Abstract
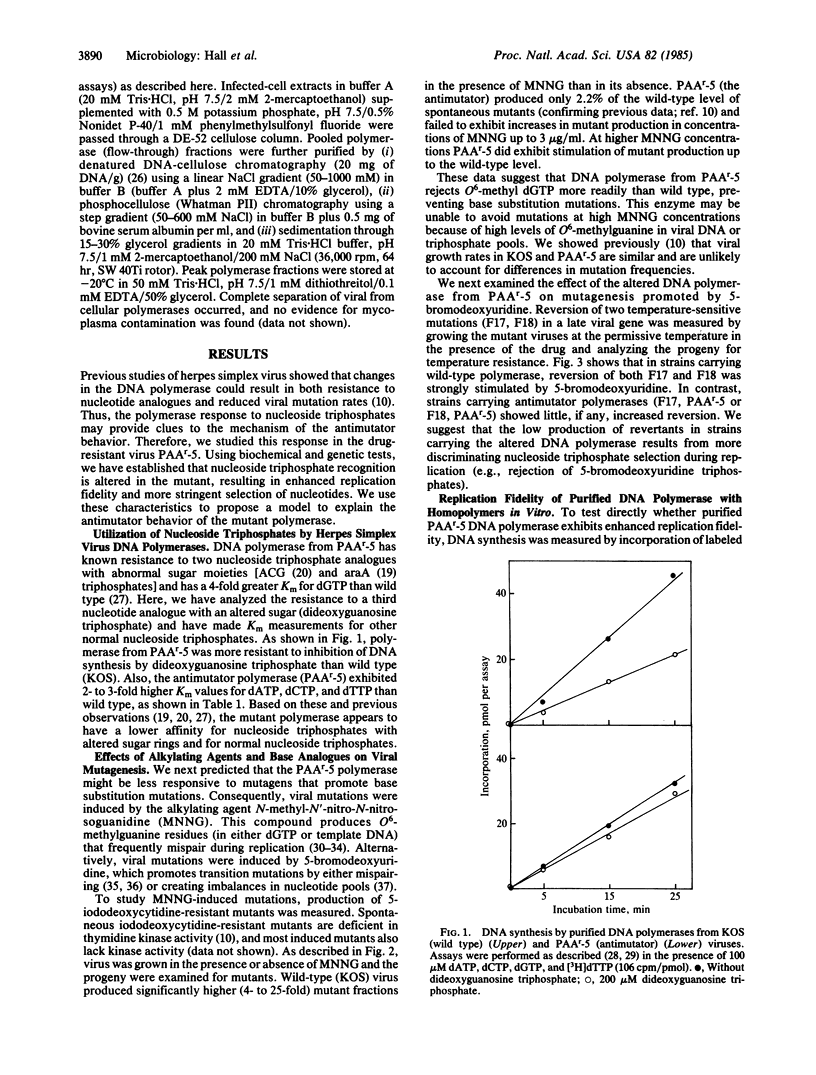
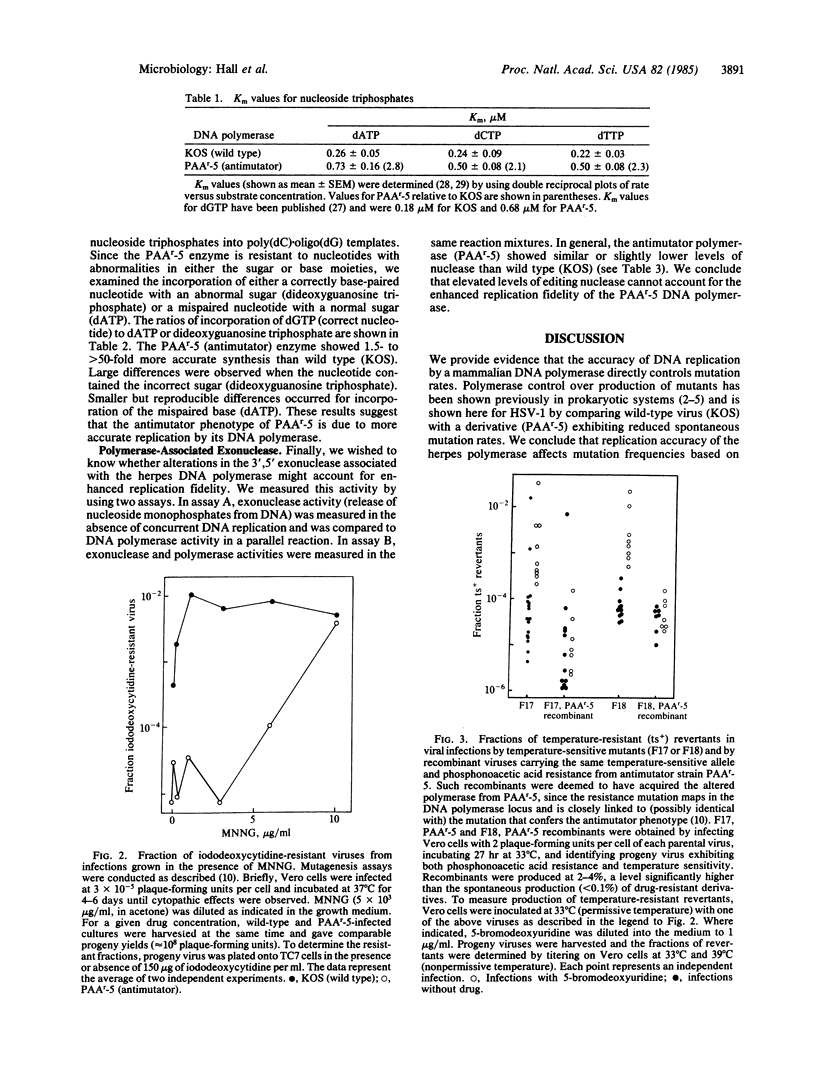
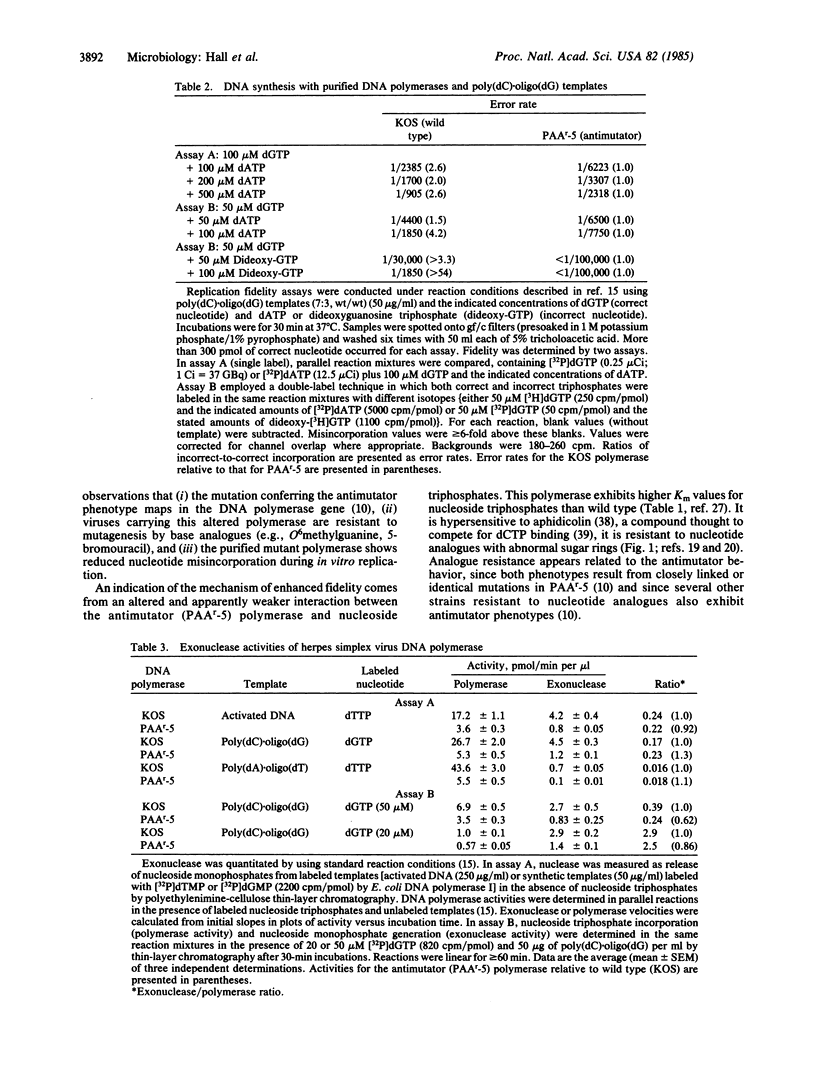
We present evidence that mutation frequencies in a mammalian system can vary according to the replication fidelity of the DNA polymerase. We demonstrated previously that several derivatives of herpes simplex virus type 1 that encode polymerases resistant to various antiviral drugs (e.g., nucleotide analogues) also produce reduced numbers of spontaneous mutants. Here we show that the DNA polymerase from one antimutator virus exhibits enhanced replication fidelity. First, the antimutator virus showed a reduced response to known mutagens that promote base mispairing during DNA replication (N-methyl-N'-nitro-N-nitrosoguanidine, 5-bromo-deoxyuridine). Second, purified DNA polymerase from the antimutator produced fewer replication errors in vitro, based on incorporation of mispaired nucleotides or analogues with abnormal sugar rings. We have investigated possible mechanisms for the enhanced fidelity of the antimutator polymerase. We show that the mutant enzyme has altered interactions with nucleoside triphosphates, as indicated by its resistance to nucleotide analogues and elevated Km values for normal nucleoside triphosphates. We present evidence against increased proofreading by an associated 3',5' exonuclease (as seen for T4 bacteriophage antimutator polymerases), based on nuclease levels in the mutant polymerase. We propose that reduced affinity of the polymerase for nucleoside triphosphates accounts for the antimutator phenotype by accentuating differences in base-pair stability, thus facilitating selection of correct nucleotides.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashman C. R., Davidson R. L. Bromodeoxyuridine mutagenesis in mammalian cells is related to deoxyribonucleotide pool imbalance. Mol Cell Biol. 1981 Mar;1(3):254–260. doi: 10.1128/mcb.1.3.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigden D., Fiddian P., Rosling A. E., Ravenscroft T. Acyclovir - a review of the preclinical and early clinical data of a new antiherpes drug. Antiviral Res. 1981 Nov;1(4):203–212. doi: 10.1016/0166-3542(81)90011-5. [DOI] [PubMed] [Google Scholar]
