Abstract
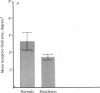
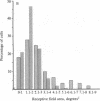
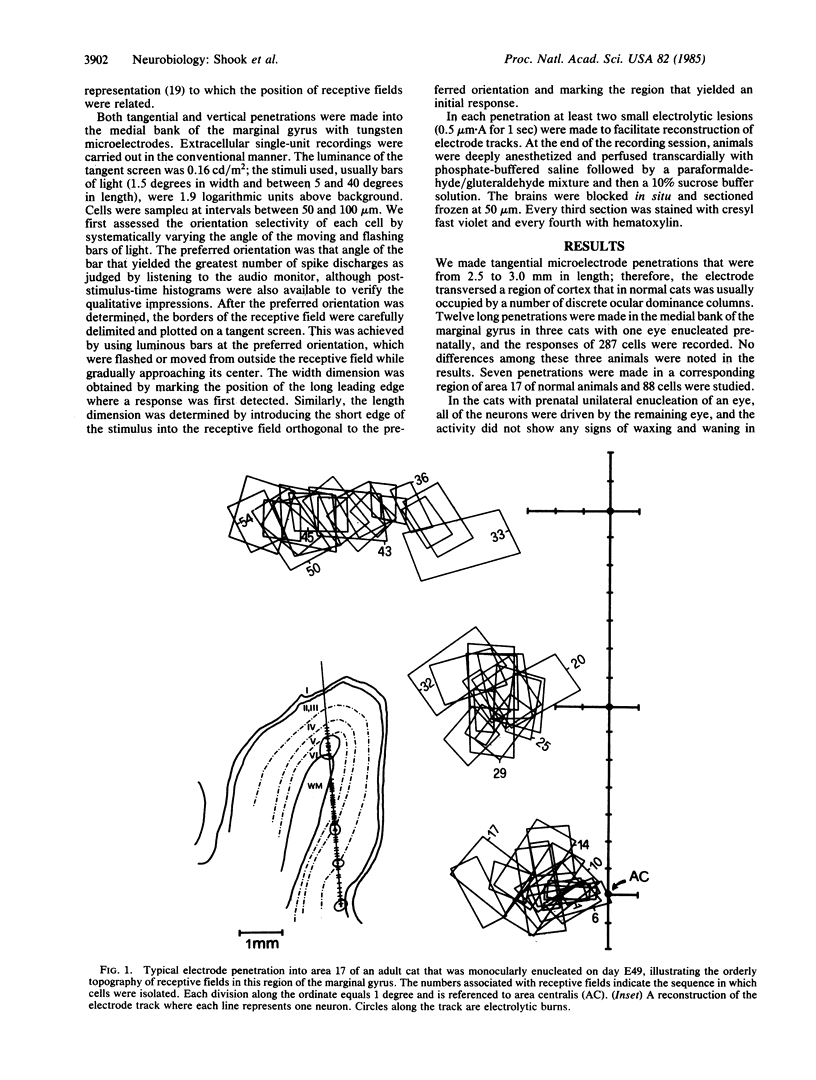
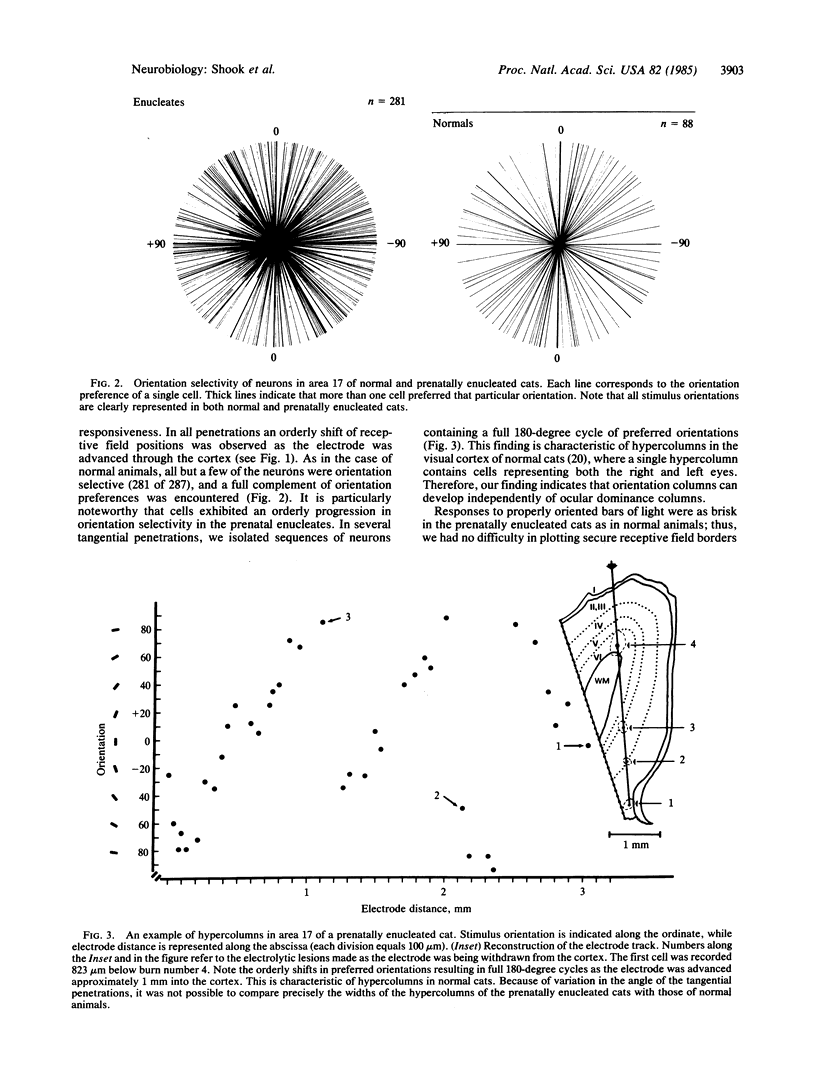
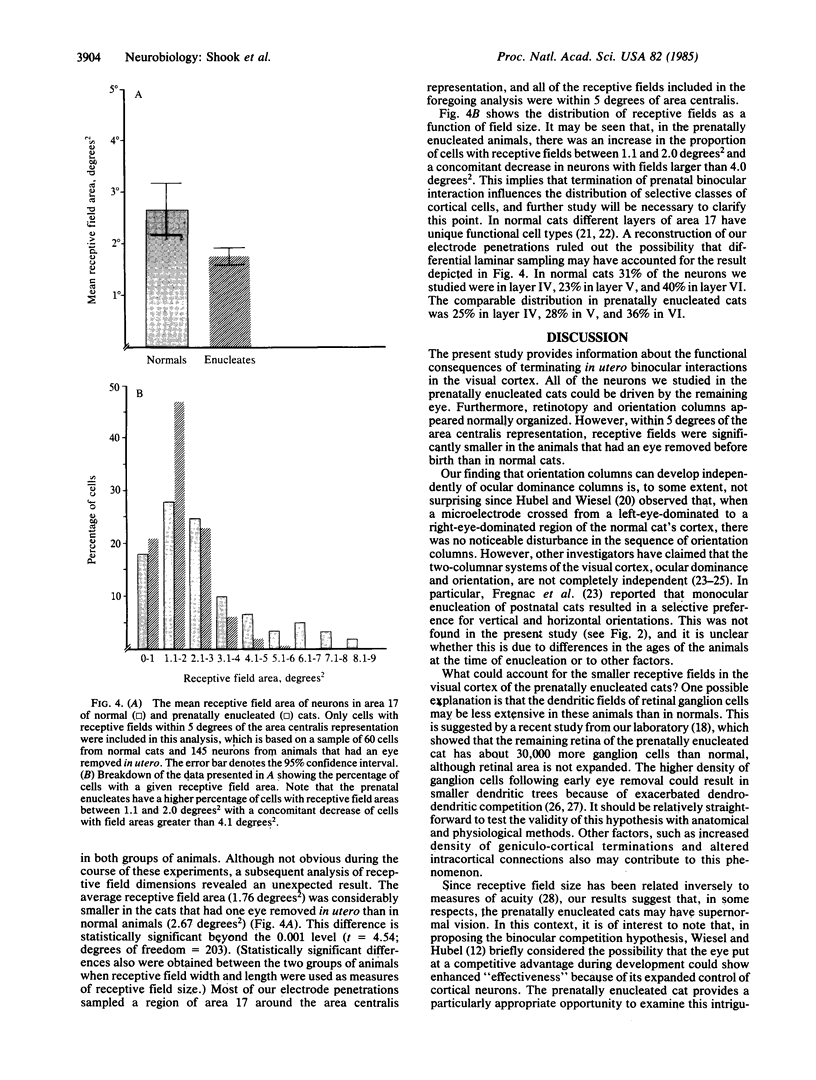
The functional consequences of interrupting in utero binocular interactions were studied by recording from single cells in area 17 of adult cats that had one eye removed at least 2 wk before birth. In these animals all cortical neurons could be driven by the remaining eye, and in tangential microelectrode penetrations, sequences of neurons containing a full 180-degree cycle of preferred orientations were encountered. Other response properties of cortical neurons in the prenatally enucleated animals were also normal with the notable exception that the dimensions of receptive fields were significantly smaller when compared with those of control animals. Our results indicate that orientation columns in the visual cortex can develop independently of ocular dominance columns, and they suggest that interruption of binocular interactions during prenatal development of the visual pathways may enhance the resolving power of the remaining eye.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BISHOP P. O., KOZAK W., VAKKUR G. J. Some quantitative aspects of the cat's eye: axis and plane of reference, visual field co-ordinates and optics. J Physiol. 1962 Oct;163:466–502. doi: 10.1113/jphysiol.1962.sp006990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalupa L. M., Williams R. W. Organization of the cat's lateral geniculate nucleus following interruption of prenatal binocular competition. Hum Neurobiol. 1984;3(2):103–107. [PubMed] [Google Scholar]
