Abstract
Background & objectives:
Patients with diabetes and vitamin-D insufficiency have increased insulin resistance. Similar observations among individuals with prediabetes are not well documented. The aim of this study was to find the occurrence of vitamin-D insufficiency/deficiency among individuals with prediabetes and to evaluate the relationship between vitamin-D status and insulin resistance.
Methods:
One hundred fifty seven individuals with prediabetes who fulfilled all the inclusion and exclusion criteria underwent clinical examination, anthropometric measurements (waist circumference, waist-hip ratio, waist-height ratio) and blood sampling after overnight fast for estimation of fasting blood glucose, fasting insulin, 25(OH)vitamin-D, intact parathyroid hormone (iPTH) and lipid profile. One hour post 75 g glucose (1hPG) blood glucose during oral glucose tolerance test was measured.
Results:
Vitamin-D deficiency/insufficiency was found in 115 (73.25%) individuals with prediabetes. Severe vitamin-D deficiency (<10 ng/ml) was seen in 14.65 per cent individuals. Individuals with the lowest vitamin-D levels (<10 ng/ml) had the highest insulin resistance (HOMA2-IR: 2.04 ± 0.67). Serum 25(OH)D had a statistically significant inverse correlation with insulin resistance (HOMA2-IR; r=-0.33; P=0.008), and positive correlation with insulin sensitivity (QUICKI; r=0.39; P=0.002), after adjusting for BMI and HbA1c. There was no correlation between vitamin-D status and estimated beta cell mass (HOMA-β). The mean waist-height ratio among individuals with prediabetes was 0.57 (normal<0.5) indicating a high risk of cardiovascular morbidity. Individuals with elevated 1hPG>155 mg/dl had significantly higher BMI and worse insulin resistance, and 1hPG correlated well with 2 hour post glucose blood glucose (r=0.57; P<0.001).
Interpretations & conclusions:
Vitamin-D deficiency/insufficiency may have some role in the development/worsening of insulin resistance in individuals with prediabetes in our country who have a high cardiovascular risk. Prospective studies on a large group of individuals need to be done to confirm the findings.
Keywords: Deficiency, insufficiency, insulin resistance, one hour post glucose blood glucose, prediabetes, vitamin-D
Individuals with impaired fasting glucose (IFG) and/or impaired glucose tolerance (IGT) are referred to as having prediabetes1. The annual risk of progression to overt diabetes from IGT ranged from 2.5 per cent in the Diabetes Prevention Trial (DPT)2, 11.5 per cent in the Chinese diabetes prevention study (CDPS)3 to 18 per cent in the Indian Diabetes Prevention Programme-1 (IDPP-1)4. Prediabetes is frequently associated with obesity and other components of metabolic syndrome. Obesity in turn is commonly associated with hypovitaminosis-D due to the capacity of adipose tissue to store 25-hydroxy vitamin-D [25(OH)D] making it biologically unavailable5. A decreased amount of serum 25(OH)D, calcitriol [1,25(OH)2D] and raised parathyroid hormone (PTH) can increase intracellular calcium in adipocytes, which can stimulate lipogenesis predisposing a patient to further weight gain and thus increasing the risk of diabetes6. In patients with diabetes, administration of vitamin-D has been shown to decrease insulin resistance7. However similar observation among individuals with prediabetes has not been documented. The aim of this study was, therefore, to find the presence of vitamin-D insufficiency/ deficiency and to study the relationship between insulin resistance and vitamin-D status among individuals with prediabetes in eastern India.
Material & Methods
Family members of consecutive patients of diabetes attending diabetic clinic of the department of Endocrinology and Metabolism, Institute of Postgraduate Medical Education & Research, (IPGMER) and SSKM hospital, Kolkata, West Bengal, India, and individuals attending the health camps conducted by the department across the city of Kolkata from August 2010 to March 2012, who gave consent were screened by finger prick blood glucose estimation using a glucometer (One Touch Ultra, Johnson & Johnson, India). Individuals with fasting blood glucose between 100-125 mg/dl or post-prandial / random blood glucose between 140-199 mg/dl were invited to the department for 75 g oral glucose tolerance test (OGTT) with blood glucose estimated at fasting, 1 and 2 h post glucose (2hPG). Individuals with IFG (100-125mg/dl) or IGT (2hPG: 140-199 mg/dl) were called for a second OGTT within a week. Only individuals 30-80 yr of age with persistent IFG or IGT over 2 OGTTs were considered for the study. It has been reported that 1 h post glucose (1hPG) blood glucose >155 mg/dl is a strong predictor for future risk of T2D8. Hence, the relation of 1hPG blood glucose levels with vitamin-D status and insulin resistance was also studied in individuals with prediabetes. The study protocol was explained to the individuals and only those who gave informed written consent were included in the study. Individuals with history of any oral antidiabetic medications or insulin use were excluded. Also individuals with associated disorders like primary hyperparathyroidism, chronic kidney disease, liver disease, any chronic illness, malignancy, chronic drug use like antiepileptic agents, oral contraceptive pills, steroids which are likely to interfere with vitamin-D metabolism, were excluded. Individuals with a history of calcium or vitamin-D supplementation in the last one year were excluded (Figure). The study protocol was approved by the institutional ethics committee.
