Abstract
Background & objectives:
Atopic diseases, including atopic dermatitis (AD), allergy and asthma, are complex diseases resulting from the effect of multiple genetic and interacting environmental factors on their pathophysiology. The genetic basis is incompletely understood; however, recent studies have shown an association between loss-of-function variants of the filaggrin gene (FLG) and atopic dermatitis. The aim of this study was to determine whether FLG variants can serve as a predictor for atopic diseases in Korean individuals.
Methods:
A total of 648 subjects were genotyped for the FLG P478S (rs11584340, C/T base change) polymorphism (322 patients and 326 controls). Serum levels of free fatty acids (FFA) and IgE were later stratified to determine the effects of the FLG polymorphism on AD.
Results:
A significant difference in genotype frequency was found between AD patients and controls in the FLG P478S polymorphism. The FLG P478S T allele carrier (TT+TC) was associated with AD risk (odds ratio = 1.877, 95% confidence interval 1.089 to 3.234). In addition, the P478S T allele was related to high levels of FFA in AD patients (471.79 ± 298.96 vs. 333.54 ± 175.82 μg eq/l, P <0.05).
Interpretation & conclusions:
The results of the present study suggest that the FLG P478S polymorphism alone and combined with other factors influences FFA levels and increases the susceptibility to AD.
Keywords: Atopic dermatitis, fatty acids, filaggrin, IgE, P478S, polymorphism
Allergic diseases such as allergic rhinitis (AR), asthma, and atopic dermatitis (AD) result from the complex interplay between genetic and environmental factors. Recently, there has been growing evidence that heritable skin barrier defects caused by mutations in the filaggrin gene (FLG) play an important role in the pathogenesis of AD1,2. Filaggrin has been reported to play an important role in skin-barrier formation and hydration. Filaggrin aggregates the keratin cytoskeleton to facilitate the collapse and flattening of keratinocytes in the outermost skin layer2. Profilaggrin, the precursor of filaggrin, accumulates in the keratohyalin granules which form the granular layers of the epidermis. During cornification, keratin filaments in keratinocytes are aggregated by filaggrin, resulting in flattening of the keratinocytes and eventually formation of the cornified cell envelope, which is crucial for the skin barrier function3. FLG-deficient mice show a predisposition to sensitization after percutaneous exposure to an allergen and develop cutaneous inflammatory infiltration and allergen-specific immune responses after allergen sensitization4. Null mutations in FLG are associated with AD in various populations, and some mutations have been shown to be associated with rhinitis and allergen sensitization in paediatric populations5,6.
To date, 20 FLG mutations have been identified in European populations. In Asian populations, an additional 17 mutations, of which eight are prevalent and nine occur at a low frequency, have been identified2. FLG is located within the epidermal differentiation complex on chromosome 1q21, a dense cluster of genes involved in the terminal differentiation of the epidermis and the formation of the stratum corneum7. In the European population, the two mutations R501X and 2282del4 are the most prevalent8,9. The nonsense mutation R501X and the frameshift mutation 2282del4 were the first mutations shown to be associated with the development of AD in a Caucasian population8,9. Subsequently, mutations R2447X, S3247X, and 3702delG were also reported to be associated with the development of AD in European population8. In Japanese population, the 3321delA frameshift mutation and the S2554X nonsense mutation were found to be associated with the development of AD, but these two mutations have not been found in European population10. The common single-nucleotide polymorphism (SNP) rs11584340 at codon 478 of the FLG, which encodes either proline (CCT) or serine (TCT), was found to be associated with AD and psoriasis in Chinese and Taiwanese populations11,12. Most FLG variants are specific to a population; and the FLG P478S polymorphism in the Korean population has not been adequately studied.
The purpose of this study was, therefore, to investigate the possible effects of FLG P478S variants in AD in Korean patients. In addition, various functional parameters were also evaluated in AD patients carrying the prevalent FLG variants.
