Abstract
Background & objectives:
Serum alanine aminotransferase (ALT) level is most commonly used as a marker for the assessment of various liver diseases. Currently upper limits of normal for aspartate aminotransferase (AST) or ALT levels used are based on the western literature. This study was conducted to determine the ALT and AST levels in healthy blood donors from western India and to determine the relation with body mass index (BMI) and waist-to-hip ratio (WHR).
Methods:
A total of 5077 voluntary blood donors were selected with strict routine inclusion and exclusion criteria. Weight, height, BMI and WHR were determined along with AST and ALT levels. BMI and WHR were correlated with liver enzymes.
Results:
Of the 5077 donors, 160 were excluded due to positive serological results. In the remaining 4917 individuals, 4643 (94.4%) were males and 274 (5.6%) were females. Majority 3024 (61.5%) showed BMI more than 23 kg/m2. WHR > 0.85 and 0.80 was found in 4046 (87.0%) males and 250 (91.2%) females. Mean AST and ALT levels in males were 23.4 ± 9.9 IU/l and 27.0 ± 17.3 IU/l and in females 19.1 ± 9.8 IU/l and 17.7 ± 11.2 IU/l, respectively. With increase in BMI, there was a significant increase in AST and ALT levels. Similar increase was also seen with WHR.
Interpretation & conclusions:
Majority of voluntary blood donors showed high BMI and WHR which was directly related to AST and ALT levels. This study highlights the magnitude of obesity in general healthy population of western India and a need to revise the current normal limits of serum ALT.
Keywords: Aminotransferase, body mass index, non-alcoholic fatty liver disease, obesity, waist to hip ratio
The serum aminotransferases discovered in 1927 by Needham, are identified as sensitive indicators of disease affecting several organ systems in particular liver, skeletal muscles, heart, lungs and brain1,2,3. Alanine aminotransferase (ALT) has been proved particularly more useful in the evaluation of hepatic disease because it is found in greater concentration in the liver whereas its concentration in heart, skeletal muscles and kidney is lower. In contrast, in men the activity of aspartate aminotransferase (AST) is highest in cardiac muscles followed by liver and skeletal muscles. It is also detectable in brain and kidney2,4. Serum ALT level is commonly used as an early marker for assessment of various liver diseases5. In past, It has been used as a surrogate marker of non A non B hepatitis before availability of anti hepatitis C virus (HCV) testing6,7. Its use has also been advocated as a surrogate non-invasive marker for diagnosis of non-alcoholic fatty liver diseases (NAFLD) in otherwise serologically negative apparently healthy persons8. Some studies have also suggested that elevated ALT level may predict overall mortality rate in general population, independent of liver disease7,9,10. The levels of AST or ALT vary widely among populations and the question has been raised whether the previously established values for normal AST and ALT range are still accurate and applicable6,7,11. There is also debate whether or not different cut-offs are required in males and females for normal range of liver transaminases11. The available upper limit of normal (ULN) thresholds of ALT and AST were determined earlier and with currently defined ULN, ALT might underestimate patients at risk of chronic liver disease12.
In India, currently used upper limits of normal for AST or ALT levels are mainly based upon western population and those provided by manufacturer of kits13. Rapidly changing lifestyle, food habits and globalization in the recent past have led to an increased incidence of obesity, metabolic syndrome, diabetes in India14. Several studies from different parts of globe have shown variation in AST and ALT levels with various clinical and demographic factors such as age, sex, race, body mass index (BMI), waist to hip ratio (WHR), chronic alcoholism, diabetes, dietary factors, etc. and have advocated that individual laboratories should determine the cut-off value for ALT in their own setting15,16.
There is a paucity of data on normal AST and ALT values in Indian subjects. The present study was undertaken to determine serum AST and ALT levels in general healthy population in western India and their relation with BMI and WHR.
Material & Methods
A prospective cross-sectional study was conducted in the department of Pathology, T.N. Medical College, Mumbai, a tertiary care hospital in Western India over a period of two years (from July 2009 to September 2010). The study was done in association with blood bank and clinical biochemistry laboratory which are the part of Pathology department. The study population consisted of voluntary blood donors from hospital based blood donation camps conducted in general community. The blood donors represent general healthy population since they have to fulfil inclusion and exclusion criteria laid down by the Government of India for blood donation17.