Fig.
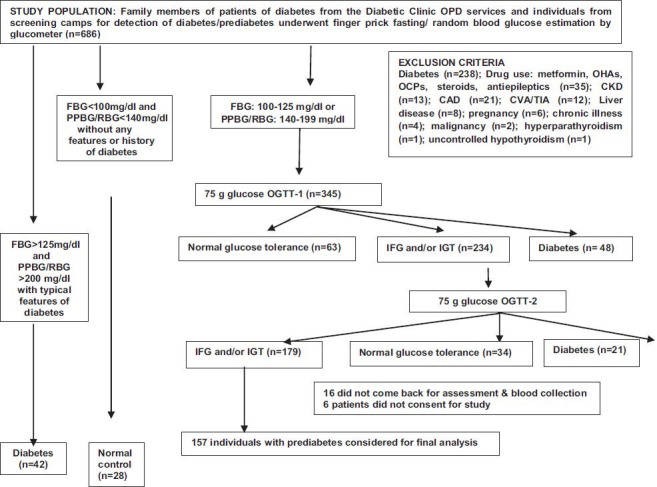
Flowchart elaborating the study protocol. FBG, fasting blood glucose; PPBG, 2 hour post-prandial blood glucose; RBG, random blood glucose; OGTT, oral glucose tolerance test; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; OHA, oral hypoglycaemic agents; OCP, oral contraceptive pill; CKD, chronic kidney disease; CAD, coronary artery disease; CVA, cerebrovascular accident; TIA, transient ischaemic attack.
Forty two newly diagnosed treatment naive T2D patients attending the Diabetic Clinic and 28 individuals with normal glucose tolerance (NGT) on 75 g OGTT, who fulfilled the inclusion and exclusion criteria and gave informed written consent, were also included in the study to compare the outcomes seen in individuals with prediabetes.
The included individuals were given an appointment to attend the OPD services after a 12 h fast. Height (to ± 0.1 cm) was measured in all the individuals using a Charder HM200PW wall-mounted stadiometer [calibrated using a 36” calibration rod (Perspective Enterprise, Portage, Michigan, USA)], and body weight (to ± 100 g) measured using an electronic calibrated scale (Tantia, Japan, Model-HA521, Lot number-860525). Waist circumference (WC), hip circumference (HC) were measured in all patients and waist-hip raio (WHR) and waist-height ratio (WHtR) were calculated as a measure of truncal obesity9. All individuals underwent detailed clinical examination. Blood samples (10 ml) were collected, serum separated and stored at -20°C.
Serum insulin was estimated using solid phase, enzyme labelled chemiluminescent immunometric assay (Immulite 1000, Siemens, Gwynedd, UK). The analytical sensitivity of the insulin assay was 2 microIU/ml with a range of 2-300 microIU/ml. The coefficient of variability (CV) of the assay ranged from 5.9-8 per cent. Serum 25(OH)D was estimated using chemiluminisent microparticle immunoassay (Architect 25-OH vitamin-D assay, Abbott, USA). The analytical sensitivity of the assay was 4ng/ml with a range of 9.4 to 165.5 ng/ml. The CV of the assay ranged from 2.8-4.6 per cent. Plasma iPTH was estimated using solid phase, enzyme labelled chemiluminescent immunometric assay (Immunite 1000, Siemens, Gwynedd, UK). The analytical sensitivity of the assay was 4pg/ml with a range of 4-2500 pg/ml. The CV of the assay ranged from 2.8-3.4 per cent. Serum lipid profile, creatinine, HbA1c, calcium, phosphorus and alkaline phosphate were estimated in all patients using clinical chemistry analyzer (Daytona, serial number-58260536, Furuno Electric, Nishnomeya, Japan).