Material & Methods
Study population: Between 2006 and 2010, the subjects were recruited consecutively from an ophthalmology, otolaryngology and dermatology clinic at the Kyung Hee University Korean Medical Center in Seoul, Korea, from an ongoing project to investigate candidate genes for atopy in the Korean population. In total, 201 patients with AD, 121 patients with AR or asthma, and 326 control subjects were enrolled in the study. AD cases were defined according to the diagnostic criteria of Hanifin and Rajka13. Asthma was diagnosed on the basis of whether patients answered that they had ever been diagnosed with asthma by a doctor.
AR was diagnosed based on typical AR symptoms as defined by the ARIA (Allergic Rhinitis and its Impact on Asthma) 2008 guidelines14 i.e., two or more AR symptoms (nasal congestion, rhinorrhoea, nasal itching, sneezing) persisting for four or more days a week during the past year. The patients with both AD and AR/asthma were excluded. The control subjects were recruited locally, primarily consisting of medical students and volunteers and had no history or clinical evidence of AD, AR or asthma. The control subjects were matched to each case subject by gender and age. Finally, 326 control subjects were available. Written informed consent was obtained from all participants. The study protocol was approved by the Kyung Hee University Korean Medical Center Ethics Committee.
The serum levels of total IgE and free fatty acids (FFA), and blood cell counts were determined by Seoul Clinical Laboratories, Seoul Medical Science Ins. (Seoul, Republic of Korea).
Genotyping: Genomic DNA was extracted from peripheral blood samples (200 μl) using the Exgene™ Blood SV Kit (GeneAll, Korea) as per the instructions from the manufacturer. The concentrations of DNA were estimated by absorbance at 260 nm. Genotyping of the FLG rs11584340 C/T polymorphism was carried out by a polymerase chain reaction (PCR) and restriction enzyme digestion. Briefly, forward (5’-CACGGAAAGGCTGGGCTGA-3’) and reverse (5’-ACCTGAGTGTCCAGACCTATT-3’) primers, designed to amplify the respective segments from exon 3 of the FLG as described previously11, was used in the amplification process. PCR was performed in a 20 μl volume containing 0.1 μM of each primer, 20 mM Tris-HCl (pH 8.4), 50 mM KCl, 1.5 mM MgCl2, 200 μM/dNTPs, 1 U of Taq DNA polymerase, and 100 ng of genomic DNA11. Reactions were amplified using the following extended PCR programme: one cycle of 95°C for 5 min; 10 cycles of 92°C for 10 sec and 49°C for 30 sec; and one cycle of 68°C for 6 min; and 28 cycles of 92°C for 10 sec, 49°C for 30 sec, and 68°C for 6 min, followed by one cycle of 68°C for 10 min. The 312 bp fragments were digested with 1 unit of HpyCH4III at 37°C overnight. The resulting products of three fragments (198, 66, and 48 bp) (T allele) and two fragments (246 and 66 bp) (C allele) are diagnostic.
Statistical analysis: Clinical characteristics of continuous variables were expressed as mean ± standard deviations (SDs) and were tested using an independent T-test. The significance of the genotype differences between groups was tested by χ2 method. Odds ratios (ORs) were determined by the Mantel-Haenszel method. A genetic Power Calculator (http://pngu.mgh.harvard.edu/~purcell/gpc)15 was used to compute the statistical power of the sample. All statistical analyses were performed using SPSS v17 (SPSS Inc., Chicago, Illinois, USA). P < 0.05 was considered significant.
