Abstract
Background & objectives:
Interstitial lung disease (ILD) is a progressive complication in patients with rheumatoid arthritis (RA). Although the precise mechanisms of ILD are not fully understood, Th2 cytokines, especially interleukin (IL)-4 may play an important role in the processes of fibrosis. We, therefore, investigated the role of Th2 cytokines, including IL-4, IL-13 and IL-5 in RA patients with or without ILD.
Methods:
Serum samples were obtained from 63 patients with RA. Among them, 29 RA patients had ILD while the remaining 34 patients were without ILD. The bronchoalveolar lavage fluids (BALF) from 11 RA patients with ILD and eight patients without ILD were also collected. Enzyme-linked immunosorbent assay (ELISA) was used to analyze the levels of IL-4, IL-13 and IL-5 both in serum and in BALF.
Results:
The levels of IL-4 were increased in the serum and BALF of RA patients with ILD compared with RA patients without ILD. There were no differences in the levels of IL-13 and IL-5 among the different groups.
Interpretation & conclusion:
The present results indicate that IL-4 seems to play an important role in the development of ILD in patients with RA.
Keywords: Interstitial lung disease, rheumatoid arthritis, Th2 cytokines
Rheumatoid arthritis (RA) is a chronic autoimmune disease and cytokines play a fundamental role in the processes of RA. Interstitial lung disease (ILD) is a progressive and lethal complication in patients with RA. ILD includes a wide range of disorders in which pulmonary fibrosis is the final pathway of pathology. Although the precise mechanisms of ILD are not fully understood, Th2 cytokines may play an important role in the processes of fibrosis1. Interleukin (IL)-4 can induce differentiation of stem/precursor cells into fibroblast-like cells and stimulate fibroblast proliferation and extracellular matrix (ECM) production in vitro1,2,3. Some studies have shown elevated levels of IL-4, IL-5 and IL-13 in patients with idiopathic pulmonary fibrosis (IPF)4,5, providing further evidence that fibrosis is often associated with the development of Th2-type responses. However, the mechanism of ILD in RA is not clear. To evaluate whether Th2 cell response has any effect on the development of ILD in patients with RA, we detected the serum and bronchoalveolar lavage fluid (BALF) levels of Th2 cytokines (IL-4, IL-13 and IL-5) in RA patients with or without ILD and compared with normal controls.
Material & Methods
This preliminary study was conducted in the Department of Rheumatology, China Medical University, Shanyang, PR China, from 2006 to 2008. Sixty three consecutive patients with RA who fulfilled the 1987 American College of Rheumatology criteria of RA6, were included in the study. Among the 63 RA patients, 16 patients were newly diagnosed while the remaining 47 though were old patients but had not taken drugs at least for the last three month. The diagnosis of ILD was based on the high resolution computed tomography (HRCT). Among them, 29 RA patients had ILD (24 women and five men; mean age 49.4 ± 10.3 yr; mean disease duration 6.4 ± 5.3 yr) while other 34 RA patients were without ILD (29 women and five men; mean age 44.9 ± 10.2 yr; mean disease duration 5.1 ± 4.6 yr). There were no differences in the medications of the RA patients with ILD versus those without ILD. Twenty healthy controls (16 women and four men; mean age 45.0 ± 8.9 yr) age and gender matched to the patients, were also recruited. The bronchoalveolar lavage fluids (BALF) from 11 RA patients with ILD and eight RA patients without ILD were also collected and stored at -80°C for further analysis. The study protocol was approved by the ethics committee in 1st Affiliated Hospital of China Medical University and written informed consents were given by all patients.
Detection of cytokines levels: Serum and BALF levels of cytokines (IL-4, IL-13 and IL-5) were measured by the enzyme-linked immunosorbent assay (ELISA) (R&D, Minneapolis, USA) and performed following the manufacturer's instructions.
Statistical analysis: Data were presented as the mean±SD or median (range) depending on Gaussian or non Gaussian population. The differences in concentration of cytokines between different groups were analyzed by One-way ANOVA test. Mann-Withney test was used to analyse the difference of BALF cytokine levels between RA patients with and without ILD. All analyses were performed using SPSS17 and GraphPad Prism 5 software San Diego, CA.
Results
The level of IL-4 was 87.1 ± 43.1 pg/ml in patients with RA and 88.2 ± 46.2 pg/ml in normal control. There was no significant difference between these two groups (Table). The differences of IL-13 and IL-5 between patients with RA and normal controls were also not significant.
