics of study population between the cases and controls. Chi-square tests were used to examine differences in proportions (e.g., periodontitis) and t-tests were used to examine difference in the means of PI. Chi-square tests for linear trend were used to examine the relationship between periodontal disease severity and DR. Multivariable logistic regression was used to examine the association between periodontal disease and DR and to adjust for potential confounding variables, including age, sex, and systemic hypertension. The adjusted OR and its 95% confidence interval (CI) were derived from the coefficients of the logistic models and the standard errors. The Statistical Package for Social Sciences (SPSS), version 18.0 was used for all the statistical analyses.
Results
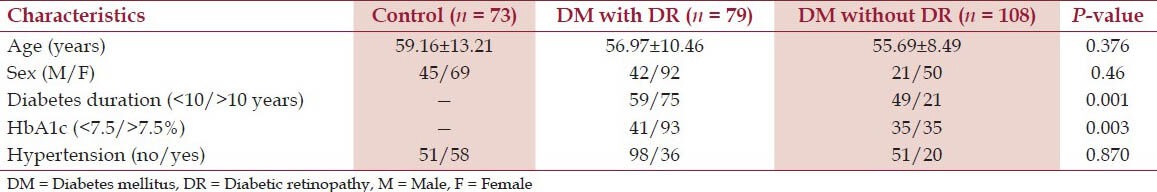
The medical history of 213 cases of diabetic patients with and without DR and 73 controls is shown in Table 1. Characteristics of subjects in each age group are similar. The percentage of females was higher than that of males. A total of 84 subjects (39.4%) were diagnosed as having DR according to medical examination and the previously mentioned criteria. The prevalence between males and females did not differ significantly. The durations of DM in individuals with and without DR were 10.5 (range 5-14 years) and 12.8 years (range 8-18 years), respectively. The patients with DR had higher levels of HbA1c (P < 0.001) compared with the patients without DR. There was a significant relationship between the severity of DR and duration of diabetes (P < 0.001).
Table 1.
Characteristics of study population
In effect, on examining the periodontal conditions of the diabetics versus the control group, no statistical significant differences were observed in terms of the PI, so the conditions of oral hygiene were similar in both groups. However, significant differences were recorded in CPITN, and periodontal disease severity. Type 2 diabetic patients with DR had higher CPITN (P < 0.001) and more severe periodontal disease (P < 0.001) compared with patients without DR in the same group [Table 2].
Table 2.
Periodontal disease and diabetic retinopathy
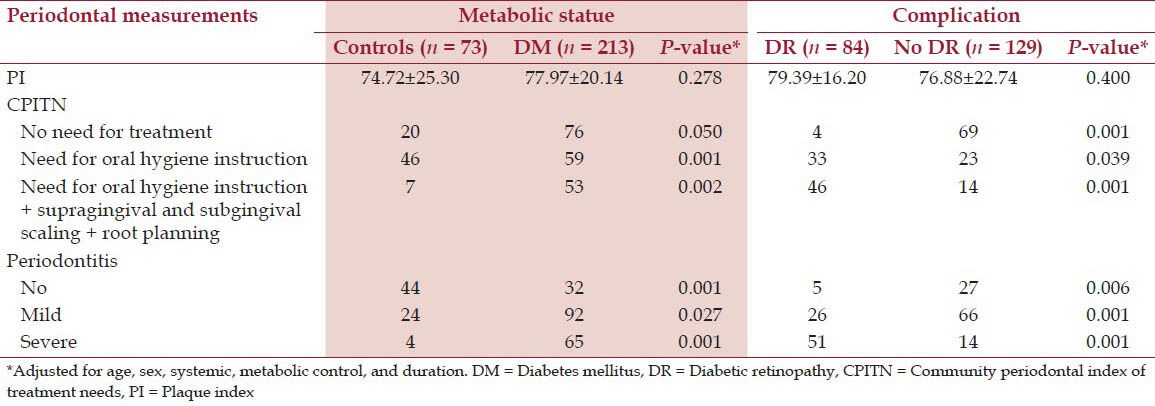
The severity of periodontal disease was significantly correlated with the severity of DR (P < 0.011), and the risk of PDR was significantly higher in the presence of periodontal disease OR = 2.80, P < 0.029). There was a significant relationship between the severity of periodontal disease and HbA1c level, but failed to identify a relationship between periodontal disease and duration of diabetes (P < 0.001 and 0.288, respectively).
