Abstract
Background & objectives:
Most studies on the clinical presentation with influenza viruses have been conducted in outpatient or inpatient medical facilities with only a few studies in community settings. Clinical differences between influenza A (H1N1) pdm 09 and influenza B virus infections have importance for community-based public health surveillance. An active community surveillance at the time of emergence of pandemic influenza provided us with an opportunity to compare the clinical features among patients infected with influenza A (H1N1) pdm09 virus and those with influenza B virus co-circulating in an active community-based weekly surveillance in three villages in Faridabad, Haryana, north India.
Methods:
Active surveillance for febrile acute respiratory infection (FARI) was carried out in a rural community (n=16,182) in the context of an inactivated trivalent influenza vaccine trial (among children <11 yr). Individuals with FARI were assessed clinically by nurses and respiratory samples collected and tested for influenza viruses by real time RT-PCR from November 2009 to August 2010. Clinical symptoms of patients with influenza A (H1N1) pdm 09 and influenza B infection were compared.
Results:
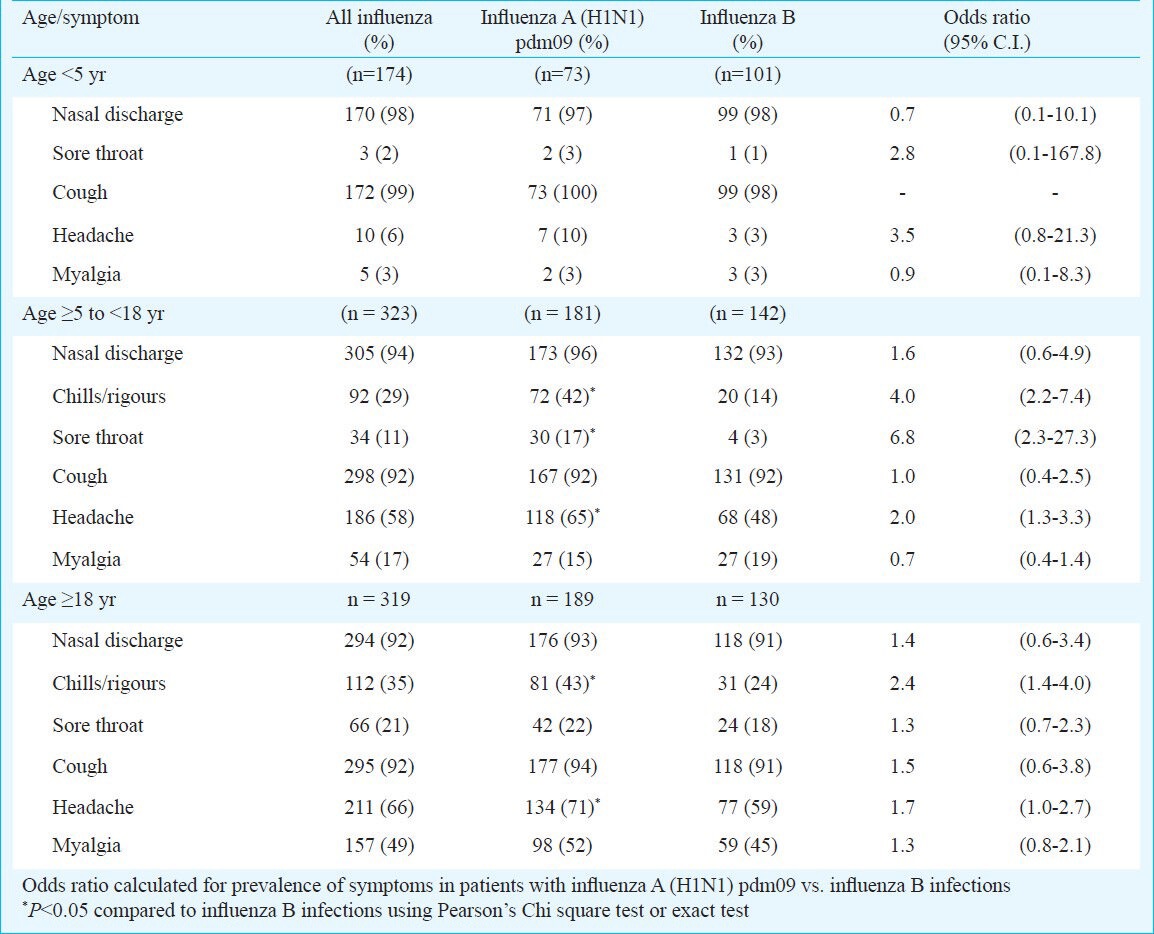
Of the 4796 samples tested, 822 (17%) were positive for influenza virus. Of these, 443 (54%) were influenza A (H1N1) pdm09, 373 (45%) were influenza B and six were other subtypes/mixed infections. The mean age was lower for patients with influenza B (16.4 yr) than influenza A (H1N1) pdm09 infection (18.7 yr; P=0.04). Among children aged 5-18 yr, chills/rigours (OR 4.0; CI 2.2, 7.4), sore throat (OR 6.8; CI 2.3, 27.3) and headache (OR2.0; CI 1.3, 3.3) were more common in influenza A (H1N1) pdm09 infection than in influenza B cases. Chills/rigours (OR 2.4; CI 1.4, 4.0) and headache (OR 1.7; CI 1.0, 2.7) were associated with influenza A (H1N1) pdm09 infection in those >18 yr. No significant differences were seen in children <5 yr.