Based on the vitamin-D status, individual with prediabetes, diabetes and normal controls were divided into four groups viz. vitamin-D sufficiency [25(OH)D ≥30 ng/ml], vitamin-D insufficiency [25(OH)D: 20-30 ng/ml], mild vitamin-D deficiency [25(OH)D: 10-20 ng/ml] and severe vitamin-D deficiency [25(OH)D <10 ng/ml10.
Insulin resistance in basal state was calculated using HOMA2-IR (homeostatic model assessment-insulin resistance) and beta cell function was estimated using HOMA2-β. No linear formula is available and HOMA2 calculator was used for calculation. (http://www.dtu.ox.ac.uk)11. QUICKI (quantitative insulin sensitivity check index) is also a validated method for estimation of insulin resistance in obese and non obese diabetic and non diabetic subjects. (https://sasl.unibas.ch/11calculators-QUICKI.php) [QUICKI score= 1/ {log (Fasting Insulin) + log (Fasting Sugar)}]. QUICKI index correlates well with glucose clamp studies (r = 0.78) and values range between 0.45 for healthy individuals and 0.30 in diabetics12.
The prevalence of vitamin-D deficiency among individuals with prediabetes in India is not known. However, a study done in Delhi showed that as many as 90 per cent of the apparently healthy adults had vitamin-D deficiency13. It was calculated that at least 98 individuals of prediabetes were to be evaluated to keep the power of the study at 80 per cent and type-I error at 5 per cent.
Statistical analysis: Student's t test was used for analysis of continuous variables, Fisher's exact test for binary variables, and the χ2 test was used for categorical variables. One way ANOVA was used to study outcomes where three or more groups were present.
Results
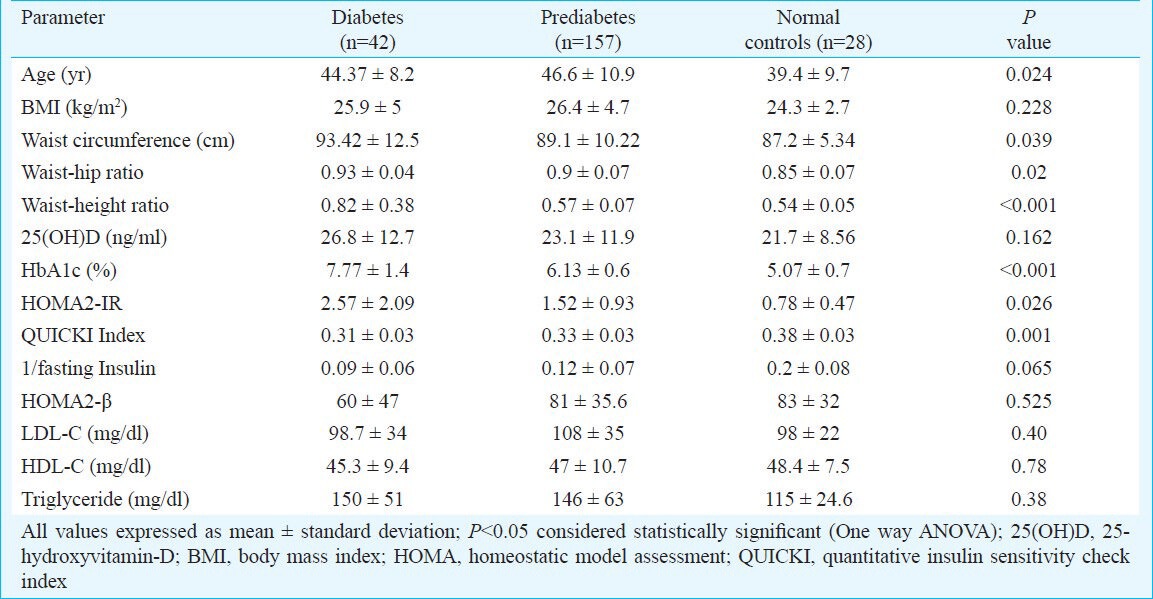
A total of 157 individuals of prediabetes along with 42 patients of diabetes and 28 normal individuals who fulfilled all the inclusion and exclusion criteria were included in the study (Figure). The presence of vitamin-D deficiency/ insufficiency was 73.25 per cent (n=115), 66.6 per cent (n=28) and 78.57 per cent (n=22) in individuals with prediabetes, diabetes and normal glucose tolerance (controls), respectively. Severe vitamin-D deficiency (<10 ng/ml) was seen in 14.65 per cent of individuals with prediabetes (n=23) and 7.14 per cent each among those in diabetes (n=3) and normal glucose tolerance groups (n=2) (Table I). Normal individuals were significantly (P<0.05) younger than individuals with prediabetes or diabetes (Table II). There was no difference in BMI among the groups. Patients with diabetes had the highest WC, WHR and WHtR as compared to individuals with prediabetes and normal individuals. Insulin resistance was significantly worse among patients with diabetes as compared to those with prediabetes or normal individuals (Table II).
