Abstract
BACKGROUND
Vitamin D attenuates uremic cardiac hypertrophy, possibly by suppressing the myocardial renin–angiotensin system (RAS) and fibroblast growth factors (FGFs). We compared the suppression of cardiac hypertrophy and myocardial expression of RAS and FGF receptor genes offered by the vitamin D analog paricalcitol (Pc) or the angiotensin-converting enzyme inhibitor enalapril (E) in experimental uremia.
METHODS
Rats with 5/6 nephrectomy received Pc or E for 8 weeks. Renal function, systolic blood pressure, and cardiac hypertrophy were evaluated. Myocardial expression of RAS genes, brain natriuretic peptide (BNP), and FGF receptor-1 (FGFR-1) were determined using quantitative reverse-transcription (pRT)-PCR.
RESULTS
Blood pressure, proteinuria, and serum creatinine were significantly higher in untreated uremic animals. Hypertension was significantly reduced by E but only modestly by Pc; however, cardiac hypertrophy in the untreated group was similarly attenuated by Pc or E. Upregulation of myocardial expressions of renin, angiotensinogen, FGFR-1, and BNP in untreated uremic animals was reduced similarly by Pc and E, while the angiotensin II type 1 receptor was downregulated only by E.
CONCLUSIONS
Uremic cardiac hypertrophy is associated with activation of the myocardial RAS and the FGFR-1. Downregulation of these genes induced by Pc and E results in similar amelioration of left ventricular hypertrophy despite the different antihypertensive effects of these drugs.
Keywords: Vitamin D, uremia, cardiac hypertrophy, renin-angiotensin system, fibroblast growth factor, paricalcitol.
Left ventricular hypertrophy (LVH) is the most frequent cardiac complication of chronic kidney disease (CKD) and constitutes a powerful mortality risk factor.1,2 The pathogenesis of LVH is multifactorial and cannot account only for traditional risk factors such as hypertension and hypervolemia.2–4 Indeed, studies in experimental uremia have demonstrated that cardiac hypertrophy may develop independent of hypertension.5,6 Furthermore, disturbances of mineral metabolism may be involved, including hyperphosphatemia, increased parathyroid hormone, and fibroblast growth factor-23.7 While the cardiovascular system is not traditionally regarded as a target of 1,25(OH)2D3 (1,25D, calcitriol), this metabolite and its receptor the vitamin D receptor (VDR) have important effects on circulation8 and may play an important role in the development of LVH in uremia.9 In renal insufficiency, the decline in renal 1α-hydroxylase activity and the increased catabolic rate of 1,25D10 result in reduced circulating levels and 1,25D deficiency.11 The latter has been linked to increased cardiovascular mortality and can be reduced by treatment with the VDR agonists calcitriol or its less-calcemic analog paricalcitol [Pc; 19-nor-1,25(OH)2D2].12 Calcitriol therapy attenuates cardiac hypertrophy in experimental uremia and in patients on dialysis.13 A likely mechanism underlying the cardioprotective effects of vitamin D is the downregulation of the renin–angiotensin system (RAS) since VDR and 1α-hydroxylase knockout mice develop hyperreninemia and LVH,14,15 treatment with VDR activators improves cardiac hypertrophy in hypertensive rats,16 1,25D suppresses renin gene transcription,17 and Pc suppresses renal renin expression in normal mice.18 Several studies, including our own,8,19 have demonstrated that renin inhibition by Pc may offer a novel mechanism for suppressing the intrarenal RAS in various experimental and clinical conditions typically characterized by RAS upregulation. These observations raise the possibility that increased myocardial RAS activity plays a significant role in the development of cardiac hypertrophy in uremia and that VDR activators are capable of attenuating these changes.
In support of this possibility, recent investigations have shown that myocardial RAS is regulated independently from the circulating RAS and plays a determinant role in the development of myocardial hypertrophy.20,21 All components of the RAS have been demonstrated in the myocardium of animals with intact renal function,21 and there is compelling evidence of the therapeutic effects of angiotensin-converting enzyme (ACE) inhibitors and angiotensin II type 1 receptor (AT1R) antagonists in experimental models of heart failure in animals with intact renal function.21 However, data on the mRNA expression of components of the RAS in the hearts of uremic animals are scarce, and the changes resulting from therapy with VDR activators or ACE inhibition in their myocardium have not been investigated.
Fibroblast growth factor (FGF)-23, the 23rd member of the FGF family, is a potent negative regulator of circulating phosphate and 1,25D levels. Also, FGF-23 induces phosphaturia and lowers serum phosphorus through reduction of the sodium–phosphate cotransporters in the kidney proximal tubules and it directly suppresses renal 1α-hydroxylase, leading to decreased conversion of 25-hydroxyvitamin D to its active metabolite 1,25D.22 In addition, increasing evidence suggests intricate hormonal interactions between FGF-23, vitamin D, and the RAS, with important consequences for patients with CKD.23 Elevated circulating FGF-23 concentrations have been strongly associated with LVH and mortality risk in adults24 and, more recently, in children with CKD.25 Activation of FGF receptors (FGFRs), such as FGFR-1, has been implicated in the development of cardiac hypertrophy, and FGF-23 administration has resulted in LVH in animals and in hypertrophy of cardiomyocytes via FGFR-dependent signaling.26 However, the specific FGFR that mediates these effects has not been identified, and neither FGF-23 nor its obligatory coreceptor Klotho are expressed in cardiomyocytes.7,27 Therefore, a mechanistic explanation for the association between elevated FGF-23 levels and LVH remains elusive.
