Abstract
Background & objectives:
Abnormalities in thyroid hormonal status is common in major psychiatric disorders. Although the relevance of thyroid dysfunction to bipolar disorder is well-recognized, yet the association between thyroid dysfunction and schizophrenia-spectrum disorders is under-emphasized. The aim of this study was to examine and compare the rates of abnormal thyroid hormonal status in patients with schizophrenia-spectrum disorders and mood disorders in an inpatient tertiary care general hospital psychiatry unit.
Methods:
This was a retrospective hospital-based study on 468 inpatient samples. Data on serum thyroid stimulating hormone (TSH), T3 (triiodothyroxine), T4 (L-thyroxine), free unbound fractions of T3 and T4 (FT3 and FT4) were obtained from records of 343 patients, 18 patients were anti-TPO (anti thyroid peroxidase antibody) positive. The rates of abnormal thyroid hormonal status were compared using the chi square test.
Results:
Abnormal thyroid hormonal status in general, and presence of hypothyroidism and hyperthyroidism, in particular were seen in 29.3, 25.17 and 4.08 per cent patients with schizophrenia spectrum disorders, respectively. These were comparable to the rates in patients with mood disorders (23.24, 21.62 and 1.62%, respectively). Eleven of the 18 patients with antiTPO positivity had a schizophrenia-spectrum disorder. There were no gender differences.
Interpretation & conclusions:
Thyroid dysfunction was present in patients with schizophrenia-spectrum disorder as well as mood disorders. Autoimmune thyroid disease was more commonly seen in patients with schizophrenia-spectrum disorders compared to mood disorders. The findings reiterate the relevance of screening patients with schizophrenia-spectrum disorders for abnormal thyroid hormonal status.
Keywords: Autoimmune thyroiditis, hyperthyroidism, hypothyroidism, mood disorder, schizophrenia
The association between thyroid dysfunction and mood disorders is well recognized. The prevalence of mood and anxiety disorders is higher in patients with thyroid dysfunction1, thyroid status predicts treatment response in major depression and bipolar disorder2, augmentation with thyroid hormone has therapeutic efficacy in treatment-resistant depression3, and additionally, thyroid hormone receptors are localized to limbic structures implicated in regulation of mood4. The nature of the association between thyroid dysfunction and schizophrenia-spectrum disorders has, however, not been well studied. Several studies have revealed a high prevalence of thyroid dysfunction in patients with schizophrenia5,6,7. There are reports available on the association of autoimmune thyroid disorders with non-affective psychosis8,9,10.
It is noteworthy that studies which have shown an association between thyroid dysfunction and bipolar disorder (BPD) have tended to choose the disorder of their interest (namely BPD) and club the other psychiatric disorders together as a psychiatric ‘control group’11,12. The presence of very low prevalence rates in some diagnostic groups within such a heterogeneous ‘control group’ would conceivably lower the mean prevalence in the ‘control group’ and thus exaggerate the difference between the diagnosis of interest and the control group.
There is limited literature on the rates of thyroid dysfunction among patients with major psychiatric disorders in the Indian population. This study was carried out to examine and compare the level of thyroid dysfunction between patients with mood disorders and schizophrenia-spectrum disorders in a hospital-based inpatient sample. The term “thyroid dysfunction” refers to abnormalities in laboratory test parameters of thyroid hormonal status, namely serum TSH (thyroid stimulating hormone), T3 (triiodothyroxine), T4 (L-thyroxine), FT3 (Free ‘unbound’ fraction of T3), FT4 (Free ‘unbound’ fraction of T4).
Material & Methods
Study design: The retrospective study was conducted at St. Johns Medical College Hospital, Bangalore, a tertiary-care, general hospital adult psychiatric unit in July 2011 - December 2011 on patients admitted between January 2008 and December 2009. The study protocol was approved by the St. John's Medical College Hospital Institutional Ethics Review Board (IERB). The patients were assigned an ICD-1013 diagnosis based on a clinical interview and diagnostic consensus between two consultant psychiatrists. The data were obtained from electronic records, and information was obtained regarding diagnosis, age, gender, medication status, thyroid function tests (serum TSH, T3, T4, FT3, FT4) and thyroid antibody titres (anti-TPO levels). Since the vast number of admissions were of patients with substance-dependence and the focus of the analysis was to examine thyroid status in patients with schizophrenia-spectrum disorders and mood disorders, a random sample of 30 patients with substance use disorders was taken as a representative sample for the group. For the other diagnostic groups (dissociative disorder, panic disorder, and other anxiety disorders), the sampling was consecutive.