Table I.
Vitamin-D status among individuals with prediabetes, diabetes and normal glucose tolerance
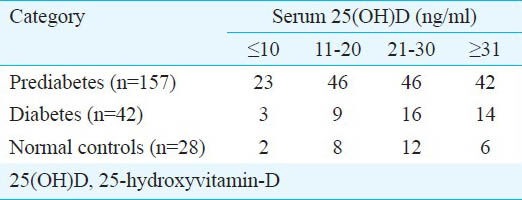
Table II.
Relationship between anthropometric parameters, insulin resistance and dyslipidaemia among individuals with diabetes, prediabetes and normal glucose tolerance
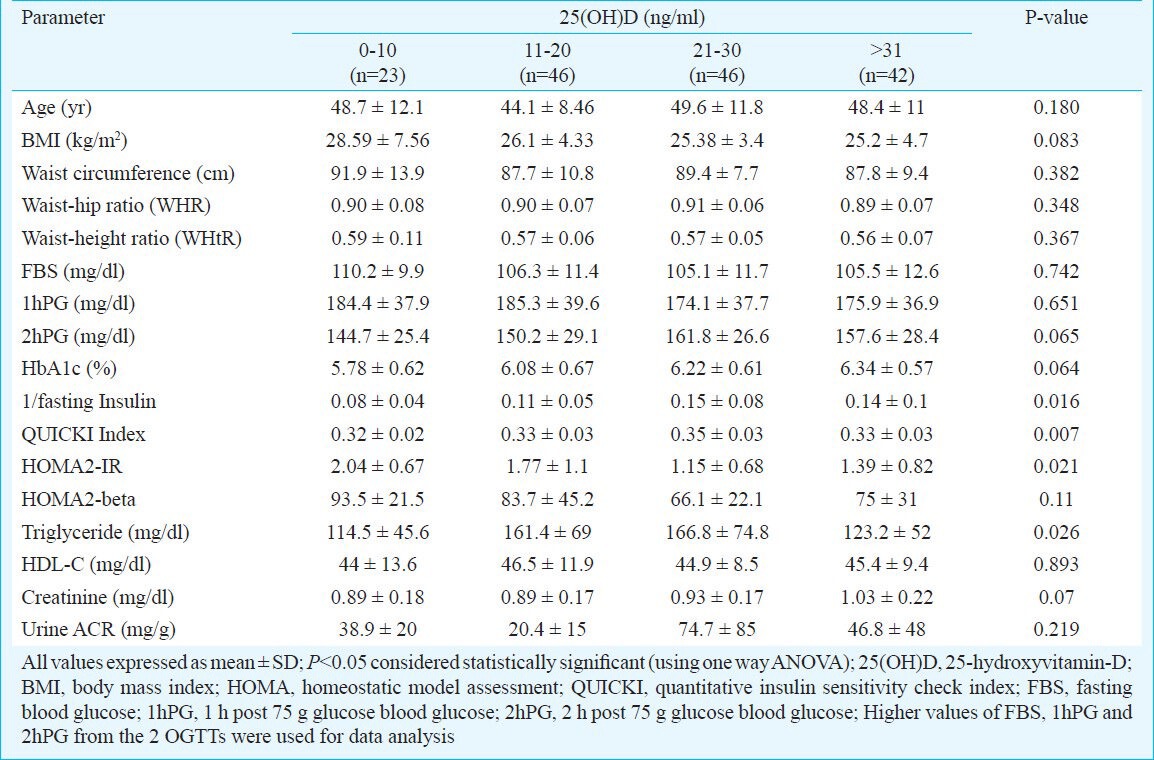
There was statistically significant difference in the insulin resistance among the 4 groups based on vitamin-D status, with individuals with severe vitamin-D deficiency (<10 ng/ml) having the highest insulin resistance (Table III). Individuals with vitamin-D insufficiency (21-30 ng/ml) had the highest triglyceride levels (Table III).