- Chan J. Y., Becker F. F. Decreased fidelity of DNA polymerase activity during N-2-fluorenylacetamide hepatocarcinogenesis. Proc Natl Acad Sci U S A. 1979 Feb;76(2):814–818. doi: 10.1073/pnas.76.2.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen D. M., Aschman D. P., Gelep P. T., Retondo M. J., Weller S. K., Schaffer P. A. Fine mapping and molecular cloning of mutations in the herpes simplex virus DNA polymerase locus. J Virol. 1984 Jan;49(1):236–247. doi: 10.1128/jvi.49.1.236-247.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen D. M., Furman P. A., Aschman D. P., Schaffer P. A. Mutations in the herpes simplex virus DNA polymerase gene conferring hypersensitivity to aphidicolin. Nucleic Acids Res. 1983 Aug 11;11(15):5287–5297. doi: 10.1093/nar/11.15.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen D. M., Furman P. A., Gelep P. T., Schaffer P. A. Mutations in the herpes simplex virus DNA polymerase gene can confer resistance to 9-beta-D-arabinofuranosyladenine. J Virol. 1982 Mar;41(3):909–918. doi: 10.1128/jvi.41.3.909-918.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen D. M., Schaffer P. A. Two distinct loci confer resistance to acycloguanosine in herpes simplex virus type 1. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2265–2269. doi: 10.1073/pnas.77.4.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echols H., Lu C., Burgers P. M. Mutator strains of Escherichia coli, mutD and dnaQ, with defective exonucleolytic editing by DNA polymerase III holoenzyme. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2189–2192. doi: 10.1073/pnas.80.8.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elion G. B., Furman P. A., Fyfe J. A., de Miranda P., Beauchamp L., Schaeffer H. J. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5716–5720. doi: 10.1073/pnas.74.12.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman P. A., Coen D. M., St Clair M. H., Schaffer P. A. Acyclovir-resistant mutants of herpes simplex virus type 1 express altered DNA polymerase or reduced acyclovir phosphorylating activities. J Virol. 1981 Dec;40(3):936–941. doi: 10.1128/jvi.40.3.936-941.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman P. A., St Clair M. H., Fyfe J. A., Rideout J. L., Keller P. M., Elion G. B. Inhibition of herpes simplex virus-induced DNA polymerase activity and viral DNA replication by 9-(2-hydroxyethoxymethyl)guanine and its triphosphate. J Virol. 1979 Oct;32(1):72–77. doi: 10.1128/jvi.32.1.72-77.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman P. A., St Clair M. H., Spector T. Acyclovir triphosphate is a suicide inactivator of the herpes simplex virus DNA polymerase. J Biol Chem. 1984 Aug 10;259(15):9575–9579. [PubMed] [Google Scholar]