Thyroid function tests were done routinely for all patients during the first admission and on subsequent admissions, if there is a suspicion of thyroid disorder. TSH was done on almost all patients; T3, T4, FT3, FT4 and anti-TPO were done when TSH level was abnormal. The assays of TSH, T3, T4, FT3, FT4 and anti-TPO were done by the Chemilumniscence (CLIA) method using Access 2 and Unicel Dxi 600 automated systems (Beckman Coulter, India). Reagents were obtained from Beckman Coulter India Pvt. Ltd., Bangalore. The sensitivity and range for the assays were as follows: T3=0.1 ng/ml (range=0.1-8 ng/ml); T4=0.5 μg/dl (range=0.5-30 μg/dl); TSH=0.003 μIU/ml (range=0.01-100 μIU/ml); FT3=0.88 pg/ml (range=0.88-30 pg/ml); FT4=0.25 ng/dl (range=0.25-6 ng/dl) and anti-TPO=0.25 IU/ml (range=0.25-1000 IU/ml).
Data were analyzed using SPSS 15.0 IBM, USA. Psychiatric disorders were grouped into schizophrenia-spectrum disorders (schizophrenia, schizoaffective disorder, acute psychosis) and mood disorders (bipolar disorder, major depressive disorder). Since serum thyroid hormonal levels have a wide range of normal variability, the data were analyzed with subjects categorized as normal vs abnormal thyroid function (TSH <0.34 μIU/ml or TSH>4.1 μIU/ml or TSH=Normal but FT4<0.61 ng/dl), positive vs negative anti-TPO. In the absence of data on physical manifestations of thyroid disease, TSH> 4.I μIU/ml with T4 < 6.09 μIU/ml was considered to represent clinically significant hypothyroidism, while TSH ≤ 0.02 μIU/ml was considered to indicate clinically significant hyperthyroidism14. The data were analyzed using Chi-square (χ2) test.
Results
Results of 468 patients were reviewed. Data on thyroid hormonal status were available in 343 subjects [Male=173 (50.4%), Female=169 (49.3%), Missing =1 (0.3%)]. The mean age of the study subjects was 37.46 ± 13.56 yr.
The distribution of psychiatric diagnosis in the sample is shown in the Table. There were 147 (42.86%) patients with schizophrenia-spectrum disorders (schizophrenia=108, schizoaffective disorder=17, acute psychosis=22) and 185 (53.94%) with mood-spectrum disorders (bipolar disorder=122, major depressive disorder=63).
Table.
Diagnosis-wise distribution of sample and rates of thyroid dysfunction
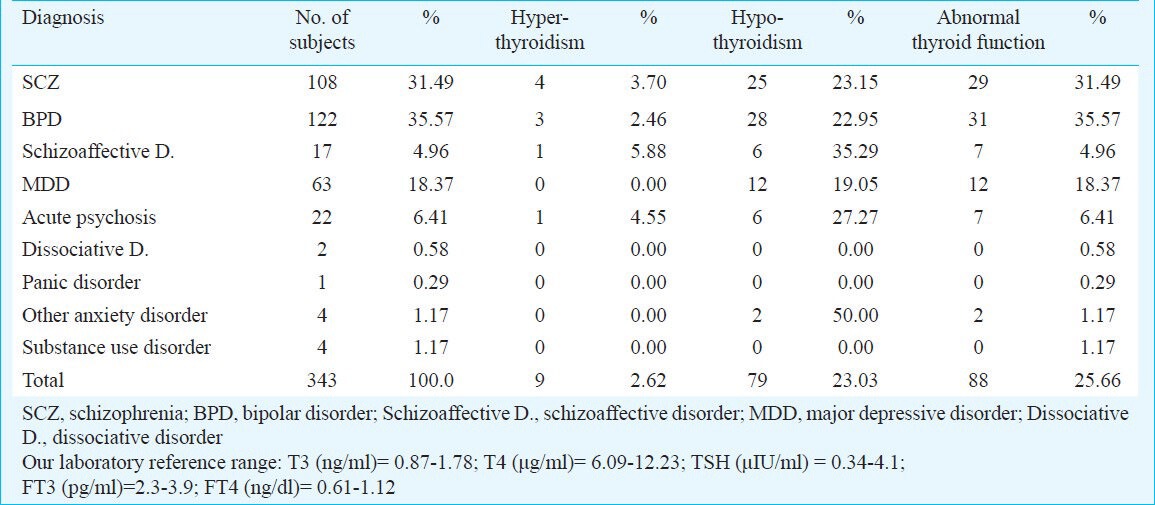
Thyroid dysfunction: Hypothyroidism was observed 37 of 147 (25.17%) patients with schizophrenia-spectrum disorders (schizophrenia=25/108, schizoaffective disorder=6/17, acute psychosis=6/22). Of the 185 patients with mood spectrum disorders 40 (21.62%) (bipolar disorder=28/122, major depressive disorder =12/63) had hypothyroidism. Three subjects with schizophrenia and two with major depressive disorder had clinically significant hypothyroidism. Hyperthyroidism was seen in six of 147 patients (4.08%) with schizophrenia-spectrum disorders (schizophrenia=4/108, schizoaffective disorder=1/17, acute psychosis=1/22). Three of 185 patients (1.62%) with mood spectrum disorders (bipolar disorder= 3/122, major depressive disorder=0/63) (Table). Two subjects with schizophrenia had TSH ≤ 0.02 μIU/ml suggestive of clinically significant hyperthyroidism. Overall, abnormal thyroid hormonal status was seen in 43 of 147 (29.3%) patients with schizophrenia-spectrum disorders (schizophrenia=29/108, schizoaffective disorder=7/17, acute psychosis=7/22) and in 23.24 per cent (43/185) of mood spectrum disorders patients (bipolar disorder=31/122, major depressive disorder=12/63) (Table).