Inclusion criteria: Voluntary blood donation, age group between 18 to 60 yr, weight more than 45 kg, body temperature not more than 37.5°C, pulse rate between 60-100/min and regular, blood pressure-systolic 100-160 mm of Hg, diastolic 60-90 mm of Hg and haemoglobin level >12.5 g/dl.
Exclusion criteria: Hepatitis B and C infections, HIV I and II infection, malignancy, heart disease, chronic nephritis, allergic disorders, leprosy, schizophrenia, polycythemia vera, abnormal bleeding tendencies, unexplained weight loss and individuals on medications (anticonvulsant, antiarrhythmic, immunosuppressive, anticoagulant, vasodilator, sedative in high doses, antithyroid & lipid lowering drugs). The blood donors who were chronic alcoholic (consumed >20 g/day) were also excluded.
Additional exclusion criteria: (i) Drugs causing fatty liver such as asparaginase, tetracycline, warfarin, amiodarone, tamoxifen, oestrogens, bleomycin, diltiazem, nifedipine, methotrexate, corticosteroids, salicylates, (ii) persons who were found to be seropositive after blood donation for hepatitis B surface antigen, anti HCV antibody, HIV 1 and 2, VDRL and malarial parasite, and (iii) serum bilirubin level more than 1.5 mg/dl.
This study protocol was approved by the institutional ethics committee. The Informed written consent was taken from all participants. Each participant was screened by a trained physician before blood donation. The following anthropometric measurements were taken: weight in kilograms; height in centimetres; waist circumference at mid-point between the lower border of the rib cage and the iliac crest in centimetres; and hip circumference at level of maximum circumference of buttock.
All individuals were sub-classified in various groups using Asian criteria where normal BMI range was between 18.0-22.9 kg/m2. BMI between 23.0-24.9 kg/m2 was considered to be overweight and >25.0 kg/m2 as obese18.
Waist to hip ratio (WHR) was calculated as waist circumference / hip circumference in cm.
WHR >0.85 in males and >0.80 in females was considered as indication of abdominal fat accumulation as per Asian criteria18.
During routine blood donation, one extra blood sample (5 ml) was collected from each donor in plain test tube. Serological testing for HBsAg, anti HCV, HIV was carried out as a routine procedure on each blood sample using standard diagnostic kits. Veneral disease research laboratory (VDRL) and malarial parasite testing was also done on every sample of blood. Those samples showing negative serology were further processed for AST and ALT levels using Olympus AU400 auto-analyser (Mishima Olympus Co. Ltd., Shizuoka-ken, Japan). To ensure accuracy and precision, internal quality control was done everyday using quality controls Lyphochek level 1 and 2 supplied by Bio-Rad Laboratories, France.
HBsAg testing was done using HBsAg ELISA test kit Microscreen supplied by Span Diagnostics Ltd. or Hepalisa supplied by J. Mitra & Co. Pvt. Ltd., India. HCV antibody testing was done using microwell ELISA test kit (Inonova supplied by Span Diagnostics Ltd. or Microlisa HCV supplied by J. Mitra & Co. Pvt. Ltd.). To rule out HIV 1 and 2 infections, HIV 1+2 ELISA kits (Enzaids of Span Diagnostics Ltd. or Microlisa HIV of J. Mitra & Co. Pvt. Ltd.) were used. VDRL testing for syphilis was performed using R.P.R. Flocculation test kits (Rapid Plasma Reagin test kits) of Beacon Diagnostics Pvt. Ltd., India. In case of icteric plasma, serum bilirubin was measured. The icteric plasma was confirmed by colorimetric reading taken at 540 nm. If serum bilirubin level was found to be more than 1.5 mg/dl, such samples were not included in the analysis.
Statistical method: The data analysis (chi square test & 2 tailed Pearson correlations) was performed using SPSS software, (version 15.0 SPSS, Inc., Chicago, IL, USA).
Results
A total of 5,077 blood samples were collected from equal number of voluntary blood donors. Of these, 160 (3.2%) samples were excluded due to positive serological results or icteric plasma. The remaining 4,917 individuals were further analysed, of them 4,643 (94.4%) were males and 274 (5.6%) were females. Of the 4917 individuals, 2581 (55.6%) were in the age group 18 to 40 yr. Mean age in the study group for males was 30.9 ± 8.8 (range 18 to 60) years whereas that in females was 32.5 ± 10.6 (range 18 to 58) years. Normal BMI (18-22.9 kg/m2 was found in 1792 (36.4%). BMI > 23 kg/m2 was found in 3024 (61.5 %) and low BMI (<18.0 kg/m2 in 101 (2.1%).