These considerations prompted the present study in rats with cardiac hypertrophy associated with experimentally induced CKD. This study was designed to compare the effects of the VDR activator Pc with those of the ACE inhibitor enalapril (E) on uremic myocardial hypertrophy.
METHODS
Experimental animals and design
Male Sprague–Dawley rats (Instituto Venezolano de Investigaciones Cientificas (IVIC), Los Teques, Altos de Pipe, Venezuela), which weighed 250–395g at the beginning of the study, were housed in temperature-controlled facilities, fed standard rat chow containing 1% calcium and 0.74% phosphorus, had free access to water, and were handled in accordance with institutional guidelines of animal care. The Committee of Animal Care and Use of the IVIC Zulia, Venezuela, approved the study protocol. Rats were assigned to have either renal ablation by 5/6 nephrectomy (Nx) or to have a sham operation. Four days after surgery, the animals were randomly assigned to the following experimental groups: (i) sham-operated group served as controls (C; n = 5), consisted of rats that underwent surgical incision similar to that of group 2, the kidneys were manipulated but left intact, and the wound was closed; (ii) 5/6 Nx (CKD) group (n = 7) received vehicle 3 times a week by intraperitoneal injection; (iii) paricalcitol (CKD-Pc) group (n = 5) received intraperitoneal Pc, 0.3 μg/kg, 3 times a week; and (iv) enalapril (CKD-E) group (n = 5) received E daily 5mg/kg/d in their drinking water. The dose of Pc has been previously demonstrated to effectively control the development and treatment of secondary hyperparathyroidism without elevation in serum ionized calcium in the 5/6 Nx model28 and is within the therapeutic dose range currently used in clinical practice.29 The administration of Pc, vehicle, and E was begun 5–6 days after surgery and maintained for 8 weeks. Plasma samples for creatinine, calcium, phosphorus, and 24-hour urine protein were evaluated by autoanalyzer methodology as previously described.19 The systolic blood pressure (SBP) was determined by tail-cuff plethysmography (IITC Life Science Inc., Woodland Hills, CA) as previously reported.19 At the end of the study, animals were euthanized under general anesthesia, and hearts were harvested, weighted, and processed for mRNA determinations.
Quantitative real-time polymerase chain reaction
The mRNA levels of angiotensinogen (AGT), ACE, AT1R, renin, brain natriuretic peptide (BNP), and FGFR-1 in the hearts of 3 rats in the experimental and control groups were determined by quantitative real-time reverse transcription polymerase chain reaction (RT-PCR). Hearts harvested from the animals were immediately placed in RNALater solution (Ambion, Austin, TX) and stored at −80 °C. Total RNAs were extracted from the left ventricle using TRIZOL reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instruction. First-strand cDNAs were reverse transcribed from total RNAs using MML-V reverse transcriptase (Invitrogen) and hexanucleotide random primers. Real time RT-PCR was performed in a Roche LightCycler 480II real-time PCR system using a SYBR green PCR reagent kit (Applied Biosystems, Foster City, CA); mRNA levels were determined based on the following formula: 2(−∆∆Ct), as previously described.19 β2-microglobulin was used as the internal control to normalize loading variations and expressed relative to the values in sham-operated animals. The PCR primers used in this study are listed in Table 1.
Table 1.
Primers used in real-time reverse-transcription polymerase chain reaction amplification
| Gene | Primer nucleotide sequences |
|---|---|
| Renin | Forward 5′ AGG ATC AGT GCT GAA TGG GGT GA 3′ |
| Reverse 5′ GGT TGT GAA TCT CAC AGG CAG TGT 3′ | |
| Angiotensinogen | Forward 5′ CAG CAC GAC TTC CTG ACT TGG AT 3′ |
| Reverse 5′ GGA TGC TGT TGA GAA CCT CTC CCA 3′ | |
| Angiotensin II type 1 receptor | Forward 5′ GCT CTG CCA CAT TCC CTG AGT TA 3′ |
| Reverse 5′ CTT GGG GCA GTC ATC TTG GAT TCT 3′ | |
| Brain natriuretic peptide | Forward 5′ AAT CCA CGA TGC AGA AGC TGC T3′ |
| Reverse 5′ GCG CCG ATC CGG TCT ATC TTC T3′ | |
| Angiotensin-converting enzyme | Forward 5′ TGC CTA GAT CCC AAG GTG ACT TTG A 3′ |
| Reverse 5′ CAA CTT CAT GGC ATC TGC CAG CA 3′ | |
| Fibroblast growth factor receptor-1 | Forward 5′ ACA GGG GAG GAG GTG GAG 3′ |
| Reverse5′ACG GTT TGG TTT GGT GTT GT 3′ | |
| β2 microglobulin | Forward 5′ ATC TGT CCT TCA GCA AGG ACT GGT 3′ |
| Reverse 5′ TGG TCC AGA TGA TTC AGA GCT CCA 3′ |
All primers were designed according to the cDNA sequence of each gene deposited in the GenBank database.