Discussion
The suggested mechanism for diabetic effects on periodontal disease is that diabetes-enhanced inflammation specifically affects periodontal tissues.[23] Moreover, the increased severity of periodontal disease in DM may indicate an alteration in the breakdown of periodontal tissues, resulting in more frequent periodontal-tissue destruction which is also affected by poor metabolic state.[24,25,26] Chronic anaerobic periodontal infections possibly influence poor glycemic control and increase the risk of diabetes complications.[14,27,28] Poor-control diabetics exhibited an increased percentage of calculus and greater risk for periodontitis.[29]
Epidemiological studies showed periodontal impairment was significantly more frequent and severe in subjects with type 2 DM.[30,31,32,33] Eldarrat described a significant association (P < 0.05) between glycemic control and oral infections and between the duration of diabetes and denture problems.[34] Periodontal therapy could improve metabolic control in diabetic patients.[35,36] However, several reports detected no significant differences in levels of periodontal disease between subjects with and without DM.[16,17] Diversity in types of subjects (i.e., race) and degree of diabetes or other medical conditions might have produced these conflicting results.
The present study investigated type 2 diabetics with presence or absence of retinopathy, which also compared with a healthy group of age-and sex-matched controls. The two groups had healthy lifestyles and presented similar oral hygiene in terms of bacterial plaque, thus contributing to homogenize the study series. In the present study, we observed a positive relationship between CPITN and the severity of periodontal diseases with retinal complications of DM. As reported by several studies a correlation between complications inherent to DM, such as DR or nephropathy, and an increased degree of periodontal inflammation.[37,38] Noma et al., described a significant relationship between the severity of periodontal disease and DR, with high IL-6 concentrations in the retinal vitreous fluid of the studied patients.[15] Although the reasons for this increase had not been appointed, the presence of immune alterations in these patients, with the subsequent increase in inflammatory mediator production, could be responsible for the damage observed both at periodontal level and elsewhere. Whether periodontal disease is a risk factor for DR or a coincidental finding remains to be determined.
In the present study, we found that despite similar PIs in both groups and control, the diabetics showed a significantly higher gingival bleeding, based on gentle probing of the gingival sulcus may be suggestive of greater periodontal vulnerability. These observations are similar with most studies that consider diabetics to show greater periodontal inflammation in response to the accumulation of bacterial plaque.[39] We also detected more periodontal attachment loss and more pathological periodontal pockets in the diabetic patients than in the control group in concordance with the observations of Oliver and Tervonen.[40]
Bajaj et al., recently demonstrated that retinal microvascular complication of DM was found in 50% of patients with oral diseases.[41]
Our results indicate a probable relationship between type 2 DM with retinal microvascular complication and periodontal disease, and a tendency for diabetics to have greater CPITN and more periodontal disease severity than nondiabetics. Hence, the current results support most previous studies that DM is associated with periodontal disease, and that the outcome of periodontal destruction is significantly more frequent and severe in subjects with DR.
There was a significant relationship between periodontal disease and severity of DR, but it was unclear whether periodontal disease directly affects the progression of DR because this was a cross-sectional study. Further prospective studies, using a large population, and rigorous systemic evaluation, are necessary to confirm the association between DR and periodontal disease.