- Frégnac Y., Trotter Y., Bienenstock E., Buisseret P., Gary-Bobo E., Imbert M. Effect of neonatal unilateral enucleation on the development of orientation selectivity in the primary visual cortex of normally and dark-reared kittens. Exp Brain Res. 1981;42(3-4):453–466. doi: 10.1007/BF00237510. [DOI] [PubMed] [Google Scholar]
- Gilbert C. D. Laminar differences in receptive field properties of cells in cat primary visual cortex. J Physiol. 1977 Jun;268(2):391–421. doi: 10.1113/jphysiol.1977.sp011863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert C. D., Wiesel T. N. Morphology and intracortical projections of functionally characterised neurones in the cat visual cortex. Nature. 1979 Jul 12;280(5718):120–125. doi: 10.1038/280120a0. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson A. E., Hunt S. P., Wu J. Y. Immunocytochemical localization of glutamic acid decarboxylase in monkey striate cortex. Nature. 1981 Aug 13;292(5824):605–607. doi: 10.1038/292605a0. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Laminar and columnar distribution of geniculo-cortical fibers in the macaque monkey. J Comp Neurol. 1972 Dec;146(4):421–450. doi: 10.1002/cne.901460402. [DOI] [PubMed] [Google Scholar]
- LeVay S., Gilbert C. D. Laminar patterns of geniculocortical projection in the cat. Brain Res. 1976 Aug 20;113(1):1–19. doi: 10.1016/0006-8993(76)90002-0. [DOI] [PubMed] [Google Scholar]
- LeVay S., Hubel D. H., Wiesel T. N. The pattern of ocular dominance columns in macaque visual cortex revealed by a reduced silver stain. J Comp Neurol. 1975 Feb 15;159(4):559–576. doi: 10.1002/cne.901590408. [DOI] [PubMed] [Google Scholar]