Results
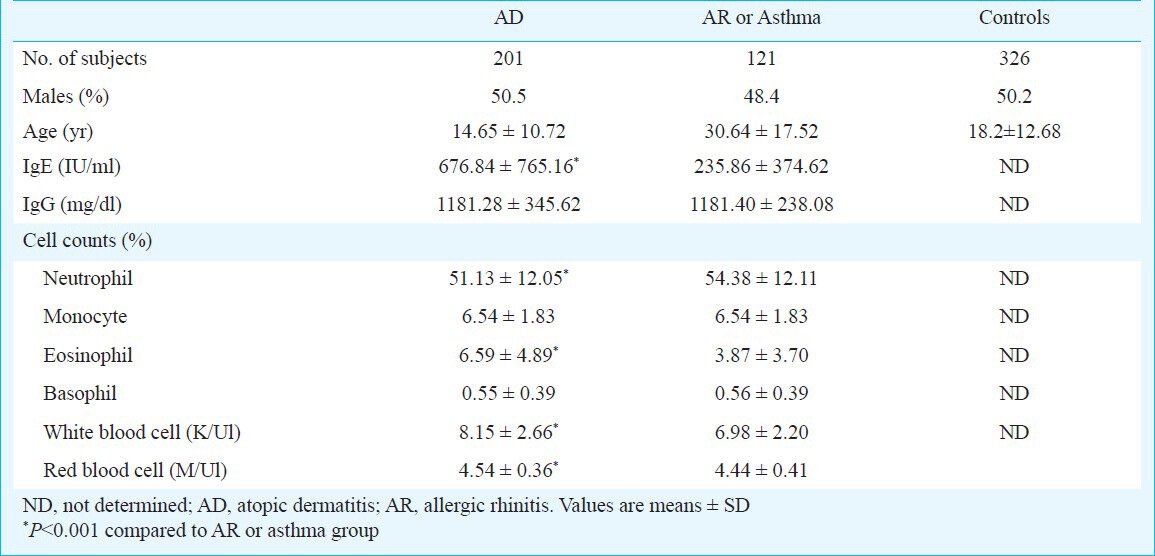
Characteristics of patients and controls: A total of 648 subjects participated in the study. These included 322 patients with a clinical diagnosis of AD, AR or asthma and 326 normal controls. Of the 322 cases, 201 (62.4%) had AD and 121 (37.6%) had AR or asthma. The AD group had 50.5 per cent male with a mean age of 14.65 ± 10.72 yr. There were 48.4 per cent male in the AR/asthma group with a mean age of 30.64 ± 17.52 yr. The controls group consisted of 326 normal individuals with a mean age of 18.2 ± 12.68 yr, and 50.2 per cent were male. The total IgE of the AD patients was higher than that of the AR or asthma patients (676.84 ± 765.16 vs. 235.86 ± 374.62 IU/ml, P=0.001). The white blood cell (WBC), red blood cell (RBC), and eosinophil counts of AD patients were also higher than that of AR or asthma patients (P<0.05) (Table I).
Table I.
Demographic and clinical characteristics of study population
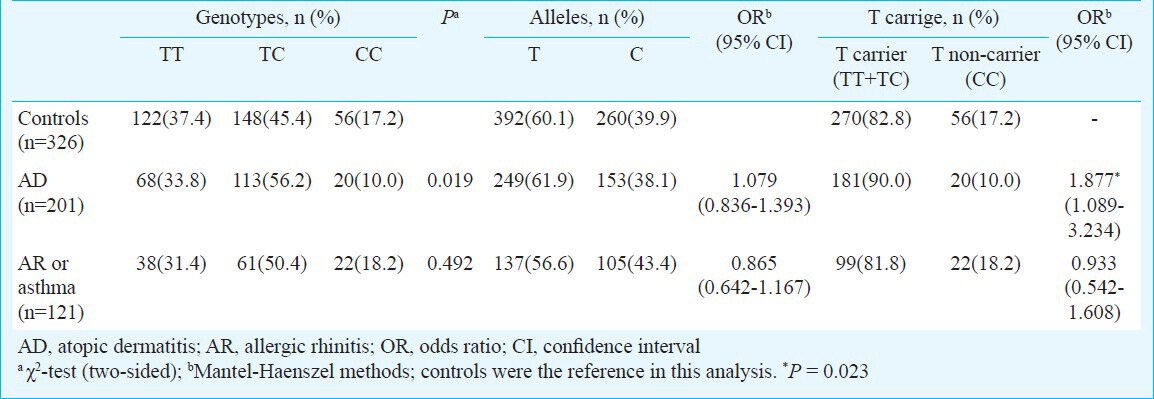
FLG genotype distribution of patients and controls: The genotype distribution in both the groups in patients and controls did not deviate from the Hardy-Weinberg equilibrium. The calculated power of the study was more than 90 per cent with these sample sizes to get 2-fold risk with α=0.05. Although distribution of genotypes TT, TC and CC in controls and AD patients were significantly different but the T and C allele frequencies in controls and patients were not significantly different (Table II). The frequencies of T allele containing genotypes were higher in the AD patients compared to the controls (90.0 vs. 82.8%), corresponding to OR of 1.877 with a 95% confidence interval (CI) 1.089-3.234. However, no significant difference was found between the AR/asthma patients and controls (81.8 vs. 82.8%). In addition, the TT + TC genotype of the P478S polymorphism was higher in the AD group than in either the AR or the asthma group (90.0 vs. 81.8%) (OR 2.011, 95% CI 1.047-3.865, P = 0.036) (Table II).