Acknowledgement
This study was supported by grants-in-aid for scientific search from the Mazandaran University of Medical Sciences, Iran. We would like to thank Dr Leila Sarparast and Mrs Soheila Shahmohammadi, CRNA, Research Fellow, of the Bu Ali-Sina Clinical Research Development Unit of the Mazandaran University of Medical Sciences, Sari, Iran for statistical analyses and technical assistance. The authors wish to sincerely thank Mr. Nakhai, Head of Laboratory and Mrs. Mosavi, Staff Head Nursery of Eye Department, Bu Ali-Sina Hospital for support during sample collection. Appreciation also goes to the Molecular Biology and Biotechnology Department, Sari Medical School for its constructive criticism after reading the manuscript.
Footnotes
Source of Support: Financial support was provided by MAZUMS/Iran.
Conflict of Interest: None declared.
References
- 1.Nelson RG. Periodontal disease and diabetes. Oral Dis. 2008;14:204–5. doi: 10.1111/j.1601-0825.2008.01443.x. [DOI] [PubMed] [Google Scholar]
- 2.Smyth S, Heron A. Diabetes and obesity: The twin epidemics. Nat Med. 2006;12:75–80. doi: 10.1038/nm0106-75. [DOI] [PubMed] [Google Scholar]
- 3.Aiello LP, Gardner TW, King GL, Blankenship G, Cavallerano JD, Ferris FL, 3rd, et al. Diabetic retinopathy. Diabetes Care. 1998;21:143–56. doi: 10.2337/diacare.21.1.143. [DOI] [PubMed] [Google Scholar]
- 4.Klein R, Lee KE, Gangnon RE, Klein BE. The 25-year incidence of visual impairment in type 1 diabetes mellitus the wisconsin epidemiologic study of diabetic retinopathy. Ophthalmology. 2010;117:63–70. doi: 10.1016/j.ophtha.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012;366:1227–39. doi: 10.1056/NEJMra1005073. [DOI] [PubMed] [Google Scholar]
- 6.Kinane DF. Causation and pathogenesis of periodontal disease. Periodontol 2000. 2001;25:8–20. doi: 10.1034/j.1600-0757.2001.22250102.x. [DOI] [PubMed] [Google Scholar]
- 7.Garcia RI, Henshaw MM, Krall EA. Relationship between periodontal disease and systemic health. Periodontol 2000. 2001;25:21–36. doi: 10.1034/j.1600-0757.2001.22250103.x. [DOI] [PubMed] [Google Scholar]
- 8.Amar S, Han X. The impact of periodontal infection on systemic diseases. Med Sci Monit. 2003;9:RA291–9. [PubMed] [Google Scholar]
- 9.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809–20. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 10.Manau C, Echeverria A, Agueda A, Guerrero A, Echeverria JJ. Periodontal disease definition may determine the association between periodontitis and pregnancy outcomes. J Clin Periodontol. 2008;35:385–97. doi: 10.1111/j.1600-051X.2008.01222.x. [DOI] [PubMed] [Google Scholar]
- 11.Campus G, Salem A, Uzzau S, Baldoni E, Tonolo G. Diabetes and periodontal disease: A case-control study. J Periodontol. 2005;76:418–25. doi: 10.1902/jop.2005.76.3.418. [DOI] [PubMed] [Google Scholar]
- 12.Khader YS, Dauod AS, El-Qaderi SS, Alkafajei A, Batayha WQ. Periodontal status of diabetics compared with non-diabetics: A meta-analysis. J Diabetes Complications. 2006;20:59–68. doi: 10.1016/j.jdiacomp.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Furukawa T, Wakai K, Yamanouchi K, Oshida Y, Miyao M, Watanabe T, et al. Associations of periodontal damage and tooth loss with atherogenic factors among patients with type 2 diabetes mellitus. Intern Med. 2007;46:1359–64. doi: 10.2169/internalmedicine.46.0106. [DOI] [PubMed] [Google Scholar]