Table.
Serum and bronchoalveolar lavage fluid (BALF) levels of interleukin (IL)-4, IL-13 and IL-5 in rheumatoid arthritis (RA) patients and normal control (NC) group
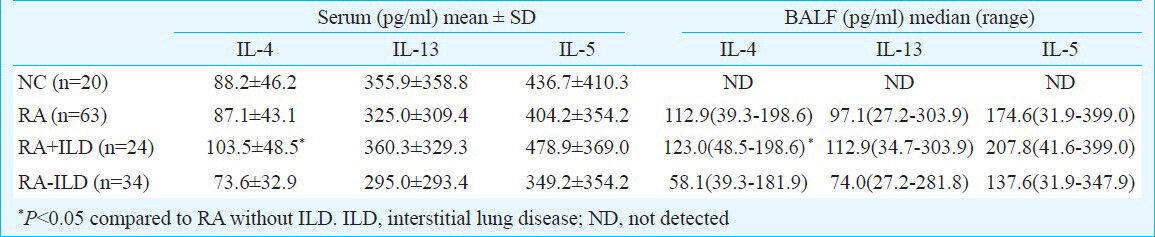
The levels of IL-4 in serum from the RA patients with ILD (103.5 ± 48.5 pg/ml) were significantly higher than those in RA patients without ILD (73.6 ± 32.9 pg/ml; P<0.05) (Table), which was not seen in IL-13 and IL-5. There were no significant differences in IL-4 levels between RA patients without ILD and healthy controls. The levels of IL-4 in BALF from RA patients with ILD (median: 123.0 pg/ml; range: 48.5 - 198.6 pg/ml) were much higher than those in RA patients without ILD (median: 58.1 pg/ml; range: 39.3 - 181.9 pg/ml; P<0.05) (Table).
Discussion
IL-4, also known as B-cell-stimulating factor, mainly promotes proliferation of T cells and induces antibody production by B cells. It can also stimulate proliferation, differentiation and activation of several other cell types, including fibroblasts, endothelial cells and epithelial cells and increase the recruitment of inflammatory cells. Levels of IL-4 and the proportion of CCR3-positive Th2 cells were elevated in the lungs of Nrf2-/- mice after bleomycin administration7. These cytokines enhance the fibrotic process by augmenting fibroblast proliferation and collagen production, and are required for the initiation and maintenance of pulmonary fibrosis.
Thus, like interferon-γ (IFN-γ), IL-4 may have both protective and pathogenic roles in RA, depending on the stage of disease8,9. This demonstrated the need for a better understanding of IL-4 functions in patients with RA. Some studies showed high levels of IL-4 in the BALF of patients with IPF and systemic scleroderma (SSc) patients with ILD10,11. Although the extent to which IL-4 participates in fibrosis varies in different diseases, it has long been considered a potent profibrotic mediator. Some RA patients will develop ILD and result in pulmonary fibrosis, which indicates a poor prognosis.
The present study did not show the difference of IL-4 levels between RA patients and normal controls. In contrast, RA patients with ILD showed higher levels of IL-4 than RA patients without ILD. The levels of IL-4 in BALF from RA patients with ILD were much higher than those without ILD. IL-4 can have both inflammatory and anti-inflammatory properties. It is, therefore, possible that in RA patients with ILD, the elevated IL-4 level is an attempt of the body to turn off the abnormal response. However, further studies are needed to clarify the exact role of IL-4 in RA and ILD.
IL-13 is a T cell specific cytokine that has a 30 per cent protein homology with IL-45. IL-4 and IL-13 have in common the IL-4 receptor, IL-4Rα, and IL-13 was presumed to have the same effector function as IL-4. Related studies have also shown a dominant role for IL-13 in the pathogenesis of pulmonary fibrosis12 and liver fibrogenesis13. Treatment with anti-IL-13 antibody markedly reduced collagen deposition in the lungs of animals challenged with Aspergilus fumigatus conidia14 or bleomycin15. IL-5 has also been shown to regulate tissue fibrogenesis16.
Though IL-13 and IL-5 are important in pulmonary fibrosis, we did not find any difference in patients with and without ILD. This suggests that different cytokines may have different effect on the development of fibrosis.
In summary, the present study demonstrated that levels of IL-4 were higher in RA patients with ILD. This suggested possible therapeutic significance of IL-4 in RA patients with ILD.