Table II.
Allele and genotype frequencies of P478S polymorphism of filaggrin gene in study population
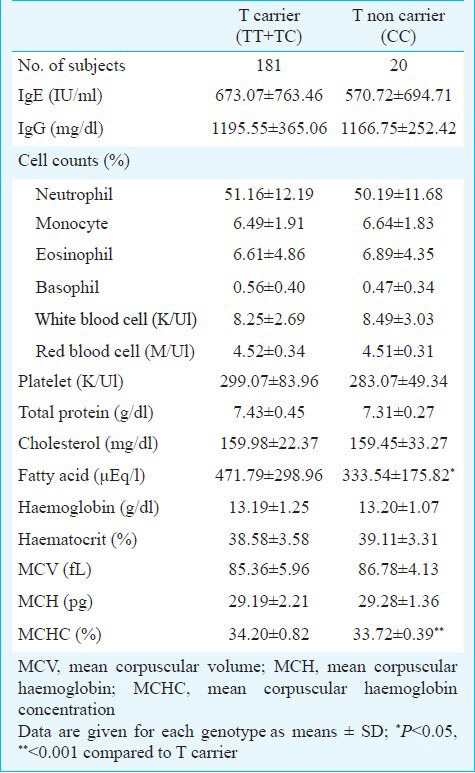
Clinical demography and genetic polymorphisms: For a better understanding of AD development, various functional parameters were investigated in AD patients carrying the prevalent FLG variants (Table III). AD subjects with the P478S TT + TC genotypes had significantly high levels of FFA compared to those carrying the CC genotype (471.79 ± 298.96 vs. 333.54 ± 175.82 μEq/l, P<0.05). In addition, the TT + TC genotypes were related to high levels of mean corpuscular haemoglobin concentrations (MCHC) in AD patients (34.20 ± 0.82 vs. 33.72 ± 0.39, P<0.001). However, no significant associations were detected from the FLG variants or other parameters such as the IgE level and peripheral eosinophil counts.
Table III.
Characteristics of AD phenotype according to filaggrin P478S T carriage
Discussion
In this study, the FLG P478S (rs11584340) polymorphism was found to be associated with AD, and affected the serum levels of FFA in AD patients. Our results further reinforce the role of the FLG P478S polymorphism in AD, which is a strong genetic factor identified in this common complex disease.
FLG P478S SNP is the most common variant of the FLG coding region in the SNP database of the NCBI10 and may serve as a good screening tool. Wang et al12 reported that the FLG P478S polymorphism was associated with AD in a Taiwanese population. Chang et al11 demonstrated the association of FLG P478S polymorphism with increased susceptibility to the development of psoriasis in Taiwanese Chinese. In this study, the frequencies of T allele containing genotypes were higher in cases than it was in the controls11, which was consistent with the findings obtained in the present study. Taiwanese Chinese and Korean have a similar allele frequency in many genetic studies, for example, the frequencies of the C and T alleles of interleukin (IL)-1 beta gene +3953 polymorphism16,17,18,19.
The significance of the functional change of FLG P478S remains unclear. Its location in exon 3 of the FLG is at the initiating portion of the first FLG repeat and very close to the first linker segment, codon 468-474, which connects the 5’-end to the truncated FLG repeat20. The 478 serine residue may be phosphorylated and hinder the action of protease cleavage, thus affecting the aggregation rate between filaggrin and keratin filaments. Another possible explanation is that an unrecognized functional mutation may be present at or adjacent to the FLG, which is in linkage disequilibrium with the P478S polymorphism, thereby contributing to the risk of AD. Further investigations will be necessary to identify the specific variants underlying the association observed here.