- 14.Taylor GW, Borgnakke WS. Periodontal disease: Associations with diabetes, glycemic control and complications. Oral Dis. 2008;14:191–203. doi: 10.1111/j.1601-0825.2008.01442.x. [DOI] [PubMed] [Google Scholar]
- 15.Noma H, Sakamoto I, Mochizuki H, Tsukamoto H, Minamoto A, Funatsu H, et al. Relationship between periodontal disease and diabetic retinopathy. Diabetes Care. 2004;27:615. doi: 10.2337/diacare.27.2.615. [DOI] [PubMed] [Google Scholar]
- 16.Arrieta-Blanco JJ, Bartolomé-Villar B, Jiménez-Martinez E, Saavedra-Vallejo P, Arrieta-Blanco FJ. Dental problems in patients with diabetes mellitus (II): Gingival index and periodontal disease. Med Oral. 2003;8:233–47. [PubMed] [Google Scholar]
- 17.Ogunbodede EO, Fatusi OA, Akintomide A, Kolawole K, Ajayi A. Oral health status in a population of Nigerian diabetics. J Contemp Dent Pract. 2005;6:75–84. [PubMed] [Google Scholar]
- 18.Lee HK, Choi SH, Won KC, Merchant AT, Song KB, Jeong SH, et al. The effect of intensive oral hygiene care on gingivitis and periodontal destruction in type 2 diabetic patients. Yonsei Med J. 2009;50:529–36. doi: 10.3349/ymj.2009.50.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 20.O’Leary TJ, Drake RB, Naylor JE. The plaque control record. J Periodontol. 1972;43:38. doi: 10.1902/jop.1972.43.1.38. [DOI] [PubMed] [Google Scholar]
- 21.Cutress TW, Ainamo J, Sardo-Infirri J. The community periodontal index of treatment needs (CPITN) procedure for population groups and individuals. Int Dent J. 1987;37:222–33. [PubMed] [Google Scholar]
- 22.Offenbacher S, Lieff S, Boggess KA, Murtha AP, Madianos PN, Champagne CM, et al. Maternal periodontitis and prematurity. Part I: Obstetric outcome of prematurity and growth restriction. Ann Periodontol. 2001;6:164–74. doi: 10.1902/annals.2001.6.1.164. [DOI] [PubMed] [Google Scholar]
- 23.Graves DT, Liu R, Oates TW. Diabetes-enhanced inflammation and apoptosis: Impact on periodontal pathosis. Periodontol 2000. 2007;45:128–37. doi: 10.1111/j.1600-0757.2007.00219.x. [DOI] [PubMed] [Google Scholar]
- 24.Nishimura F, Iwamoto Y, Soga Y. The periodontal host response with diabetes. Periodontol 2000. 2007;43:245–53. doi: 10.1111/j.1600-0757.2006.00171.x. [DOI] [PubMed] [Google Scholar]
- 25.Southerland JH, Taylor GW, Moss K, Beck JD, Offenbacher S. Commonality in chronic inflammatory diseases: Periodontitis, diabetes, and coronary artery disease. Periodontol 2000. 2006;40:130–43. doi: 10.1111/j.1600-0757.2005.00138.x. [DOI] [PubMed] [Google Scholar]
- 26.Ebersole JL, Holt SC, Hansard R, Novak MJ. Microbiologic and immunologic characteristics of periodontal disease in Hispanic americans with type 2 diabetes. J Periodontol. 2008;79:637–46. doi: 10.1902/jop.2008.070455. [DOI] [PubMed] [Google Scholar]
- 27.Genco RJ, Grossi S, Ho A, Nishimura F, Murayama Y. A proposed model linking inflammation to obesity, diabetes, and periodontal infections. J Periodontol. 2005;76:2075–84. doi: 10.1902/jop.2005.76.11-S.2075. [DOI] [PubMed] [Google Scholar]