Table III.
Relationship between anthropometric parameters, insulin resistance, and dyslipidaemia in individuals of prediabetes with respect to their vitamin-D status
Serum 25(OH)D had a moderately strong inverse correlation with measures of insulin resistance (HOMA2-IR) and a positive correlation with measures of insulin sensitivity (QUICKI, 1/fasting insulin) among individuals with prediabetes, even after adjusting for BMI and HbA1c (Table IV). Similar correlations were not seen among individuals with diabetes and normal glycaemia due to the small number of individuals in each group.
Table IV.
Correlation between vitamin-D status and insulin resistance, systemic inflammation and dyslipidaemia in individuals with prediabetes
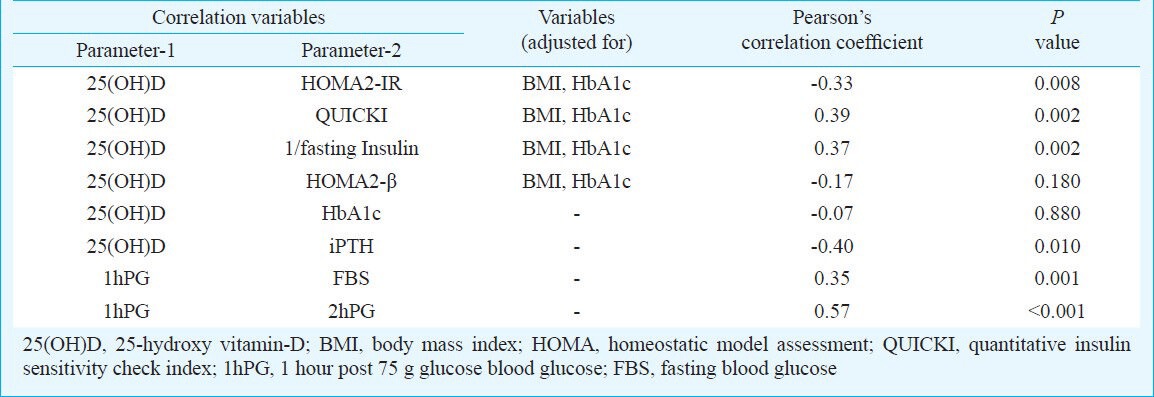
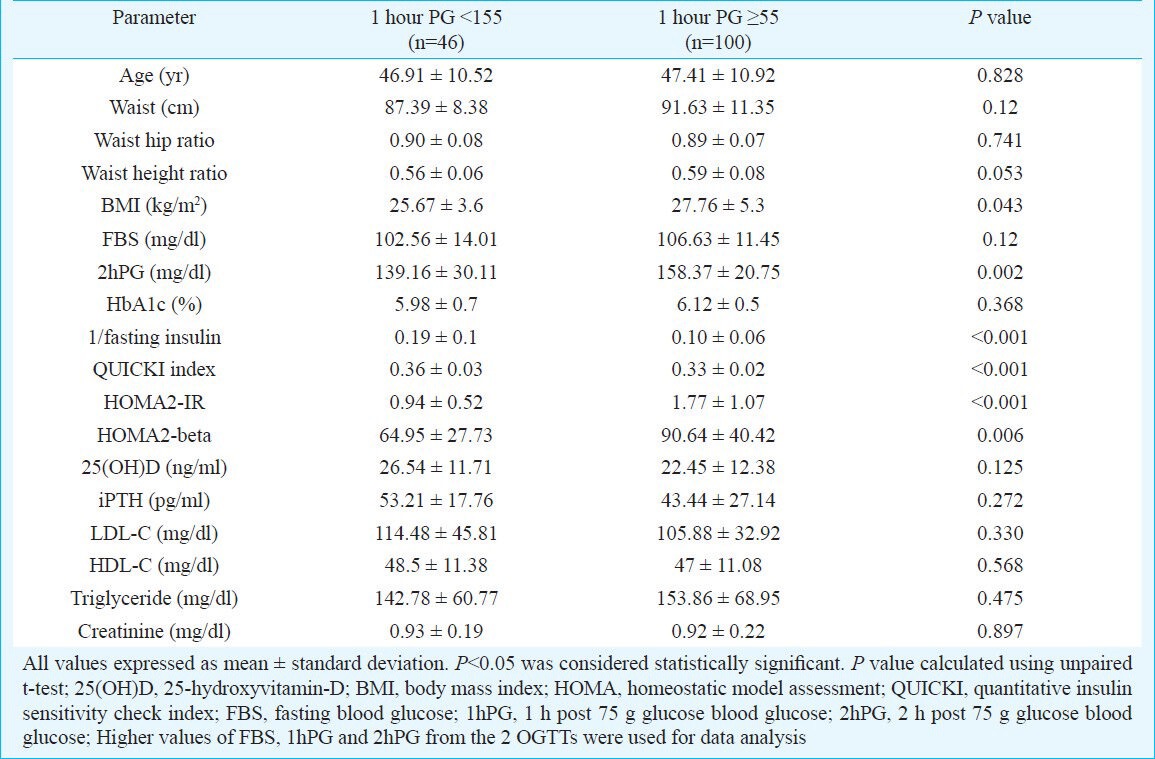
Among individuals with prediabetes, 1hPG blood glucose values were available in only 146 individuals. Of these, 100 (68.5%) individuals had 1hPG >155 mg/dl. Those with 1hPG ≥155 mg/dl had significantly higher BMI, 2hPG blood glucose and significantly worse measures of insulin resistance, as compared to those with 1hPG <155 mg/dl (Table V). Prediabetes individuals with 1hPG <155 mg/dl had higher but statistically insignificant levels of serum vitamin-D (Table V). Further, 1hPG blood glucose had statistically significant positive correlation with FBS and 2hPG blood glucose (Table IV).
Table V.
Anthropometry, insulin resistance, vitamin-D levels, lipid parmeters in individuals of prediabetes with elevated 1 hour post glucose blood sugar (>155mg/dl) as compared to those having normal 1 hour post glucose sugar (≤155 mg/dl)
Discussion
Vitamin-D deficiency is a significant problem in our country as has been documented previously13. Our study showed that vitamin-D insufficiency/deficiency was common among individuals with prediabetes. Vitamin-D deficiency/insufficiency is associated with increased insulin resistance, systemic inflammation and HbA1c in patients of T2D which improved with vitamin-D supplementation7. However, the relation of vitamin-D status with insulin resistance has not been well studied among individuals with prediabetes. Ford et al14 in a study of 7904 individuals reported 25(OH)D to be inversely associated with metabolic syndrome14. In contrast, Reis et al15 and Wareham et al16 reported no association