Anti-TPO positivity: Data on anti-TPO status were available from 210 patients (schizophrenia=52, schizoaffective disorder=13, acute psychosis=16, major depressive disorder=44, bipolar disorder=81, dissociative disorder=2, anxiety disorder=2). Eighteen patients were anti-TPO positive. Of the 18 anti-TPO positive patients, 11 had a schizophrenia-spectrum disorders (schizophrenia=8, schizoaffective disorder=0, acute psychosis=3), while seven had a mood disorder (bipolar disorder=5, major depressive disorder=2). The rate of anti-TPO positivity in the schizophrenia spectrum disorder group was 13.58 per cent (11/81) vs 5.6 per cent (7/125) in the mood disorder group.
Effect of gender: Overall, there was no difference in the abnormal thyroid hormonal levels (M=40/166; F=46/165), hypothyroidism (M=35/166; F=42/165) or hyperthyroidism (M=5/166; F=4/165) between men and women. There was no gender difference in individual psychiatric diagnostic categories.
Effect of medications: Limited data were available regarding antipsychotic and mood-stabilizer medications as follows: lithium (n= 32), valproate (n= 27), risperidone (n= 68), olanzapine (n= 13), quetiapine (n=9), haloperidol (n=6), clozapine (n=6). There was no significant difference in TSH levels among the patients on different classes of antipsychotics although levels of TSH were least with quetiapine (2.09±1.74) and highest with Olanzapine (7.29 ± 20.05). Patients on lithium had higher scores on TSH (5.37 ± 8.71 μIU/ml, n=32) compared to patients on valproate (3.79 ± 3.21 μIU/ml, n=27) although the difference was not statistically significant.
Discussion
Our results indicated that thyroid abnormalities were present in patients with schizophrenia-spectrum disorders and mood disorders in an inpatient population admitted to a tertiary-care general hospital unit. Autoimmune thyroid disease was more frequent in schizophrenia-spectrum disorders compared to mood-disorders. There was no gender difference. There was no significant effect of medication on TSH levels in our sample, although data on medication status were limited.
Abnormal thyroid hormonal status was observed in 29.3 per cent patients with schizophrenia-spectrum disorders in our study. This was comparable with that reported in a similar study in a hospital sample in South-East Asia which showed that 36.4 per cent of patients with schizophrenia had thyroid dysfunction6. Poyraz et al8 found that in a sample of 74 consecutive subjects with schizophrenia, 11 (14.86%) were serum positive for autoimmune thyroiditis which is comparable to our data. Among the general population in India, the rates of thyroid dysfunction are, clinical hypothyroidism=3.9 per cent, subclinical hypothyroidism=9.4 per cent15.
Thyroid dysfunction in bipolar disorder seen in our study was (25.41%) which was lower than that shown by Bartalena et al16 (32%) and higher than that of Cassidy et al17 (11.51%). While a few studies show that autoimmune thyroid disorders are associated with bipolar disorder18,19, others fail to find an association20. Eller et al21 reported the rate of autoimmune thyroiditis in depressive disorders as 8.9 per cent comparable to our finding of 5.6 per cent.