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA). All data are expressed as mean ± standard error of the mean and median with ranges. Comparisons between experimental and control groups were done using the Kruskal–Wallis nonparametric multigroup 1-way analysis of variance followed by Dunn multiple comparison tests. Two-tailed P values <0.05 were considered indicative of significant differences.
RESULTS
Blood pressure, biochemical data, and cardiac weight
The baseline body weights, SBP, plasma creatinine, calcium, phosphorus concentrations, and proteinuria in all groups of rats were normal, and the values were similar between groups (Table 2). As expected, after 8 weeks, the SBP increased in all groups with renal ablation (CKD, CKD-Pc, and CKD-E) compared with values from sham-operated (C) rats, and the highest BP values were observed in the untreated CKD group (204±18mm Hg; P < 0.001 vs. C; Table 3). Hypertension was corrected by E treatment and modestly ameliorated by Pc treatment (Table 3). Consistent with the experimental model, plasma creatinine concentrations were significantly higher in the 5/6 Nx group, indicating substantial renal dysfunction (Table 3). Treatment with E or Pc reduced similarly the plasma creatinine concentrations to values comparable to those in the sham animals (Table 3). Development of proteinuria in the CKD group (P < 0.05 vs. C) was similarly attenuated by treatment with Pc and E (Table 3). Calcium concentrations in both treated groups were similar to those in the untreated CKD group; however, Pc-treated animals displayed higher Ca levels compared with the sham-operated C group (P < 0.05; Table 3). Phosphorus concentrations were unmodified by either treatment and were similar in all groups (Table 3).
Table 2.
Baseline characteristics in normal and uremic rats
| Characteristic | Group 1, Sham- C (n = 5) | Group 2, CKD (n = 7) | Group 3, CKD-Pc (n = 5) | Group 4, CKD-E (n = 5) |
|---|---|---|---|---|
| Weight, grams | 346±18 (340, 295–405) | 319±7.7 (325, 290–340) | 325±25 (355, 250–380) | 356±13 (365, 320–390) |
| Systolic blood pressure, mm Hg | 133±0.9 (133, 129–135) | 133±0.6 (133, 130–135) | 131±0.9 (132, 128–133) | 132±0.4 (132, 131–134) |
| Creatinine, mg/dL | 0.38±0.04 (0.4, 0.3–0.5) | 0.41±0.03 (0.4, 0.3–0.5) | 0.38±0.04 (0.4, 0.3–0.5) | 0.38±0.04 (0.4, 0.3–0.5) |
| Calcium, mg/dL | 8.4±0.5 (8.3, 7.1–10.0) | 8.6±0.2 (8.4, 8.0–9.8) | 8.5±0.3 (8.6, 7.6–9.1) | 8.8±0.2 (9.0, 8.1–9.2) |
| Phosphorus, mg/dL | 4.4±0.3 (4.7, 3.3–5.2) | 5.4±0.4 (5.6, 3.4–6.8) | 4.8±0.2 (4.6, 4.2–5.4) | 5.6±0.4 (5.5, 4.8–6.9) |
| Proteinuria, mg/24 hours | 2.0±0.3 (1.7, 1.3–2.9) | 1.5±0.1 (1.4, 1.2–2.0) | 1.9±0.2 (1.8, 1.3–2.6) | 0.9±0.1 (0.9, 0.6–1.2) |
Rats were divided into 4 groups (groups 1–4) and studied for 8 weeks. Sham (control, C), untreated 5/6 nephrectomy (chronic kidney disease (CKD)) received vehicle, CKD-Pc (paricalcitol), CKD-E (enalapril). Results are expressed as mean ± standard error of the mean (median, range), n = number of animals in each group.
Table 3.