of 25(OH)D with metabolic syndrome or 2hPG respectively. This discordance in observations may be explained by the differences in race, BMI, blood sugars, sunlight exposure and obesity15,16. In our study, among individuals with prediabetes, those having severe vitamin D deficiency (<10 ng/ml), had the worst insulin resistance (HOMA2-IR, QUICKI and 1/fasting insulin) as compared to those having higher levels, with an inverse correlation between vitamin-D status and insulin resistance after adjusting for BMI and glycaemia (HbA1c). Adjustment for BMI was necessary as vitamin-D is predominantly stored in the adipose tissue and individuals with higher BMI tend to have lower serum vitamin-D. Vitamin-D deficiency/insufficiency thus may have some role in the development/worsening of insulin resistance in individuals with prediabetes in our country. Individuals with prediabetes and elevated 1hPG have been shown to have increased risk of progression to diabetes8. In our study prediabetes individuals with 1hPG ≥155mg/dl had significantly higher BMI and insulin resistance. They also had lower serum vitamin-D but not statistically significant. The cross-sectional nature of our study is one of the major limitations and hence the association between insulin resistance and vitamin-D status in prediabetes individuals cannot be commented upon. Longitudinal prospective studies are warrented to assess whether this worsened insulin resistance in prediabetes individuals with lower vitamin-D actually results in increased progression to diabetes. The current data on interventional studies of vitamin-D in prediabetes are hampered by the extremely small number of patients and use of variable preparations and dosages of vitamin-D or calcitriol. Most of the studies evaluated the outcomes over short periods of two weeks to three months, with most of them showing a lack of significant improvement in glycaemic and insulin resistance outcomes17,18,19,20.
Scragg et al21 reported 25(OH)D to be significantly lower in individuals with newly diagnosed IGT or diabetes as compared to normal individuals. In our study vitamin-D levels were not significantly different in individuals with prediabetes as compared to those with diabetes or normoglycemia. This observation is limited by the small number of individuals in the diabetes and normoglycaemia group.