Previous studies hypothesized that a disturbance of the epidermal barrier in subjects with AD would subsequently lead to the development of AR and asthma in what is known as the atopic march21. However, our data did not show an association between AR and asthma and the P478S polymorphism. This result raises important questions about the mechanisms behind the association between a skin barrier gene and AR/asthma. In fact, as FLG is not expressed in the bronchial mucosa, it does not appear to affect asthma pathogenesis directly by regulating allergen permeability in the bronchial mucosa22. Hudson23 have reported that the loss-of-function mutations in the gene encoding filaggrin are important risk factors for atopic dermatitis, and interestingly, for asthma in association with atopic dermatitis, but not asthma in the absence of atopic dermatitis. Additional population-based studies are needed to evaluate whether the association between the FLG P478S polymorphism and AR and asthma is independent of the association with AD.
We investigated a possible association between FLG P478S variant alleles and various functional parameters in AD patients. An association between the FLG genotypes and FFA levels in subjects with AD was found. The WBC, RBC, and eosinophil counts of AD patients were also higher than that of AR or asthma patients. However, no significant difference was found between the FLG P478S genotypes and these parameters. Epidermal de novo fatty acid synthesis plays an important role in permeability barrier homeostasis24. Disruption of the permeability barrier results in a rapid and marked increase in fatty acid synthesis. Barrier disruption increases the activity and mRNA levels of both of the key enzymes required for de novo fatty acid synthesis, acetyl CoA carboxylase and fatty acid synthase. Moreover, following acute barrier disruption, inhibition of fatty acid synthesis delays the recovery of the permeability barrier function25. Further, the elongation of fatty acids is important, as animals deficient in the elongation of very-long-chain fatty acid-like 4 (ELOVL4) have severely compromised permeability barriers and do not survive after birth26,27,28. Our study showed significantly increased amounts of FFA in the serum of the FLG P478S mutation carriers compared to non-carriers in AD patients. In contrast to our findings, earlier studies found no differences or found reduced FFA in atopic skin29,30. These findings can be explained by the abnormal expressions of sphingomyelin acylase and deacylase and the altered sphingomyelin metabolism in AD31,32. The increase in serum fatty acid levels could be due to other factors such as decreased metabolic utilization or decreased uptake by cells.
In conclusion, our study showed that the FLG P478S polymorphism was associated with increased susceptibility to AD. Our findings pertaining to the contribution of a genetic factor to susceptibility to AD in individuals will be useful for developing a predictive test and for informing diagnostics and therapeutic advice.
Acknowledgment
This work was supported by the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MEST) (No. 2011-0006220).
References
- 1.Elias PM, Hatano Y, Williams ML. Basis for the barrier abnormality in atopic dermatitis: outside-inside-outside pathogenic mechanisms. J Allergy Clin Immunol. 2008;121:1337–43. doi: 10.1016/j.jaci.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Regan GM, Sandilands A, McLean WH, Irvine AD. Filaggrin in atopic dermatitis. J Allergy Clin Immunol. 2008;122:689–93. doi: 10.1016/j.jaci.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Candi E, Schmidt R, Melino G. The cornified envelope: a model of cell death in the skin. Nat Rev Mol Cell Biol. 2005;6:328–40. doi: 10.1038/nrm1619. [DOI] [PubMed] [Google Scholar]