Acknowledgment
Authors acknowledge the National Nature Science Foundation of China under Grant: No. 81172867, Scientific Research of The First Hospital of China Medical University: fsfh1007 and Higher Education Department of Liaoning Province Research Grant: L2010613, L2010604, PR China, for financial support.
References
- 1.Wynn TA. Integrating mechanisms of pulmonary fibrosis. J Exp Med. 2011;208:1339–50. doi: 10.1084/jem.20110551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Homer RJ, Elias JA, Lee CG, Herzog E. Modern concepts on the role of inflammation in pulmonary fibrosis. Arch Pathol Lab Med. 2011;135:780–8. doi: 10.5858/2010-0296-RA.1. [DOI] [PubMed] [Google Scholar]
- 3.Sato T, Liu X, Basma H, Togo S, Sugiura H, Nelson A, et al. IL-4 induces differentiation of human embryonic stem cells into fibrogenic fibroblast-like cells. J Allergy Clin Immunol. 2011;127:1595–603. doi: 10.1016/j.jaci.2011.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bringardner BD, Baran CP, Eubank TD, Marsh CB. The role of inflammation in the pathogenesis of idiopathic pulmonary fibrosis. Antioxid Redox Signal. 2008;10:287–301. doi: 10.1089/ars.2007.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shao DD, Suresh R, Vakil V, Gomer RH, Pilling D. Pivotal Advance: Th-1 cytokines inhibit, and Th-2 cytokines promote fibrocyte differentiation. J Leukoc Biol. 2008;83:1323–33. doi: 10.1189/jlb.1107782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 7.Kikuchi N, Ishii Y, Morishima Y, Yageta Y, Haraguchi N, Itoh K, et al. Nrf2 protects against pulmonary fibrosis by regulating the lung oxidant level and Th1/Th2 balance. Respir Res. 2010;11:31. doi: 10.1186/1465-9921-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor PC. Anti-cytokines and cytokines in the treatment of rheumatoid arthritis. Curr Pharm Des. 2003;9:1095–106. doi: 10.2174/1381612033454991. [DOI] [PubMed] [Google Scholar]
- 9.Ohmura K, Nguyen LT, Locksley RM, Mathis D, Benoist C. Interleukin-4 can be a key positive regulator of inflammatory arthritis. Arthritis Rheum. 2005;52:1866–75. doi: 10.1002/art.21104. [DOI] [PubMed] [Google Scholar]
- 10.Emura M, Nagai S, Takeuchi M, Kitaichi M, Izumi T. In vitro production of B cell growth factor and B cell differentiation factor by peripheral blood mononuclear cells and bronchoalveolar lavage T lymphocytes from patients with idiopathic pulmonary fibrosis. Clin Exp Immunol. 1990;82:133–9. doi: 10.1111/j.1365-2249.1990.tb05416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmidt K, Martinez-Gamboa L, Meier S, Witt C, Meisel C, Hanitsch LG, et al. Bronchoalveoloar lavage fluid cytokines and chemokines as markers and predictors for the outcome of interstitial lung disease in systemic sclerosis patients. Arthritis Res Ther. 2009;11:R111. doi: 10.1186/ar2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuschiotti P. Role of IL-13 in systemic sclerosis. Cytokine. 2011;56:544–9. doi: 10.1016/j.cyto.2011.08.030. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Munker S, Mullenbach R, Weng HL. IL-13 Signaling in liver fibrogenesis. Front Immunol. 2012;3:116. doi: 10.3389/fimmu.2012.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blease K, Jakubzick C, Westwick J, Lukacs N, Kunkel SL, Hogaboam CM. Therapeutic effect of IL-13 immunoneutralization during chronic experimental fungal asthma. J Immunol. 2001;166:5219–24. doi: 10.4049/jimmunol.166.8.5219. [DOI] [PubMed] [Google Scholar]
- 15.Belperio JA, Dy M, Burdick MD, Xue YY, Li K, Elias JA, et al. Interaction of IL-13 and C10 in the pathogenesis of bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2002;27:419–27. doi: 10.1165/rcmb.2002-0009OC. [DOI] [PubMed] [Google Scholar]
- 16.Ochkur SI, Jacobsen EA, Protheroe CA, Biechele TL, Pero RS, McGarry MP, et al. Coexpression of IL-5 and eotaxin-2 in mice creates an eosinophil-dependent model of respiratory inflammation with characteristics of severe asthma. J Immunol. 2007;178:7879–89. doi: 10.4049/jimmunol.178.12.7879. [DOI] [PubMed] [Google Scholar]