Effects of treatment on blood pressure and chemical parameters in rats with renal ablation
| Examined Parameter | Group 1, Sham- C (n = 5) | Group 2, CKD (n = 7) | Group 3, CKD-Pc (n = 5) | Group 4, CKD-E (n = 5) |
|---|---|---|---|---|
| Systolic blood pressure, mm Hg | 132±1.9 (133, 130–135) | 204±18* (207, 168–222) | 156±8.0 (156, 143–164) | 143±9.5** (144, 133–157) |
| Plasma creatinine, mg/dL | 0.58±0.04 (0.6, 0.5–0.7) | 1.33±0.25c (1.0, 0.9–2.8) | 0.76±0.05 (0.8, 0.6–0.9) | 0.76±0.03 (0.8, 0.7–0.8) |
| Proteinuria, mg/24 hours | 4.2±1.7 (5.5, 0.1–8.7) | 62±9.0a (63, 30–89) | 17±13 (4.8, 2.3–68) | 11±4.8 (7.1, 4.8–30) |
| Calcium, mg/dL | 7.3±0.1 (7.4, 7.0–7.7) | 8.5±0.3 (8.3, 7.8–9.7) | 9.9±0.8b (10.1, 7.3–11.9) | 7.7±0.3 (7.7, 6.7–8.3) |
| Phosphorus, mg/dL | 6.7±0.2 (6.8, 6.0–7.3) | 7.3±0.7 (7.6, 3.5–9.6) | 6.5±0.8 (5.8, 4.8–8.7) | 6.3±0.8 (6.2, 3.9–8.7) |
Blood pressure, plasma creatinine, calcium, phosphorus, and proteinuria after 8 weeks of treatment with paricalcitol (Pc) or enalapril (E) compared with untreated 5/6 nephrectomy (chronic kidney disease (CKD)) and sham control animals (C). Results are expressed as mean ± standard error of the mean (median, range); n = number of animals in each group.
a P < 0.05 vs. C and Pc; b P < 0.05 vs. C; c P < 0.001 vs. C.
*P < 0.001 vs. C; **P < 0.05 vs. CKD.
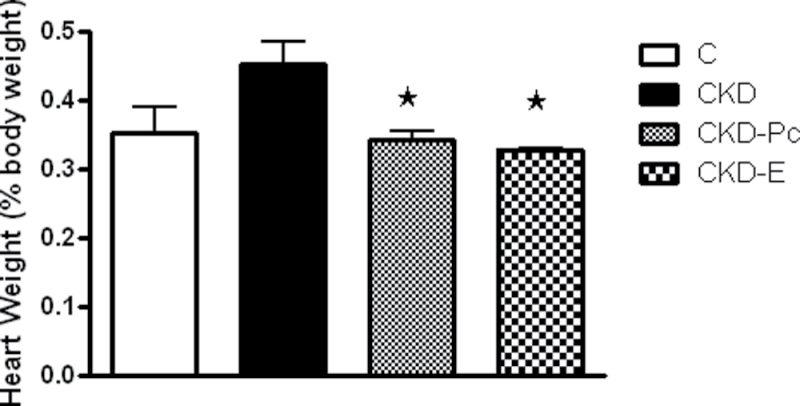
Cardiac weight (expressed as percent of body weight) was higher in the CKD group (by 30%) compared with the C group (0.45±0.03% and 0.35±0.04% body weight, respectively), denoting the presence of cardiac hypertrophy (Figure 1). Treatment with Pc or E significantly reduced (P < 0.05) the heart-to-body weight ratio in the rats with renal ablation to values similar to those in sham-operated control rats (Figure 1), indicating prevention of cardiac hypertrophy.
Figure 1.
Effects of paricalcitol and enalapril treatment on cardiac hypertrophy in uremic rats. Development of cardiac hypertrophy in the untreated chronic kidney disease (CKD) group (CKD), manifested by increased heart weight (expressed as percent of body weight) compared with sham controls (C), was effectively averted following 8 weeks of treatment with paricalcitol (Pc) or enalapril (E). Data represent mean ± standard error of the mean. * P < 0.05 vs. CKD.
Effects on myocardial gene expression
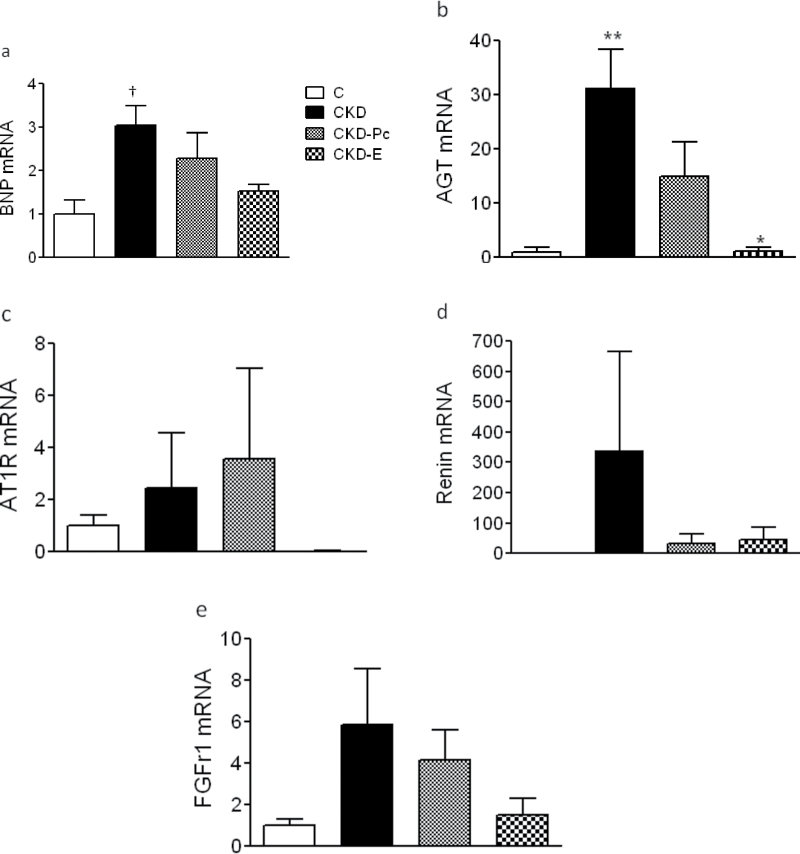
Consistent with cardiac hypertrophy, the mRNA levels of BNP, which is a biomarker of ventricular stress and hypertrophy, were significantly increased in the hearts of uremic rats of the CKD group (P < 0.05 vs. C group) and reduced similarly by Pc or E to levels comparable to those in sham-operated C rats (Figure 2a). Figure 2b shows that after 8 weeks of uremia, the left ventricular mRNA expression of AGT was increased 30 fold over values in the controls (P < 0.01). Pc treatment attenuated substantially AGT expression (50% reduction), but E treatment reduced AGT mRNA levels more drastically and significantly (P < 0.01; Figure 2b). The expression of AT1R was upregulated in uremia, but no significant differences were seen between the groups (Figure 2c), and the ACE expression was not modified by uremia (data not shown). Figure 2d shows a sharp increase in myocardial mRNA expression of renin in the untreated uremic CKD group (>300 fold), with marked reduction following treatment with E or Pc to mean levels comparable to those in the sham-operated C group. The mRNA expression of FGFR-1, which is the most abundant FGFR in the myocardium, was notably increased (6 fold) in the heart of the rats in the CKD group (Figure 2e). Both Pc and E treatment clearly reduced FGFR-1 levels (by 30% and 75%, respectively).
Figure 2.