- 4.Fallon PG, Sasaki T, Sandilands A, Campbell LE, Saunders SP, Mangan NE, et al. A homozygous frameshift mutation in the mouse FLG gene facilitates enhanced percutaneous allergen priming. Nat Genet. 2009;41:602–8. doi: 10.1038/ng.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imoto Y, Enomoto H, Fujieda S, Okamoto M, Sakashita M, Susuki D, et al. S2554X mutation in the filaggrin gene is associated with allergen sensitization in the Japanese population. J Allergy Clin Immunol. 2010;125:498–500. doi: 10.1016/j.jaci.2009.10.062. [DOI] [PubMed] [Google Scholar]
- 6.Henderson J, Northstone K, Lee SP, Liao H, Zhao Y, Pembrey M, et al. The burden of disease associated with filaggrin mutations: a population-based, longitudinal birth cohort study. J Allergy Clin Immunol. 2008;121:872–87. doi: 10.1016/j.jaci.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 7.Hoffjan S, Stemmler S. On the role of the epidermal differentiation complex in ichthyosis vulgaris, atopic dermatitis and psoriasis. Br J Dermatol. 2007;157:441–9. doi: 10.1111/j.1365-2133.2007.07999.x. [DOI] [PubMed] [Google Scholar]
- 8.Sandilands A, Terron-Kwiatkowski A, Hull PR, O’Regan GM, Clayton TH, Watson RM, et al. Comprehensive analysis of the gene encoding filaggrin uncovers prevalent and rare mutations in ichthyosis vulgaris and atopic eczema. Nat Genet. 2007;39:650–4. doi: 10.1038/ng2020. [DOI] [PubMed] [Google Scholar]
- 9.Akiyama M. FLG mutations in ichthyosis vulgaris and atopic eczema: spectrum of mutations and population genetics. Br J Dermatol. 2010;162:472–7. doi: 10.1111/j.1365-2133.2009.09582.x. [DOI] [PubMed] [Google Scholar]
- 10.Ma L, Zhang L, Di ZH, Zhao LP, Lu YN, Xu J, et al. Association analysis of filaggrin gene mutations and atopic dermatitis in Northern China. Br J Dermatol. 2010;162:225–7. doi: 10.1111/j.1365-2133.2009.09539.x. [DOI] [PubMed] [Google Scholar]
- 11.Chang YC, Wu WM, Chen CH, Hu CF, Hsu LA. Association between P478S polymorphism of the filaggrin gene and risk of psoriasis in a Chinese population in Taiwan. Arch Dermatol Res. 2008;300:133–7. doi: 10.1007/s00403-007-0821-2. [DOI] [PubMed] [Google Scholar]
- 12.Wang IJ, Lin TJ, Kuo CF, Lin SL, Lee YL, Chen PC. Filaggrin polymorphism P478S, IgE level, and atopic phenotypes. Br J Dermatol. 2011;164:791–6. doi: 10.1111/j.1365-2133.2011.10212.x. [DOI] [PubMed] [Google Scholar]
- 13.Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol (Stockh) 1980;92(Suppl):44–7. [Google Scholar]
- 14.Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen) Allergy. 2008;63:8–160. doi: 10.1111/j.1398-9995.2007.01620.x. [DOI] [PubMed] [Google Scholar]
- 15.Purcell S, Cherny SS, Sham PC. Genetic Power Calculator: design of linkage and association genetic mapping studies of complex traits. Bioinformatics. 2003;19:149–50. doi: 10.1093/bioinformatics/19.1.149. [DOI] [PubMed] [Google Scholar]
- 16.Um JY, Chung HS, Song MY, Shin HD, Kim HM. Association of interleukin-1β gene polymorphism with body mass index in women. Clin Chem. 2004;50:647–50. doi: 10.1373/clinchem.2003.025858. [DOI] [PubMed] [Google Scholar]
- 17.Um JY, Jang CH, Kim HL, Cho YB, Park J, Lee SJ, et al. Proinflammatory cytokine IL-1 β polymorphisms in sudden sensorineural hearing loss. Immunopharmacol Immunotoxicol. 2013;35:52–6. doi: 10.3109/08923973.2012.719523. [DOI] [PubMed] [Google Scholar]