- LeVay S., Stryker M. P., Shatz C. J. Ocular dominance columns and their development in layer IV of the cat's visual cortex: a quantitative study. J Comp Neurol. 1978 May 1;179(1):223–244. doi: 10.1002/cne.901790113. [DOI] [PubMed] [Google Scholar]
- LeVay S., Wiesel T. N., Hubel D. H. The development of ocular dominance columns in normal and visually deprived monkeys. J Comp Neurol. 1980 May 1;191(1):1–51. doi: 10.1002/cne.901910102. [DOI] [PubMed] [Google Scholar]
- Payne B. R., Berman N. Functional organization of neurons in cat striate cortex: variations in preferred orientation and orientation selectivity with receptive-field type, ocular dominance, and location in visual-field map. J Neurophysiol. 1983 Apr;49(4):1051–1072. doi: 10.1152/jn.1983.49.4.1051. [DOI] [PubMed] [Google Scholar]
- Perry V. H., Linden R. Evidence for dendritic competition in the developing retina. Nature. 1982 Jun 24;297(5868):683–685. doi: 10.1038/297683a0. [DOI] [PubMed] [Google Scholar]
- Rakic P. Development of visual centers in the primate brain depends on binocular competition before birth. Science. 1981 Nov 20;214(4523):928–931. doi: 10.1126/science.7302569. [DOI] [PubMed] [Google Scholar]
- Rakic P. Prenatal development of the visual system in rhesus monkey. Philos Trans R Soc Lond B Biol Sci. 1977 Apr 26;278(961):245–260. doi: 10.1098/rstb.1977.0040. [DOI] [PubMed] [Google Scholar]
- Rakic P. Prenatal genesis of connections subserving ocular dominance in the rhesus monkey. Nature. 1976 Jun 10;261(5560):467–471. doi: 10.1038/261467a0. [DOI] [PubMed] [Google Scholar]