- Gillin F. D., Nossal N. G. Control of mutation frequency by bacteriophage T4 DNA polymerase. I. The CB120 antimutator DNA polymerase is defective in strand displacement. J Biol Chem. 1976 Sep 10;251(17):5219–5224. [PubMed] [Google Scholar]
- Hall J. D., Almy R. E. Evidence for control of herpes simplex virus mutagenesis by the viral DNA polymerase. Virology. 1982 Jan 30;116(2):535–543. doi: 10.1016/0042-6822(82)90146-5. [DOI] [PubMed] [Google Scholar]
- Hall J. D., Coen D. M., Fisher B. L., Weisslitz M., Randall S., Almy R. E., Gelep P. T., Schaffer P. A. Generation of genetic diversity in herpes simplex virus: an antimutator phenotype maps to the DNA polymerase locus. Virology. 1984 Jan 15;132(1):26–37. doi: 10.1016/0042-6822(84)90088-6. [DOI] [PubMed] [Google Scholar]
- Hall J. D., Featherston J. D., Almy R. E. Evidence for repair of ultraviolet light-damaged herpes virus in human fibroblasts by a recombination mechanism. Virology. 1980 Sep;105(2):490–500. doi: 10.1016/0042-6822(80)90049-5. [DOI] [PubMed] [Google Scholar]
- Hopkins R. L., Goodman M. F. Deoxyribonucleotide pools, base pairing, and sequence configuration affecting bromodeoxyuridine- and 2-aminopurine-induced mutagenesis. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1801–1805. doi: 10.1073/pnas.77.4.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jofre J. T., Schaffer P. A., Parris D. S. Genetics of resistance to phosphonoacetic acid in strain KOS of herpes simplex virus type 1. J Virol. 1977 Sep;23(3):833–836. doi: 10.1128/jvi.23.3.833-836.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaina B., Heindorff K., Aurich O. O6-methylguanine, but not N7-methylguanine or N3-methyladenine, induces gene mutations, sister-chromatid exchanges and chromosomal aberrations in Chinese hamster cells. Mutat Res. 1983 Mar;108(1-3):279–292. doi: 10.1016/0027-5107(83)90126-4. [DOI] [PubMed] [Google Scholar]