- 18.Kang L, Chen CH, Yu CH, Chang CH, Chang FM. Interleukin-1β gene is not associated with preeclampsia in Taiwanese. Taiwan J Obstet Gynecol. 2012;51:240–4. doi: 10.1016/j.tjog.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 19.Cheng HH, Chang CS, Wang HJ, Wang WC. Interleukin-1beta and -10 polymorphisms influence erosive reflux esophagitis and gastritis in Taiwanese patients. J Gastroenterol Hepatol. 2010;25:1443–51. doi: 10.1111/j.1440-1746.2010.06310.x. [DOI] [PubMed] [Google Scholar]
- 20.Presland RB, Haydock PV, Fleckman P, Nirunsuksiri W, Dale BA. Characterization of the human epidermal profilaggrin gene. Genomic organization and identification of an S-100-like calcium binding domain at the amino terminus. J Biol Chem. 1992;267:23772–81. [PubMed] [Google Scholar]
- 21.Marenholz I, Nickel R, Rüschendorf F, Schulz F, Esparza-Gordillo J, Kerscher T, et al. Filaggrin loss-of-function mutations predispose to phenotypes involved in the atopic march. J Allergy Clin Immunol. 2006;118:866–71. doi: 10.1016/j.jaci.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 22.Ying S, Meng Q, Corrigan CJ, Lee TH. Lack of filaggrin expression in the human bronchial mucosa. J Allergy Clin Immunol. 2006;118:1386–8. doi: 10.1016/j.jaci.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 23.Hudson TJ. Skin barrier function and allergic risk. Nat Genet. 2006;38:399–400. doi: 10.1038/ng0406-399. [DOI] [PubMed] [Google Scholar]
- 24.Feingold KR. The outer frontier: the importance of lipid metabolism in the skin. J Lipid Res. 2009;50(Suppl):S417–22. doi: 10.1194/jlr.R800039-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feingold KR. Thematic review series: skin lipids. The role of epidermal lipids in cutaneous permeability barrier homeostasis. J Lipid Res. 2007;48:2531–46. doi: 10.1194/jlr.R700013-JLR200. [DOI] [PubMed] [Google Scholar]
- 26.Cameron DJ, Tong Z, Yang Z, Kaminoh J, Kamiyah S, Chen H, et al. Essential role of Elovl4 in very long chain fatty acid synthesis, skin permeability barrier function, and neonatal survival. Int J Biol Sci. 2007;3:111–9. doi: 10.7150/ijbs.3.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li W, Sandhoff R, Kono M, Zerfas P, Hoffmann V, Ding BC, et al. Depletion of ceramides with very long chain fatty acids causes defective skin permeability barrier function, and neonatal lethality in ELOVL4 deficient mice. Int J Biol Sci. 2007;3:120–8. doi: 10.7150/ijbs.3.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vasireddy V, Uchida Y, Salem N, Jr, Kim SY, Mandal MN, Reddy GB, et al. Loss of functional ELOVL4 depletes very long-chain fatty acids (> or =C28) and the unique omega-O-acylceramides in skin leading to neonatal death. Hum Mol Genet. 2007;16:471–82. doi: 10.1093/hmg/ddl480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamamoto A, Serizawa S, Ito M, Sato Y. Stratum corneum lipid abnormalities in atopic dermatitis. Arch Dermatol Res. 1991;283:219–23. doi: 10.1007/BF01106105. [DOI] [PubMed] [Google Scholar]
- 30.Bleck O, Abeck D, Ring J, Hoppe U, Vietzke JP, Wolber R, et al. Two ceramide subfractions detectable in Cer(AS) position by HPTLC in skin surface lipids of non-lesional skin of atopic eczema. J Invest Dermatol. 1999;113:894–900. doi: 10.1046/j.1523-1747.1999.00809.x. [DOI] [PubMed] [Google Scholar]
- 31.Hara J, Higuchi K, Okamoto R, Kawashima M, Imokawa G. High-expression of sphingomyelin deacylase is an important determinant of ceramide deficiency leading to barrier disruption in atopic dermatitis. J Invest Dermatol. 2000;115:406–13. doi: 10.1046/j.1523-1747.2000.00072.x. [DOI] [PubMed] [Google Scholar]
- 32.Murata Y, Ogata J, Higaki Y, Kawashima M, Yada Y, Higuchi K, et al. Abnormal expression of sphingomyelin acylase in atopic dermatitis: an etiologic factor for ceramide deficiency? J Invest Dermatol. 1996;106:1242–9. doi: 10.1111/1523-1747.ep12348937. [DOI] [PubMed] [Google Scholar]