Patients with diabetes had the worst waist circumference (WC), WHR and WHtR in our study as compared to prediabetes and normal controls, inspite of having a comparable BMI. Increased WC, WHR and WHtR are markers of visceral adiposity. These increased WC, WHR and WHtR may contribute to the dysglycaemia in patients with diabetes. WHtR is considered to be the best predictor of cardiovascular (CV) morbidity and mortality and is believed to be superior to WHR and waist circumference22. BMI is considered to be a poor predictor of CV morbidity21. For people under 40, a WHtR of over 0.5 is critical for increased CV morbidity; for people in the age group between 40 and 50 the critical value is between 0.5 and 0.6, and for people over 50 the critical value starts at 0.6, in terms of prediction of risk for heart attack, stroke and all cause mortality22,23,24. The mean WHtR among individuals with diabetes, prediabetes and normoglycaemia in our study was 0.82, 0.57 and 0.54, respectively indicating the high risk of CV events across the spectrum of glycaemia among Indians. Among individuals with prediabetes, those with the lowest serum 25(OH)D had the highest WHtR but not statistically significant.
In our study, 1hPG blood glucose value had a positive correlation with both FBS and 2hPG blood glucose values. Hence, 1hPG blood glucose can be a useful measure of severity of hyperglycaemia. Further studies are warranted in large groups of individuals before routine use of 1hPG can be advocated in clinical practice especially as a replacement for 2hPG values, which would in turn decrease waiting time for patients undergoing OGTT and thus ensure better patient compliance.
To summarize, vitamin-D deficiency/insufficiency is common among individuals with prediabetes in our country, a reflection of the vitamin-D deficiency state in the general population. However, this vitamin-D deficiency/insufficiency was associated with worsened insulin resistance in individuals with prediabetes. One hour post glucose blood glucose levels can be a useful measure of hyperglycaemia especially among individuals with prediabetes. In view of the worsening diabetic epidemic in India and the associated high risk of cardiovascular morbidity in normal individuals, those with prediabetes and diabetes (as evidenced by WHtR), urgent prospective studies are needed to evaluate whether supplementation of vitamin-D and correction of deficiency/insufficiency delays the development of diabetes.
References
- 1.Executive summary: Standards of medical care in diabetes-2010. Diabetes Care. 2010;33(Suppl 1):S4–10. doi: 10.2337/dc10-S004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang W, Lin L, Qi J. The preventive effect of acarbose and metformin on the IGT population from becoming diabetes mellitus: a 3-year multi-centric prospective study. Chin J Endocrinol Metab. 2001;17:131–6. [Google Scholar]
- 4.Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V. Indian Diabetes Prevention Programme (IDPP). The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1) Diabetologia. 2006;49:289–97. doi: 10.1007/s00125-005-0097-z. [DOI] [PubMed] [Google Scholar]
- 5.Palomer X, Gonzalez-Clemente JM, Blanco-Vaca F, Mauricio D. Role of vitamin D in the pathogenesis of type 2 diabetes mellitus. Diabetes Obes Metab. 2008;10:185–97. doi: 10.1111/j.1463-1326.2007.00710.x. [DOI] [PubMed] [Google Scholar]
- 6.Takiishi T, Gysemans C, Bouillon R, Mathieu C. Vitamin D and diabetes. Endocrinol Metab Clin North Am. 2010;39:419–46. doi: 10.1016/j.ecl.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Cigolini M, Iagulli MP, Miconi V, Galiotto M, Lombardi S, Targher G. Serum 25-hydroxyvitamin D3 concentrations and prevalence of cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2006;29:722–4. doi: 10.2337/diacare.29.03.06.dc05-2148. [DOI] [PubMed] [Google Scholar]