Activation of brain natriuretic peptide (BNP), renin–angiotensin system (RAS), and fibroblast growth factor receptor-1 (FGFR-1) gene expression in the myocardium of rats with renal ablation. After 8 weeks of renal ablation, left ventricle mRNA levels of (a) BNP, (b) angiotensinogen (AGT), (c) angiotensin II type 1 receptor (AT1R), (d) renin, and (e) FGFR-1) were determined by real-time reverse-transcription polymerase chain reaction. The relative amounts of the sample mRNA were normalized to the β2-microglobulin mRNA. Levels are presented as values relative to the sham (C) group. Data are presented as mean ± standard error of the mean. † P < 0.05 vs. C; * P < 0.01 vs. untreated chronic kidney disease (CKD); ** CKD < 0.01 vs. C.
DISCUSSION
The central findings in this study are the demonstration of upregulation of myocardial mRNA levels of BNP, renin, angiotensinogen, AT1R, and FGFR-1 in chronic renal insufficiency. Also, we found that treatment with Pc or E corrected overexpression of these genes and prevented cardiac hypertrophy.
Our study confirms the renoprotective effects of Pc and E in the renal ablation model,19,30 as evidenced by the substantial reductions of plasma creatinine and proteinuria in the treated animals. The severity of hypertension in the CKD group was similar to that found in our previous studies19 and, as predicted, was effectively reduced with E treatment.6,30 In agreement with most studies in rats with renal ablation, treatment with the D analog Pc4,30 had only a modest effect on hypertension. Nevertheless, both Pc and E corrected the cardiac hypertrophy in this experimental model. Thus, improvement of LVH cannot be attributed only to the improvement in blood pressure. These results are in line with investigations that have shown that reversal of LVH in uremic animals5,31 is independent of BP changes.
Cardiac hypertrophy is accompanied by activation of the fetal gene program in the cardiac ventricles, including the gene encoding BNP. Since higher mRNA BNP expression may also result from a chronic increase in pressure overload32 and since BP was significantly lower in the E group, it is likely that the reduced BP may have played a role in the improvement in cardiac hypertrophy observed in the E-treated group. However, improvements in myocardial hypertrophy and fibrosis have been observed with E but not with other drugs of comparable antihypertensive effects,6 underlining the beneficial effects of factors other than hemodynamic stress.
The finding that cardiac hypertrophy in renal failure can be effectively prevented by the administration of either E or Pc is consistent with the notion that the RAS plays an important role in the regulation of cardiomyocytes cell size and number6,9,33 and that these changes can be abrogated by therapeutic agents capable of modulating the RAS cascade.6 The significant attenuation of the heart-to-body weight following E is in line with past observations that demonstrated prevention of the development and progression of LVH with ACEI treatment in uremia.33
Paricalcitol also significantly ameliorated myocardial hypertrophy, a novel observation in experimental uremia. Our findings differ from those of Mizobuchi et al.9 who showed only a modest and nonsignificant reduction in cardiac hypertrophy with Pc treatment in uremia. The disagreement may be due to the use of lower doses of Pc and shorter periods of administration, as well as more severe uremia in the animals studied by these investigators.9 In a recent study,34 adults with moderate CKD treated with Pc showed only a modest improvement in LVH after 1 year of observation. However, many other variables that drive cardiac hypertrophy are difficult to control in clinical studies.