Amongst 4643 males, 2881 (62 %) were either overweight or obese and only 98 (1.1%) were underweight. In females, 143 (52 %) were overweight and obese. BMI was normal in 128 (35.8%) females and only three (2.1%) were underweight (Table I). Mean WHR in males and females were 0.89 ± 0.045 (range 0.63 to 1.12) and 0.87 ± 0.050 (range 0.72 to 1.05), respectively. Maximum number of males 4046 (87.0%) and females 250 (91.2%) have WHR more than 0.85 and 0.80, respectively.
Table I.
Body mass index (BMI) & waist to hip ratio in study population

Mean AST and ALT levels in males were 23.4 ± 9.9 and 27.0 ± 17.3 IU/l and in females 19.1 ± 9.8 and 17.7 ± 11.2 IU/l, respectively. In males, AST and ALT levels were slightly higher in age group 31 to 40 yr (mean 24.5 ± 10.8 and 30.4±19.6 IU/l, respectively). While in females peak for AST and ALT was found in 41 to 50 yr group (mean 22.1 ± 12.5 & 21.4 ± 13.6 IU/l, respectively).
Mean AST at normal BMI was found to be 21.4 ± 8.7 and 18.0 ± 8.8 IU/l for males and females respectively. As BMI increased, there was an increase in the AST levels. Mean AST for BMI >23.0 kg/m2 was 24.7 ± 10.4 IU/l in males which was significantly higher (P<0.001) as compared to individuals with BMI <23 kg/m2. For females, AST level was high with increasing BMI but it was not found to be significant.
Mean ALT levels for normal BMI was 20.5 ± 11.8 IU/l in males and 15.0 ± 7.8 IU/l in females. With increase in BMI, there was increase in ALT level in both males and females. For BMI >23 kg/m2 mean ALT was 31.1± 18.8 and 20.4±13.1 IU/l in males and females, respectively which was significant (2 tailed Pearson correlation value 0.352) (P<0.001) as compared to normal BMI (Fig. 1). Similarly with increasing WHR there was an increase in mean AST and ALT. In males ALT levels significantly increased with increase in WHR (2 tailed Pearson correlation value 0.352) (P<0.001) but such significant correlation was not seen in females. After initial increase in ALT, a slight decline in mean ALT level was noted in females when WHR was more than 0.94 (Fig. 2).
Fig. 1.
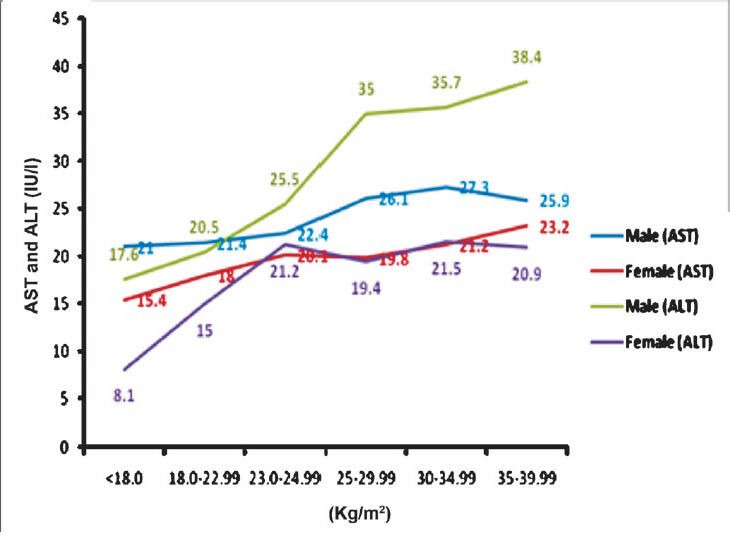
Correlation of BMI with aspartate and alanine aminotransferases (AST and ALT).
Fig. 2.