- 28.Mealey BL, Ocampo GL. Diabetes mellitus and periodontal disease. Periodontol 2000. 2007;44:127–53. doi: 10.1111/j.1600-0757.2006.00193.x. [DOI] [PubMed] [Google Scholar]
- 29.Awartani F. Evaluation of the relationship between type 2 diabetes and periodontal disease. Odontostomatol Trop. 2009;32:33–9. [PubMed] [Google Scholar]
- 30.Tsai C, Hayes C, Taylor GW. Glycemic control of type 2 diabetes and severe periodontal disease in the US adult population. Community Dent Oral Epidemiol. 2002;30:182–92. doi: 10.1034/j.1600-0528.2002.300304.x. [DOI] [PubMed] [Google Scholar]
- 31.Gursoy UK, Marakoglu I, Oztop AY. Relationship between neutrophil functions and severity of periodontitis in obese and/or type 2 diabetic chronic periodontitis patients. Quintessence Int. 2008;39:485–9. [PubMed] [Google Scholar]
- 32.Lalla E, Park DB, Papapanou PN, Lamster IB. Oral disease burden in northern Manhattan patients with diabetes mellitus. Am J Public Health. 2004;94:755–8. doi: 10.2105/ajph.94.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rylander H, Ramberg P, Blohme G, Lindhe J. Prevalence of periodontal disease in young diabetics. J Clin Periodontol. 1987;14:38–43. doi: 10.1111/j.1600-051x.1987.tb01511.x. [DOI] [PubMed] [Google Scholar]
- 34.Eldarrat AH. Awareness and attitude of diabetic patients about their increased risk for oral diseases. Oral Health Prev Dent. 2011;9:235–41. [PubMed] [Google Scholar]
- 35.Moeintaghavi A, Arab H, Bozorgnia Y, Kianoush K, Alizadeh M. Non-surgical periodontal therapy affects metabolic control in diabetics: A randomized controlled clinical trial. Aust Dent J. 2012;57:31–7. doi: 10.1111/j.1834-7819.2011.01652.x. [DOI] [PubMed] [Google Scholar]
- 36.Kiran M, Arpak N, Unsal E, Erdoğan MF. The effect of improved periodontal health on metabolic control in type 2 diabetes mellitus. J Clin Periodontol. 2005;32:266–72. doi: 10.1111/j.1600-051X.2005.00658.x. [DOI] [PubMed] [Google Scholar]
- 37.Silvestre FJ, Miralles L, Llambes F, Bautista D, Solá-Izquierdo E, Hernández-Mijares A. Type 1 diabetes mellitus and periodontal disease: Relationship to different clinical variables. Med Oral Patol Oral Cir Bucal. 2009;14:E175–9. [PubMed] [Google Scholar]
- 38.Sadzeviciene R, Paipaliene P, Zekonis G, Zilinskas J. The influence of microvascular complications caused by diabetes mellitus on the inflammatory pathology of periodontal tissues. Stomatologija. 2005;7:121–4. [PubMed] [Google Scholar]
- 39.Llambés F, Silvestre FJ, Hernández-Mijares A, Guiha R, Caffesse R. Effect of non-surgical periodontal treatment with or without doxycycline on the periodontium of type 1 diabetic patients. J Clin Periodontol. 2005;32:915–20. doi: 10.1111/j.1600-051X.2005.00736.x. [DOI] [PubMed] [Google Scholar]
- 40.Oliver RC, Tervonen T. Periodontitis and tooth loss: Comparing diabetics with the general population. J Am Dent Assoc. 1993;124:71–6. doi: 10.14219/jada.archive.1993.0247. [DOI] [PubMed] [Google Scholar]
- 41.Bajaj S, Prasad S, Gupta A, Singh VB. Oral manifestations in type-2 diabetes and related complications. Indian J Endocrinol Metab. 2012;16:777–9. doi: 10.4103/2230-8210.100673. [DOI] [PMC free article] [PubMed] [Google Scholar]