All the components required for angiotensin II (ANG II) production are normally present in the heart, and increased cardiac RAS components have been linked to cardiac hypertrophy (CH) and fibrosis in hypertensive models with intact renal function.14,21,35 We are not aware of any studies that directly evaluated the simultaneous mRNA expression of renin and other key components of the RAS in the myocardium of animals with renal insufficiency, nor the potential changes induced by ACEI or VDR activator therapy. Cardiac mRNA levels of renin were markedly increased (337 fold) in animals with chronic renal insufficiency compared with the C group with normal renal function. Since systemic renin is suppressed in the renal ablation model and cardiac renin expression is very low in normal rodents, the renin elevation in the 5/6 Nx animals suggests de novo renin synthesis due to renin gene activation within the heart.21,36 ANG II is normally synthesized locally within the myocardium with low expression levels under physiological conditions.21 High ANG II expression has been demonstrated in the myocardium of uremic rats with cardiac hypertrophy, likely resulting from increased AGT formation and decreased degradation.21 Increased AGT mRNA, in concert with increased renin mRNA shown here, is a likely explanation for the generation of high ANG II in the uremic heart. The suppression of the genes of both renin and AGT by Pc and E favor a downstream suppressive effect with the potential to ameliorate the profibrotic and hypertrophic properties of ANG II in the myocardium.21
The effects of Pc downregulating the mRNA of genes associated with cardiac hypertrophy in the uremic heart are in line with previous work from our laboratories that demonstrated similar effects in the remnant kidney.19 In addition, Pc may also exert antihypertrophic activity through nonrenin-dependent mechanisms by direct effects on the cardiac myocyte VDR.37 Untreated uremic rats also displayed elevated AT1R expression in the myocardium, which is in concordance with studies showing that overexpression of the AT1R in the heart of uremic animals induces massive cardiac hypertrophy without hypertension, an effect that can be reversed by AT1R blocker therapy.38 In the present study, the lack of AT1R downregulation in the Pc group and the absence of AT1R upregulation reported in VDR-null animals support the notion that VDR activators are unlikely to directly regulate the AT1R and predominantly abrogate RAS activation by suppressing renin gene expression.8,17–19 Cardiac ACE mRNA expression (data not shown) was present in all 4 groups but did not appear to be affected by uremia or by treatment with E or Pc. While ACE expression is present in the myocardium of patients with advanced heart failure, it is doubtful that it promotes cardiac ANG II production or contributes to cardiac hypertrophy.21
Alterations of mineral homeostasis are recognized risk factors for progressive LVH in patients with CKD.2,3 Hyperphosphatemia contributes to increased cardiomyocyte cell size and interstitial cell proliferation and is a strong risk factor for the development of LVH.7 In the present study, all animals were fed a diet containing normal amounts of calcium and phosphorus, hyperphosphatemia was not observed in any group with renal ablation, and treatment with E or Pc did not affect phosphorus concentrations. Elevated FGF-23 levels have been independently associated with greater left ventricular mass and LVH in adults and children;24,25 however, its obligatory coreceptor Klotho is conspicuously absent in normal cardiomyocytes. Recently, Custódio et al.,7 using immunohistochemical analysis, evaluated different dietary phosphorus loads and detected elevated FGFR-1 in the myocardium of uremic rats. Whether increased levels of FGF-23 could bind nonselectively to this receptor remains undefined. We determined myocardial mRNA levels of this receptor in experimental uremia and found the highest FGFR-1 mRNA levels in untreated uremia (Figure 2e). Whether the reduced expression, albeit not statistically significant, observed with Pc and E treatments is, in part, related to improved renal function in the remnant kidney model deserves further study.
We conclude that cardiac hypertrophy in experimental uremia is associated with upregulated RAS and FGFR-1 genes in the myocardium and that treatments with the VDR activator Pc or the ACE inhibitor E correct the overexpression of these genes and ameliorate cardiac hypertrophy. These findings provide further insight on the cardioprotective properties of Pc and E observed in patients with CKD.
DISCLOSURE
All authors declared no conflict of interest.
ACKNOWLEDGMENT
This work was partly supported by funding by a grant from the Fondo Nacional de Ciencia y Tecnologia (FONACIT) grant 2005000283, Venezuela (to B.R.I.), by National Institutes of Health grants DK073183 and HL085793 (to Y.C.L.), and from Clinical Practice Funds (to M.F.).