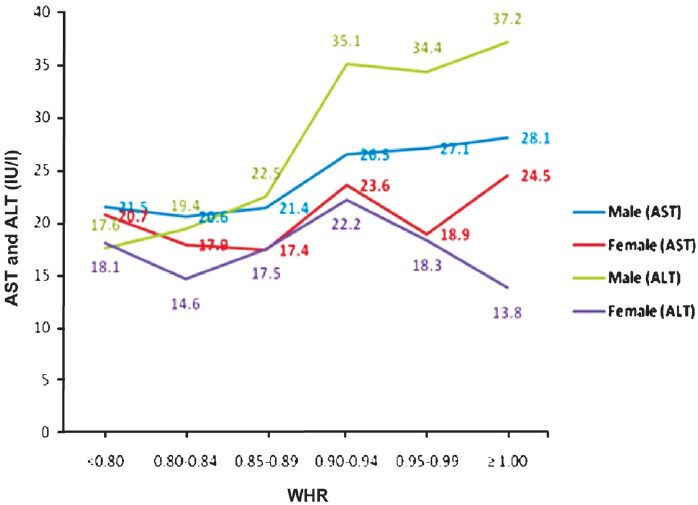
Correlation of WHR with aspartate and alanine aminotransferases (AST and ALT).
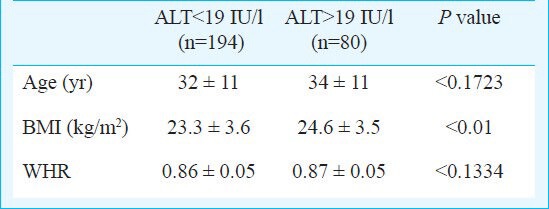
ALT level of 30 IU/l was found to be upper limit of normal for males which was calculated as 95th percentile using standard commercial kits. In this study 1435 (31%) males showed ALT levels > 30IU/l. On comparing these with ALT <30 IU/l there was significant difference in age, BMI and WHR in the two groups (P<0.0001). (Table II). In females, 19 IU/l was considered to be upper limit of normal. In this study 80 (29.1%) females showed ALT >19 IU/l. BMI was significantly high in females with ALT >19 IU/l as compared to <19 IU/l (P=0.01) (Table III).
Table II.
Serum alanine aminotransferase (ALT) values with BMI and WHR in males
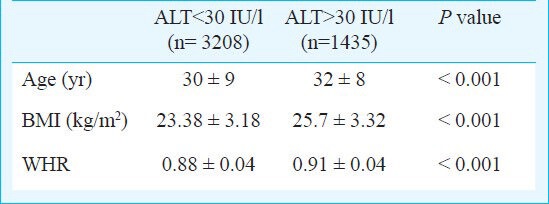
Table III.
Serum alanine aminotransferase (ALT) values with BMI and WHR in females
Discussion
BMI and WHR are good indicators of obesity. WHR determines abdominal obesity and it has been increasingly recognized as an important cardiovascular risk factor19. In this study, 2881 (62%) males and 143 (52%) females showed high BMI (>23.0 kg/m2. WHR was higher in 4046 (87%) males and 250 (91.2%) females. This indicates magnitude of obesity in general healthy population. Various studies have suggested that Asian Indians are at risk of developing obesity related co-morbidities at lower levels of BMI and WHR14,20,21. In a cross sectional survey in five cities in India, the prevalence of obesity was 6.8 per cent and overweight was 33.5 per cent in general population. The overall prevalence of BMI >23 kg/m2 was 50.8 per cent and central obesity was 52.6 per cent22.
In this study, 31 per cent individuals had ALT >30 IU/l. While correlating BMI and WHR with liver enzymes it was found that with increase in WHR and BMI there was significant increase in liver enzymes in males. In females, there was slight decline in mean ALT level with WHR more than 0.94. The similar observation has been noted by Piton et al16. This may be due to limited number of females in our study.
Lozano et al23 analysed 579 male and 457 female blood donors. The mean ALT levels were 25.3 ± 14.5 IU/l for males and 16.3 ± 7.9 IU/l in females. BMI was >27 kg/m2 in 53.3 per cent individuals. They have shown that factors such as obesity accounts for increased ALT values indicating the low specificity of the test and hence there is a need to have different cut-off values for ALT levels in males and females. Another study has shown that mean ALT levels of obese (BMI >30 kg/m2 compared with two categories of normal subjects (BMI ≤20 and BMI = 20.1 - 25 kg/ m2) was increased by 2.8 and 1.96 times, respectively24. The authors suggested to correct ALT values for BMI instead of using actual ALT values.
Xia et al25 studied the association between the liver enzymes and metabolic syndrome. Optimal cut-off values for liver enzymes in metabolic syndrome were determined. Logistic regression analysis revealed that within normal range of liver enzymes, the frequency of metabolic syndrome was significantly increased. They have also shown that a slight elevation of liver enzymes within normal limits indicates presence of metabolic syndromes24.