Thyroid hormones play an important role in neurodevelopment, specifically in neurogenesis, myelination, dendrite proliferation and formation of synapses22. Animal studies have shown that treatment with antipsychotics, like clozapine and haloperidol is associated with changes in expression of nuclear receptors and genes involved in thyroid hormone function23. Most antipsychotic medications block dopaminergic transmission and result in elevated rates of TSH (quetiapine being an exception). Lithium concentrates in the thyroid gland and can cause inhibition of the uptake of iodine into follicular cells, alteration of the structure of thyroglobulin by interfering with the coupling of iodotyrosine residues to form iodothyronines, and inhibition of thyroid hormone secretion24. It has also been observed that conversion of T4 to active T3 is decreased in both animal and human models25. Lithium use has also been associated with hyperthyroidism resulting from mechanisms such as overflow of thyroid hormone following increase in the intrathyroid iodine pool, Jod-Basedow-like phenomenon, and release of thyroglobulin due to direct toxicity to thyroid follicles26. Lithium exacerbates pre-existing thyroid autoimmunity by activating lymphocytes rather than inducing antithyroid peroxidase (TPO) on its own24. If TPO antibodies are present, continuation of thyroxine will be required even if lithium is discontinued24.
Another possibility is that thyroid hormone abnormalities may represent nonthyroidal illness such as “euthyroid sick syndrome” and “euthyroid hyperthyroxinaemia” which are a response to chronic systemic illness. The prevalence of euthyroid sick syndrome ranges from 7 to 33 per cent in psychiatric inpatients27,28 while euthyroid hyperthyroxinaemia is thought to be more common in mood disorders29. Although it is difficult to distinguish nonthyroidal illness from true thyroid dysfunction in an in-patient sample, the TSH level in euthyroid sick syndrome is upto 15-20 μIU/ml30. The mean values of TSH in the schizophrenia-spectrum and mood disorders groups were higher than the range of 15-20 microIU/ml in our sample. Further, the presence of autoimmune markers in some of our cases suggests that nonthyroidal illness cannot explain all of the results.
The high rate of thyroid dysfunction in schizophrenia-spectrum disorders makes a case for screening, and has implications for cognition and treatment response. Thyroid hormones have been directly implicated in working memory performance in schizophrenia31. TSH levels have been shown to correlate with performance on tasks of attention32. Also relevant to treatment is the role of thyroid hormones in the regulation of dopamine D2 receptors. Hypothyroidism induces increased dopamine receptor sensitivity33.
The limitations of the present study include retrospective study design, inpatient sample, the lack of control for medication status and lack of data on clinical signs of hypothyroidism or co-morbid medical conditions. Also these results may not reflect the prevalence of thyroid dysfunction in psychiatric disorders in general. Additionally, our findings do not reflect causality, i.e. whether thyroid dysfunction is a cause or a result of psychiatric disorder and its treatment. Moreover, majority of the cases were subclinical and the distinction from nonthyroidal illness further compounds the issue of interpretation of this data. A small number of patients received L-thyroxine. Since the study was retrospective and cross-sectional in design, follow up data on whether medication changes modified treatment response or the course of the illness were not available. The data on medication status was limited. Also, since the study was a retrospective review of electronic records, data on duration of illness, severity of symptoms, physical co-morbidity and timing of thyroid assessment were not available, though may be relevant to thyroid status.
Despite these limitations, the study highlighted the fact that abnormal thyroid hormonal status was frequently seen in this patient population. The implications with regard to screening/treatment of abnormal thyroid hormonal status and cost-effectiveness in the management of schizophrenia-spectrum disorders warrants further study.