- Karran P., Lindahl T., Griffin B. Adaptive response to alkylating agents involves alteration in situ of O6-methylguanine residues in DNA. Nature. 1979 Jul 5;280(5717):76–77. doi: 10.1038/280076a0. [DOI] [PubMed] [Google Scholar]
- Knopf K. W. Properties of herpes simplex virus DNA polymerase and characterization of its associated exonuclease activity. Eur J Biochem. 1979 Jul;98(1):231–244. doi: 10.1111/j.1432-1033.1979.tb13181.x. [DOI] [PubMed] [Google Scholar]
- Linn S., Kairis M., Holliday R. Decreased fidelity of DNA polymerase activity isolated from aging human fibroblasts. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2818–2822. doi: 10.1073/pnas.73.8.2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litman R. M. A deoxyribonucleic acid polymerase from Micrococcus luteus (Micrococcus lysodeikticus) isolated on deoxyribonucleic acid-cellulose. J Biol Chem. 1968 Dec 10;243(23):6222–6233. [PubMed] [Google Scholar]
- Liu P. K., Chang C. C., Trosko J. E., Dube D. K., Martin G. M., Loeb L. A. Mammalian mutator mutant with an aphidicolin-resistant DNA polymerase alpha. Proc Natl Acad Sci U S A. 1983 Feb;80(3):797–801. doi: 10.1073/pnas.80.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb L. A., Kunkel T. A. Fidelity of DNA synthesis. Annu Rev Biochem. 1982;51:429–457. doi: 10.1146/annurev.bi.51.070182.002241. [DOI] [PubMed] [Google Scholar]
- Muzyczka N., Poland R. L., Bessman M. J. Studies on the biochemical basis of spontaneous mutation. I. A comparison of the deoxyribonucleic acid polymerases of mutator, antimutator, and wild type strains of bacteriophage T4. J Biol Chem. 1972 Nov 25;247(22):7116–7122. [PubMed] [Google Scholar]
- Nagata C., Takeda E., Aida M. Why O6-alkylguanine is specifically promutagenic? Ab initio molecular orbital consideration. Mutat Res. 1982 Aug;105(1-2):1–8. doi: 10.1016/0165-7992(82)90199-3. [DOI] [PubMed] [Google Scholar]
- Newbold R. F., Warren W., Medcalf A. S., Amos J. Mutagenicity of carcinogenic methylating agents is associated with a specific DNA modification. Nature. 1980 Feb 7;283(5747):596–599. doi: 10.1038/283596a0. [DOI] [PubMed] [Google Scholar]
- North T. W., Cohen S. S. Aranucleosides and aranucleotides in viral chemotherapy. Pharmacol Ther. 1979;4(1):81–108. doi: 10.1016/0163-7258(79)90016-0. [DOI] [PubMed] [Google Scholar]