Several other population based studies have found slight increase in ALT levels within current normal range to be related to co-morbidity and mortality26,27. Current range of normal serum ALT value might underestimate prevalence of chronic liver disease. Hence there is a need to update the cut-off values for ALT levels.
Our study had certain limitations such as ALT and AST levels were assessed only once. Other drawbacks were number of female participants were significantly lower than males and there was no assessment of fatty liver by ultrasonography or liver biopsy.
In conclusion, this study shows magnitude of obesity in general healthy population in western India. There is need to revise current normal limits of serum ALT so that potential metabolic disorders can be identified.
Acknowledgment
Authors acknowledge the Research Society, TNMC & BYL Nair Charitable Hospital, Mumbai, for financial assistance, and thank Shrimati Ajita Kulkarni and Shri V.D. Rane, senior biochemists for providing laboratory technical assistance.
References
- 1.Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalaloacetic and glutamic pyruvic transaminases. A J Clin Pathol. 1957;28:56–62. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 2.Cuccherini B, Nussbaum SJ, Seeff LB, Lukacs L, Zimmerman HJ. Stability of aspartate aminotransferase and alanine aminotransferase activities. J Lab Clin Med. 1983;102:370–6. [PubMed] [Google Scholar]
- 3.Pincus MR, Tierno PM, Dufour DR. Evaluation of liver function. In: McPherson RA, Pincus MR, editors. Henry's clinical diagnosis and management by laboratory methods. 21st ed. Philadelphia, USA: Elsevier; 2007. pp. 263–78. [Google Scholar]
- 4.Wroblewski F. The clinical significance of transaminases activities of serum. Am J Med. 1959;27:911–8. doi: 10.1016/0002-9343(59)90175-5. [DOI] [PubMed] [Google Scholar]
- 5.Prati D, Taioli E, Zanella A, Della Torre E, Butelli S, Del Vecchio E, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137:1–10. doi: 10.7326/0003-4819-137-1-200207020-00006. [DOI] [PubMed] [Google Scholar]
- 6.Marshall MK. Alanine aminotransferase levels: what's normal? Ann Intern Med. 2002;137:49–51. doi: 10.7326/0003-4819-137-1-200207020-00012. [DOI] [PubMed] [Google Scholar]
- 7.Jamali R, Khonsari M, Merat S, Khoshnia M, Jafari E, Bahram Kalhori A, et al. Persistent alanine aminotransferase elevation among the general Iranian population: prevalence and causes. World J Gastroenterol. 2008;14:2867–71. doi: 10.3748/wjg.14.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kariv R, Leshno M, Beth-Or A, Strul H, Blendis L, Kokia E, et al. Re-evaluation of serum alanine aminotransferase upper normal limit and its modulating factors in a large-scale population study. Liver Int. 2006;26:445–50. doi: 10.1111/j.1478-3231.2006.01197.x. [DOI] [PubMed] [Google Scholar]
- 9.Kim WR, Flamm SL, Di Bisceglie AM, Bodenheimer HC. Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology. 2008;47:1363–70. doi: 10.1002/hep.22109. [DOI] [PubMed] [Google Scholar]
- 10.Dutta A, Saha C, Johnson CS, Chalasani N. Variability in the upper limit of normal for serum alanine aminotransferase levels: a state-wide study. Hepatology. 2009;50:1957–62. doi: 10.1002/hep.23200. [DOI] [PubMed] [Google Scholar]
- 11.Khedmat H, Fallahian F, Abolghasemi H, Hajibeigi B, Attarchi Z, Alaeddini F, et al. Serum gamma-glutamyltransferase, alanine aminotransferase, and aspartate aminotransferase activity in Iranian healthy blood donor men. World J Gastroenterol. 2007;13:889–94. doi: 10.3748/wjg.v13.i6.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schindhelm RK, Dekker JM, Nijpels G, Stehouwer CD, Bouter LM, Heine RJ, et al. Alanine aminotransferase and the 6-year risk of the metabolic syndrome in Caucasian men and women: the Hoorn Study. Diabet Med. 2007;24:430–5. doi: 10.1111/j.1464-5491.2007.02100.x. [DOI] [PubMed] [Google Scholar]