REFERENCES
- 1. Foley RN, Parfrey PS, Harnett JD, Kent GM, Martin CJ, Murray DC, Barre PE. Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int 1995; 47:186–192 [DOI] [PubMed] [Google Scholar]
- 2. Shroff R, Weaver DJ, Jr, Mitsnefes MM. Cardiovascular complications in children with chronic kidney disease. Nat Rev. Nephrol 2011; 7:642–649 [DOI] [PubMed] [Google Scholar]
- 3. London GM. Left ventricular alterations and end-stage renal disease. Nephrol Dial Transplant 2002; 17(Suppl):29–36 [DOI] [PubMed] [Google Scholar]
- 4. Husain K, Ferder L, Mizobuchi M, Finch J, Slatopolsky E. Combination therapy with paricalcitol and enalapril ameliorates cardiac oxidative injury in uremic rats. Am J Nephrol 2009; 29:465–472 [DOI] [PubMed] [Google Scholar]
- 5. Siedlecki AM, Jin X, Muslin AJ. Uremic cardiac hypertrophy is reversed by rapamycin but not by lowering of blood pressure. Kidney Int 2009; 75:800–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tyralla K, Adamczak M, Benz K, Campean V, Gross ML, Hilgers KF, Ritz E, Amann K. High-dose enalapril treatment reverses myocardial fibrosis in experimental uremic cardiomyopathy. Plos One 2011; 6:e15287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Custódio MR, Koike MK, Neves KR, dos Reis LM, Graciolli FG, Neves CL, Batista DG, Magalhães AO, Hawlitschek P, Oliveira IB, Dominguez WV, Moysés RM, Jorgetti V. Parathyroid hormone and phosphorus overload in uremia: impact on cardiovascular system. Nephrol Dial Transplant 2012; 27:1437–1445 [DOI] [PubMed] [Google Scholar]
- 8. Li YC. Vitamin D: roles in renal and cardiovascular protection. Curr Opin Nephrol Hypertens 2012; 21:72–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mizobuchi M, Nakamura H, Tokumoto M, Finch J, Morrissey J, Liapis H, Slatopolsky E. Myocardial effects of VDR activators in renal failure. J Steroid Biochem Mol Biol 2010; 121(1–2):188–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Helvig CF, Cuerrier D, Hosfield CM, Ireland B, Kharebov AZ, Kim JW, Ramjit NJ, Ryder K, Tabash SP, Herzenberg AM, Epps TM, Petkovich M. Dysregulation of renal vitamin D metabolism in the uremic rat. Kidney Int 2010; 78:463–472 [DOI] [PubMed] [Google Scholar]
- 11. Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, Andress DL. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int 2007; 71:31–38 [DOI] [PubMed] [Google Scholar]
- 12. Teng M, Wolf M, Lowrie E, Ofsthun N, Lazarus JM, Thadhani R. Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N Engl J Med 2003; 349:446–456 [DOI] [PubMed] [Google Scholar]
- 13. Kim HW, Park CW, Shin YS, Kim YS, Shin SJ, Kim YS, Choi EJ, Chang YS, Bang BK. Calcitriol regresses cardiac hypertrophy and QT dispersion in secondary hyperparathyroidism on hemodialysis. Nephron Clin Pract 2006; 102:c21–29 [DOI] [PubMed] [Google Scholar]
- 14. Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest 2002; 110:229–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhou C, Lu F, Cao K, Xu D, Goltzman D, Miao D. Calcium-independent and 1,25(OH)2D3-dependent regulation of the renin-angiotensin system in 1alpha-hydroxylase knockout mice. Kidney Int 2008; 74:170–179 [DOI] [PubMed] [Google Scholar]
- 16. Kong J, Kim GH, Wei M, Sun T, Li G, Liu SQ, Li X, Bhan I, Zhao Q, Thadhani R, Li YC. Therapeutic effects of vitamin D analogs on cardiac hypertrophy in spontaneously hypertensive rats. Am J Pathol 2010; 177:622–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yuan W, Pan W, Kong J, Zheng W, Szeto FL, Wong KE, Cohen R, Klopot A, Zhang Z, Li YC. 1,25-dihydroxyvitamin D3 suppresses renin gene transcription by blocking the activity of the cyclic AMP response element in the renin gene promoter. J Biol Chem 2007; 282:29821–29830 [DOI] [PubMed] [Google Scholar]
- 18. Fryer RM, Rakestraw PA, Nakane M, Dixon D, Banfor PN, Koch KA, Wu-Wong JR, Reinhart GA. Differential inhibition of renin mRNA expression by paricalcitol and calcitriol in C57/BL6 mice. Nephron Physiol 2007; 106:76–81 [DOI] [PubMed] [Google Scholar]
- 19. Freundlich M, Quiroz Y, Zhang Z, Zhang Y, Bravo Y, Weisinger JR, Li YC, Rodriguez-Iturbe B. Suppression of renin-angiotensin gene expression in the kidney by paricalcitol. Kidney Int 2008; 74:1394–1402 [DOI] [PubMed] [Google Scholar]
- 20. Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev 2006; 86:747–803 [DOI] [PubMed] [Google Scholar]
- 21. Wollert KC, Drexler H. The renin-angiotensin system and experimental heart failure. Cardiovasc Res 1999; 43:838–849 [DOI] [PubMed] [Google Scholar]