References
- 1.Placidi GP, Boldrini M, Patronelli A, Fiore E, Chiovato L, Perugi G, et al. Prevalence of psychiatric disorders in thyroid diseased patients. Neuropsychobiology. 1998;38:222–5. doi: 10.1159/000026545. [DOI] [PubMed] [Google Scholar]
- 2.Gitlin M, Altshuler LL, Frye MA, Suri R, Huynh EL, Fairbanks L, et al. Peripheral thyroid hormones and response to selective serotonin reuptake inhibitors. J Psychiatry Neurosci. 2004;29:383–6. [PMC free article] [PubMed] [Google Scholar]
- 3.Carvalho AF, Machado JR, Cavalcante JL. Augmentation strategies for treatment-resistant depression. Curr Opin Psychiatry. 2009;22:7–12. doi: 10.1097/YCO.0b013e32831be9ef. [DOI] [PubMed] [Google Scholar]
- 4.Bauer M, London ED, Silverman DH, Rasgon N, Kirchheiner J, Whybrow PC. Thyroid, brain and mood modulation in affective disorder: insights from molecular research and functional brain imaging. Pharmacopsychiatry. 2003;36(Suppl 3):S215–21. doi: 10.1055/s-2003-45133. [DOI] [PubMed] [Google Scholar]
- 5.Kelly DL, Conley RR. Thyroid function in treatment-resistant schizophrenia patients treated with quetiapine, risperidone, or fluphenazine. J Clin Psychiatry. 2005;66:80–4. doi: 10.4088/jcp.v66n0111. [DOI] [PubMed] [Google Scholar]
- 6.Sim K, Chong SA, Chan YH, Lum WM. Thyroid dysfunction in chronic schizophrenia within a state psychiatric hospital. Ann Acad Med Singapore. 2002;31:641–4. [PubMed] [Google Scholar]
- 7.Santos NC, Costa P, Ruano D, Macedo A, Soares MJ, Valente J, et al. Revisiting thyroid hormones in schizophrenia. J Thyroid Res 2012. 2012 doi: 10.1155/2012/569147. 569147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poyraz BC, Aksoy C, Balcioglu I. Increased incidence of autoimmune thyroiditis in patients with antipsychotic-induced hyperprolactinemia. Eur Neuropsychopharmacol. 2008;18:667–72. doi: 10.1016/j.euroneuro.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 9.Lin YT, Liao SC. Hashimoto encephalopathy presenting as schizophrenia-like disorder. Cogn Behav Neurol. 2009;22:197–201. doi: 10.1097/WNN.0b013e318197926e. [DOI] [PubMed] [Google Scholar]
- 10.Eaton WW, Byrne M, Ewald H, Mors O, Chen CY, Agerbo E, et al. Association of schizophrenia and autoimmune diseases: linkage of Danish national registers. Am J Psychiatry. 2006;163:521–8. doi: 10.1176/appi.ajp.163.3.521. [DOI] [PubMed] [Google Scholar]
- 11.Thomsen AF, Kessing LV. Increased risk of hyperthyroidism among patients hospitalized with bipolar disorder. Bipolar Disorders. 2005;7:351–7. doi: 10.1111/j.1399-5618.2005.00205.x. [DOI] [PubMed] [Google Scholar]
- 12.Kupka RW, Nolen WA, Post RM, McElroy SL, Altshuler LL, Denicoff KD, et al. High rate of autoimmune thyroiditis in bipolar disorder: lack of association with lithium exposure. Biol Psychiatry. 2002;51:305–11. doi: 10.1016/s0006-3223(01)01217-3. [DOI] [PubMed] [Google Scholar]
- 13.Geneva: World Health Organization; 1992. World Health Organization. The ICD-10 classification of mental and behavioural disorders: Clinical descriptions and diagnostic guidelines. [Google Scholar]
- 14.Unnikrishnan AG, Kalra S, Baruah M, Nair G, Nair V, Bantwal G, et al. Endocrine Society of India management guidelines for patients with thyroid nodules: A position statement. Indian J Endocrinol Metab. 2011;15:2–8. doi: 10.4103/2230-8210.77566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Unnikrishnan AG, Menon UV. Thyroid disorders in India: An epidemiological perspective. Indian J Endocrinol Metab. 2011;15(Suppl 2):S78–81. doi: 10.4103/2230-8210.83329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartalena L, Pellegrini L, Meschi M, Antonangeli L, Bogazzi F, Dell’Osso L, et al. Evaluation of thyroid function in patients with rapid-cycling and non-rapid-cycling bipolar disorder. Psychiatry Res. 1990;34:13–7. doi: 10.1016/0165-1781(90)90054-9. [DOI] [PubMed] [Google Scholar]
- 17.Cassidy F, Ahearn EP, Carroll BJ. Thyroid function in mixed and pure manic episodes. Bipolar Disord. 2002;4:393–7. doi: 10.1034/j.1399-5618.2002.01214.x. [DOI] [PubMed] [Google Scholar]