- Rakic P., Riley K. P. Regulation of axon number in primate optic nerve by prenatal binocular competition. Nature. 1983 Sep 8;305(5930):135–137. doi: 10.1038/305135a0. [DOI] [PubMed] [Google Scholar]
- Shatz C. J. The prenatal development of the cat's retinogeniculate pathway. J Neurosci. 1983 Mar;3(3):482–499. doi: 10.1523/JNEUROSCI.03-03-00482.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel T. N., Hubel D. H., Lam D. M. Autoradiographic demonstration of ocular-dominance columns in the monkey striate cortex by means of transneuronal transport. Brain Res. 1974 Oct 18;79(2):273–279. doi: 10.1016/0006-8993(74)90416-8. [DOI] [PubMed] [Google Scholar]
- Williams R. W., Bastiani M. J., Chalupa L. M. Loss of axons in the cat optic nerve following fetal unilateral enucleation: an electron microscopic analysis. J Neurosci. 1983 Jan;3(1):133–144. doi: 10.1523/JNEUROSCI.03-01-00133.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. W., Chalupa L. M. Development of the retinal pathway to the pretectum of the cat. Neuroscience. 1983 Dec;10(4):1249–1267. doi: 10.1016/0306-4522(83)90111-2. [DOI] [PubMed] [Google Scholar]
- Williams R. W., Chalupa L. M. Prenatal development of retinocollicular projections in the cat: an anterograde tracer transport study. J Neurosci. 1982 May;2(5):604–622. doi: 10.1523/JNEUROSCI.02-05-00604.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wässle H., Peichl L., Boycott B. B. Dendritic territories of cat retinal ganglion cells. Nature. 1981 Jul 23;292(5821):344–345. doi: 10.1038/292344a0. [DOI] [PubMed] [Google Scholar]