- 13.Siest G, Schiele F, Galteau MM, Pane KE, Steinmetz J, Fagnani F, et al. Aspartate aminotransferase and almine aminotransferase activities in plasma: statistical distributions, individual variations and reference values. Clin Chem. 1975;21:1077–8. [PubMed] [Google Scholar]
- 14.Misra A, Khurana L. The metabolic syndrome in South Asians: epidemiology, determinants, and prevention. Metab Syndr Relat Disord. 2009;6:497–514. doi: 10.1089/met.2009.0024. [DOI] [PubMed] [Google Scholar]
- 15.Kim HC, Nam CM, Jee SH, Han KH, Oh DK, Suh I. Normal serum aminotransferase concentration and risk of mortality from liver diseases prospective cohort study. BMJ. 2004;328:983. doi: 10.1136/bmj.38050.593634.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piton A, Poynard T, Imbert Bismut F, Khalil L, Delattre J, Pelissier E, et al. Factors associated with serum alanine transaminase activity in healthy subjects: consequences for the definition of normal values for selection of blood donors and for patients with chronic hepatitis MIU/LTIVIRC Group. Hepatology. 1998;27:1213–9. doi: 10.1002/hep.510270505. [DOI] [PubMed] [Google Scholar]
- 17.Saran RK. Transfusion medicine technical manual. 2nd ed. New Delhi: Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India; 2003. Donors’ selection and blood collection; pp. 7–22. [Google Scholar]
- 18.Misra A, Chowbey P, Makkar BM, Vikram NK, Wasir JS, Chadha D, et al. Consensus statement for diagnosis of obesity, abdominal obesity and the metabolic syndrome for Asian Indians and recommendations for physical activity, medical and surgical management. J Assoc Physicians India. 2009;57:163–70. [PubMed] [Google Scholar]
- 19.Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–7. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 20.Vikram NK, Pandey RM, Misra A, Sharma R, Devi JR, Khanna N. Non-obese (body mass index <25 kg/m2) Asian Indians with normal waist circumference have high cardiovascular risk. Nutrition. 2003;19:503–9. doi: 10.1016/s0899-9007(02)01083-3. [DOI] [PubMed] [Google Scholar]
- 21.Misra A. Revisions of cut-offs of body mass index to define overweight and obesity are needed for the Asian-ethnic groups. Int J Obes Relat Metab Disord. 2003;27:1294–6. doi: 10.1038/sj.ijo.0802412. [DOI] [PubMed] [Google Scholar]
- 22.Singh RB, Pella D. Prevalence of obesity, physical inactivity and under nutrition, a triple burden of diseases during transition in a developing economy. The Five City Study Group. Acta Cardiol. 2007;62:119–27. doi: 10.2143/AC.62.2.2020231. [DOI] [PubMed] [Google Scholar]
- 23.Lozano M, Cid J, Bedini JL, Mazzara R, Gimenez N, Mas E, et al. Study of serum alanine-aminotransferase levels in blood donors in Spain. Haematologica. 1998;83:237–9. [PubMed] [Google Scholar]
- 24.Ramesh V, Saraswat S, Choudhury N, Gupta RK. Relationship of serum alanine aminotransferase (ALT) to body mass index (BMI) in blood donors: the need to correct ALT for BMI in blood donor screening. Transfusion Med. 1995;5:273–4. doi: 10.1111/j.1365-3148.1995.tb00213.x. [DOI] [PubMed] [Google Scholar]
- 25.Xia MF, Yan HM, Lin HD, Bian H, Pan BS, Yao XZ, et al. Elevation of liver enzymes within the normal limits and metabolic syndrome. Clin Exp Pharmacol Physiol. 2011;38:373–9. doi: 10.1111/j.1440-1681.2011.05519.x. [DOI] [PubMed] [Google Scholar]
- 26.Kang HS, Um SH, Seo YS, An H, Lee KG, Hyun JJ, et al. Healthy range for serum ALT and the clinical significance of “unhealthy” normal ALT levels in the Korean population. J Gastroenterol Hepatol. 2011;26:292–9. doi: 10.1111/j.1440-1746.2010.06481.x. [DOI] [PubMed] [Google Scholar]
- 27.Chang Y, Ryu S, Sung E, Jang Y. Higher concentrations of alanine aminotransferase within the reference interval predict nonalcoholic fatty liver disease. Clin Chem. 2007;53:686–92. doi: 10.1373/clinchem.2006.081257. [DOI] [PubMed] [Google Scholar]