- Pedrali-Noy G., Spadari S. Mechanism of inhibition of herpes simplex virus and vaccinia virus DNA polymerases by aphidicolin, a highly specific inhibitor of DNA replication in eucaryotes. J Virol. 1980 Nov;36(2):457–464. doi: 10.1128/jvi.36.2.457-464.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reha-Krantz L. J., Bessman M. J. Studeis on the biochemical basis of mutation. IV. Effect of amino acid substitution on the enzymatic and biological properties of bacteriophage T4 DNA polymerase. J Mol Biol. 1977 Oct 15;116(1):99–113. doi: 10.1016/0022-2836(77)90121-8. [DOI] [PubMed] [Google Scholar]
- Reha-Krantz L. J., Bessman M. J. Studies on the biochemical basis of mutation VI. Selection and characterization of a new bacteriophage T4 mutator DNA polymerase. J Mol Biol. 1981 Feb 5;145(4):677–695. doi: 10.1016/0022-2836(81)90309-0. [DOI] [PubMed] [Google Scholar]
- Schaffer P. A., Aron G. M., Biswal N., Benyesh-Melnick M. Temperature-sensitive mutants of herpes simplex virus type 1: isolation, complementation and partial characterization. Virology. 1973 Mar;52(1):57–71. doi: 10.1016/0042-6822(73)90398-x. [DOI] [PubMed] [Google Scholar]
- Schaffer P. A., Carter V. C., Timbury M. C. Collaborative complementation study of temperature-sensitive mutants of herpes simplex virus types 1 and 2. J Virol. 1978 Sep;27(3):490–504. doi: 10.1128/jvi.27.3.490-504.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer P. A., Tevethia M. J., Benyesh-Melnick M. Recombination between temperature-sensitive mutants of herpes simplex virus type 1. Virology. 1974 Mar;58(1):219–228. doi: 10.1016/0042-6822(74)90156-1. [DOI] [PubMed] [Google Scholar]
- Schendel P. F., Robins P. E. Repair of O6-methylguanine in adapted Escherichia coli. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6017–6020. doi: 10.1073/pnas.75.12.6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springgate C. F., Loeb L. A. Mutagenic DNA polymerase in human leukemic cells. Proc Natl Acad Sci U S A. 1973 Jan;70(1):245–249. doi: 10.1073/pnas.70.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Clair M. H., Miller W. H., Miller R. L., Lambe C. U., Furman P. A. Inhibition of cellular alpha DNA polymerase and herpes simplex virus-induced DNA polymerases by the triphosphate of BW759U. Antimicrob Agents Chemother. 1984 Feb;25(2):191–194. doi: 10.1128/aac.25.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]