- 22. Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T. Targeted ablation of FgF23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest 2004; 113:561–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. De Borst MH, Vervloet MG, ter Wee PM, Navis G. Cross talk between the renin-angiotensin-aldosterone system and vitamin D-FGF-23-klotho in chronic kidney disease. J Am Soc Nephrol 2011; 22:1603–1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gutiérrez OM, Januzzi JL, Isakova T, Laliberte K, Smith K, Collerone G, Sarwar A, Hoffmann U, Coglianese E, Christenson R, Wang TJ, deFilippi C, Wolf M. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation 2009; 119:2545–2552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Seeherunvong W, Abitbol CL, Chandar J, Rusconi P, Zilleruelo GE, Freundlich M. Fibroblast growth factor 23 and left ventricular hypertrophy in children on dialysis. Pediatr Nephrol 2012; 27:2129–2136 [DOI] [PubMed] [Google Scholar]
- 26. Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutiérrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro-O M, Kusek JW, Keane MG, Wolf M. FGF23 induces left ventricular hypertrophy. J Clin Invest 2011; 121:4393–4408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Saji F, Shiizaki K, Shimada S, Okada T, Kunimoto K, Sakaguchi T, Hatamura I, Shigematsu T. Regulation of fibroblast growth factor 23 production in bone in uremic rats. Nephron Physiol 2009; 111:61–68 [DOI] [PubMed] [Google Scholar]
- 28. Slatopolsky E, Cozzolino M, Lu Y, Finch J, Dusso A, Staniforth M, Wein Y, Webster J. Efficacy of 19-Nor-1,25-(OH)2D2 in the prevention and treatment of hyperparathyroid bone disease in experimental uremia. Kidney Int 2003; 63:2020–2027 [DOI] [PubMed] [Google Scholar]
- 29. Seeherunvong W, Nwobi O, Abitbol CL, Chandar J, Strauss J, Zilleruelo G. Paricalcitol versus calcitriol treatment for hyperparathyroidism in pediatric hemodialysis patients. Pediatr Nephrol 2006; 21:1434–1439 [DOI] [PubMed] [Google Scholar]
- 30. Mizobuchi M, Morrissey J, Finch JL, Martin DR, Liapis H, Akizawa T, Slatopolsky E. Combination therapy with an angiotensin-converting enzyme inhibitor and a vitamin D analog suppresses the progression of renal insufficiency in uremic rats. J Am Soc Nephrol 2007; 18:1796–1806 [DOI] [PubMed] [Google Scholar]
- 31. Amman K, Tyralla K, Gross ML, Schwarz U, Törnig J, Haas CS, Ritz E, Mall G. Cardiomyocyte loss in experimental renal failure: prevention by ramipril. Kidney Int 2003; 63:1708–1713 [DOI] [PubMed] [Google Scholar]
- 32. Ritchie RH, Rosenkranz AC, Kaye DM. B-type natriuretic peptide: endogenous regulator of myocardial structure, biomarker and therapeutic target. Curr Mol Med 2009; 9:814–825 [DOI] [PubMed] [Google Scholar]
- 33. Törnig J, Amann K, Ritz E, Nichols C, Zeier M, Mall G. Arteriolar wall thickening, capillary rarefaction and interstitial fibrosis in the heart of rats with renal failure: the effects of ramipril, nifedipine and moxonidine. J Am Soc Nephrol 1996; 7:667–675 [DOI] [PubMed] [Google Scholar]
- 34. Thadhani R, Appelbaum E, Pritchett Y, Chang Y, Wenger J, Tamez H, Bhan I, Agarwal R, Zoccali C, Wanner C, Lloyd-Jones D, Cannata J, Thompson BT, Andress D, Zhang W, Packham D, Singh B, Zehnder D, Shah A, Pachika A, Manning WJ, Solomon SD. Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: the PRIMO randomized controlled trial. JAMA 2012; 307:674–684 [DOI] [PubMed] [Google Scholar]
- 35. Bodyak N, Ayus JC, Achinger S, Shivalingappa V, Ke Q, Chen YS, Rigor DL, Stillman I, Tamez H, Kroeger PE, Wu-Wong RR, Karumanchi SA, Thadhani R, Kang PM. Activated vitamin D attenuates left ventricular abnormalities induced by dietary sodium in Dahl salt-sensitive animals. Proc Natl Acad Sci U S A 2007; 104:16810–16815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Passier RC, Smits JF, Verluyten MJ, Daemen MJ. Expression and localization of renin and angiotensinogen in rat heart after myocardial infarction. Am J Physiol 1996; 271(3 Pt 2):H1040–1048 [DOI] [PubMed] [Google Scholar]
- 37. Chen S, Gardner DG. Liganded vitamin D receptor displays anti-hypertrophic activity in the murine heart. J Steroid Biochem Mol Biol 2013; 136:150–155 [DOI] [PubMed] [Google Scholar]
- 38. Li Y, Takemura G, Okada H, Miyata S, Maruyama R, Esaki M, Kanamori H, Li L, Ogino A, Ohno T, Kondo T, Nakagawa M, Minatoguchi S, Fujiwara T, Fujiwara H. Molecular signaling mediated by angiotensin II type 1A receptor blockade leading to attenuation of renal dysfunction-associated heart failure. J Card Fail 2007; 13:155–162 [DOI] [PubMed] [Google Scholar]