- 18.Carta MG, Loviselli A, Hardoy MC, Massa S, Cadeddu M, Sardu C, et al. The link between thyroid autoimmunity (antithyroid peroxidase autoantibodies) with anxiety and mood disorders in the community: a field of interest for public health in the future. BMC Psychiatry. 2004;4:25. doi: 10.1186/1471-244X-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vonk R, van der Schot AC, Kahn RS, Nolen WA, Drexhage HA. Is autoimmune thyroiditis part of the genetic vulnerability (or an endophenotype) for bipolar disorder? Biol Psychiatry. 2007;62:135–40. doi: 10.1016/j.biopsych.2006.08.041. [DOI] [PubMed] [Google Scholar]
- 20.Engum A, Bjoro T, Mykletun A, Dahl AA. Thyroid autoimmunity, depression and anxiety; are there any connections? An epidemiological study of a large population. J Psychosom Res. 2005;59:263–8. doi: 10.1016/j.jpsychores.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Eller T, Metskula K, Talja I, Maron E, Uibo R, Vasar V. Thyroid autoimmunity and treatment response to escitalopram in major depression. Nord J Psychiatry. 2010;64:253–7. doi: 10.3109/08039480903487533. [DOI] [PubMed] [Google Scholar]
- 22.Williams GR. Neurodevelopmental and neurophysiological actions of thyroid hormone. J Neuroendocrinol. 2008;20:784–94. doi: 10.1111/j.1365-2826.2008.01733.x. [DOI] [PubMed] [Google Scholar]
- 23.Langlois MC, Beaudry G, Zekki H, Rouillard C, Levesque D. Impact of antipsychotic drug administration on the expression of nuclear receptors in the neocortex and striatum of the rat brain. Neuroscience. 2001;106:117–28. doi: 10.1016/s0306-4522(01)00248-2. [DOI] [PubMed] [Google Scholar]
- 24.Livingstone C, Rampes H. Lithium: a review of its metabolic adverse effects. J Psychopharmacol. 2006;20:347–55. doi: 10.1177/0269881105057515. [DOI] [PubMed] [Google Scholar]
- 25.Terao T, Oga T, Nozaki S, Ohta A, Otsubo Y, Yamamoto S, et al. Possible inhibitory effect of lithium on peripheral conversion of thyroxine to triiodothyronine: a prospective study. Int Clin Psychopharmacol. 1995;10:103–5. doi: 10.1097/00004850-199506000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Chakrabarti S. Thyroid functions and bipolar affective disorder. J Thyroid Res 2011. 2011 doi: 10.4061/2011/306367. 306367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nader S, Warner MD, Doyle S, Peabody CA. Euthyroid sick syndrome in psychiatric inpatients. Biol Psychiatry. 1996;40:1288–93. doi: 10.1016/0006-3223(95)00626-5. [DOI] [PubMed] [Google Scholar]
- 28.Dickerman AL, Barnhill JW. Abnormal thyroid function tests in psychiatric patients: a red herring? Am J Psychiatry. 2012;169:127–33. doi: 10.1176/appi.ajp.2011.11040631. [DOI] [PubMed] [Google Scholar]
- 29.Lambert TJ, Davidson R, McLellan GH. Euthyroid hyperthyroxinaemia in acute psychiatric admissions. Aust N Z J Psychiatry. 1987;21:608–12. doi: 10.3109/00048678709158931. [DOI] [PubMed] [Google Scholar]
- 30.Chopra IJ. Clinical review 86: Euthyroid sick syndrome: is it a misnomer? J Clin Endocrinol Metab. 1997;82:329–34. doi: 10.1210/jcem.82.2.3745. [DOI] [PubMed] [Google Scholar]
- 31.Zoeller RT, Rovet J. Timing of thyroid hormone action in the developing brain: clinical observations and experimental findings. J Neuroendocrinol. 2004;16:809–18. doi: 10.1111/j.1365-2826.2004.01243.x. [DOI] [PubMed] [Google Scholar]
- 32.Johnson LA, Hobson V, Jenkins M, Dentino A, Ragain RM, O’Bryant S. The influence of thyroid function on cognition in a sample of ethnically diverse, rural-dwelling women: a project FRONTIER study. J Neuropsychiatry Clin Neurosci. 2011;23:219–22. doi: 10.1176/jnp.23.2.jnp219. [DOI] [PubMed] [Google Scholar]
- 33.Crocker AD, Overstreet DH, Crocker JM. Hypothyroidism leads to increased dopamine receptor sensitivity and concentration. Pharmacol Biochem Behav. 1986;24:1593–7. doi: 10.1016/0091-3057(86)90491-0. [DOI] [PubMed] [Google Scholar]