- 8.Manco M, Panunzi S, Macfarlane DP, Golay A, Melander O, Konrad T, et al. Relationship between Insulin Sensitivity and Cardiovascular Risk (RISC) Consortium. One-hour plasma glucose identifies insulin resistance and beta-cell dysfunction in individuals with normal glucose tolerance: cross-sectional data from the Relationship between Insulin Sensitivity and Cardiovascular Risk (RISC) study. Diabetes Care. 2010;33:2090–7. doi: 10.2337/dc09-2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar S, Mukherjee S, Mukhopadhyay P, Pandit K, Raychaudhuri M, Sengupta N, et al. Prevalence of diabetes and impaired fasting glucose in a selected population with special reference to influence of family history and anthropometric measurements - The Kolkata policeman study. J Assoc Physicians India. 2008;56:841–4. [PubMed] [Google Scholar]
- 10.Holick MF. Vitamin D: Importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79:362–71. doi: 10.1093/ajcn/79.3.362. [DOI] [PubMed] [Google Scholar]
- 11.Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21:2191–2. doi: 10.2337/diacare.21.12.2191. [DOI] [PubMed] [Google Scholar]
- 12.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–10. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 13.Goswami R, Gupta N, Goswami D, Marwaha RK, Tandon N, Kochupillai N. Prevalence and significance of low 25- hydroxyvitamin D concentrations in healthy subjects in Delhi. Am J Clin Nutr. 2000;72:472–5. doi: 10.1093/ajcn/72.2.472. [DOI] [PubMed] [Google Scholar]
- 14.Ford ES, Ajani UA, McGuire LC, Liu S. Concentrations of serum vitamin D and the metabolic syndrome among U.S. adults. Diabetes Care. 2005;28:1228–30. doi: 10.2337/diacare.28.5.1228. [DOI] [PubMed] [Google Scholar]
- 15.Reis JP, von Mühlen D, Kritz-Silverstein D, Wingard DL, Barrett-Connor E. Vitamin D, parathyroid hormone levels, and the prevalence of metabolic syndrome in community-dwelling older adults. Diabetes Care. 2007;30:1549–55. doi: 10.2337/dc06-2438. [DOI] [PubMed] [Google Scholar]
- 16.Wareham NJ, Byrne CD, Carr C, Day NE, Boucher BJ, Hales CN. Glucose intolerance is associated with altered calcium homeostasis: A possible link between increased serum calcium concentration and cardiovascular disease mortality. Metabolism. 1997;46:1171–7. doi: 10.1016/s0026-0495(97)90212-2. [DOI] [PubMed] [Google Scholar]
- 17.Kotsa K, Yavropoulou MP, Anastasiou O, Yovos JG. Role of vitamin D treatment in glucose metabolism in polycystic ovary syndrome. Fertil Steril. 2009;92:1053–8. doi: 10.1016/j.fertnstert.2008.07.1757. [DOI] [PubMed] [Google Scholar]
- 18.Nagpal J, Pande JN, Bhartia A. A double-blind, randomized, placebo-controlled trial of the short-term effect of vitamin D3 supplementation on insulin sensitivity in apparently healthy, middle-aged, centrally obese men. Diabet Med. 2009;26:19–27. doi: 10.1111/j.1464-5491.2008.02636.x. [DOI] [PubMed] [Google Scholar]
- 19.Ljunghall S, Lind L, Lithell H, Skarfors E, Selinus I, Sorensen OH, et al. Treatment with one-alpha-hydroxycholecalciferol in middle-aged men with impaired glucose tolerance - a prospective randomized double-blind study. Acta Med Scand. 1987;222:361–7. doi: 10.1111/j.0954-6820.1987.tb10684.x. [DOI] [PubMed] [Google Scholar]
- 20.Lind L, Pollare T, Hvarfner A, Lithell H, Sorensen OH, Ljunghall S. Long-term treatment with active vitamin D (alphacalcidol) in middle-aged men with impaired glucose tolerance. Effects on insulin secretion and sensitivity, glucose tolerance and blood pressure. Diabetes Res. 1989;11:141–7. [PubMed] [Google Scholar]
- 21.Scragg R, Holdaway I, Singh V, Metcalf P, Baker J, Dryson E. Serum 25-hydroxyvitamin D3 levels decreased in impaired glucose tolerance and diabetes mellitus. Diabetes Res Clin Pract. 1995;27:181–8. doi: 10.1016/0168-8227(95)01040-k. [DOI] [PubMed] [Google Scholar]
- 22.Schneider Friedrich N, Klotsche J, Pieper L, Nauck M, John U, Dörr M, et al. The predictive value of different measures of obesity for incident cardiovascular events and mortality. J Clin Endocrinol Metab. 2010;95:1777–85. doi: 10.1210/jc.2009-1584. [DOI] [PubMed] [Google Scholar]
- 23.Lee CM, Huxley RR, Wildman RP, Woodward M. Indices of abdominal obesity are better discriminators of cardiovascular risk factors than BMI: a meta-analysis. J Clin Epidemiol. 2008;61:646–53. doi: 10.1016/j.jclinepi.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 24.Browning Lucy M, Hsieh SD, Ashwell A systematic review of waist-to-height ratio as a screening tool for the prediction of cardiovascular disease and diabetes: 0·5 could be a suitable global boundary value. Nutr Res Rev. 2010;23:247–69. doi: 10.1017/S0954422410000144. [DOI] [PubMed] [Google Scholar]