Conclusion:
Our findings show that the differences in the clinical presentation of influenza A(H1N1)pdm09 and influenza B infections are not likely to be of clinical or public health significance.
Keywords: Diagnosis, influenza A, influenza B, H1N1 subtype, respiratory tract infections, signs and symptoms
The world experienced the emergence of influenza A (H1N1) pdm091 virus in April 2009 which spread worldwide resulting in a pandemic2. India witnessed its first influenza A (H1N1) pdm09 case in May 20093. Various aspects of the pandemic influenza A (H1N1) pdm09 virus have been described including transmission efficiency, secondary attack rates, disease severity, risk factors for severe disease, and effectiveness of antiviral treatment4,5,6,7,8,9. While the initial epidemiology and presentation of the disease were severe during the peak pandemic phase, the influenza A (H1N1) pdm09 has now become the circulating seasonal strain of H1N1 in the post pandemic phase10. Most reports of clinical presentation of influenza A (H1N1) pdm09 infections have been based on studies carried out in inpatient or outpatient medical facilities11,12,13,14,15,16,17,18, with only a few studies reporting on the clinical presentation in a community setting7,19,20,21,22. Further, most of these studies have only compared clinical symptoms of influenza A(H1N1)pdm09 with seasonal influenza A strains11,12,16,18,23,24. The current evidence shows that the initial presentations of novel influenza A (H1N1) pdm09 do not differ significantly from that of seasonal influenza A. Most studies have not compared separately the clinical presentation of influenza B with influenza A(H1N1) pdm09 infection14,19,20,25 and among non-hospitalized cases.
We carried out this study to compare the clinical features among patients infected with influenza A(H1N1) pdm09 virus and those with influenza B virus identified by a community-based weekly active surveillance system in three villages in northern India as part of an influenza vaccine trial.
Material & Methods
Study setting: In November 2009, children aged 6 months - 10 yr were vaccinated with either inactivated trivalent influenza vaccine (TIV) or the control vaccine (inactivated polio vaccine) as part of a three-year prospective household randomized controlled observer-blinded study initiated in three villages (Dayalpur, Chandawali and Atali) located in Ballabgarh block of Faridabad district in Haryana, north India26. These villages are a part of the rural field practice area of All India Institute of Medical Sciences (AIIMS), New Delhi. The population under surveillance included 16,182 individuals (of all age groups) in November 2009, with an estimated 3,700 children aged 6 months-10 years. The vaccination was done in November-December 2009.
Surveillance methodology: Active surveillance for febrile acute respiratory infection (FARI) was conducted through weekly household screening by field workers27. FARI was defined as a patient's self or proxy report of fever with at least one of the following symptoms: cough, shortness of breath, sore throat, rhinorrhoea or ear ache, during the preceding seven days. Each identified FARI episode was evaluated by a nurse who recorded the history of fever, chills or rigour, headache, sore throat, cough, runny nose, malaise, myalgia, vomiting, diarrhoea and abdominal pain. A brief clinical examination of the subjects was performed. Throat and nasal swabs from FARI patients were collected and transported in viral transport media to the virology laboratory at AIIMS, New Delhi, within 24 h. Patients were offered appropriate treatment by the physicians. The quality control standards included supervision of 10 per cent of screening visits by medical social workers and 10 per cent of medical assessments by physicians26.
Laboratory diagnosis: The samples were tested by real time RT-PCR for detection of seasonal influenza A and B viruses and influenza A(H1N1) pdm09 using the US Centers for Disease Control and Prevention protocol28. All influenza A positive samples were further sub-typed using primers and probes for A/H1 and A/H329.
Data analysis: Data from FARI cases identified during November 2009 - August 2010, in whom influenza viruses were detected, were entered into MS-Excel. Odds ratios (OR), independent sample t-tests and Chi-square test were used to compare demographic and clinical features of patients infected with influenza A(H1N1) pdm09 and influenza B viruses. Interaction between age and clinical features was assessed by stratifying the patients in three groups: <5 yr, ≥5 to 18 yr and ≥18 yr.
The Institutional Review Board of the University of Alabama, USA and Ethics Committee of AIIMS, New Delhi, India approved the study protocol for the vaccine trial. Written informed consent and/or ascent (in age 7-17 yr) was obtained from each individual participant/legal guardians at the initiation of the study. The clinical trial was enrolled in the Clinical Trials Registry of India (CTRI/2010/091/001235, 13-10-2010) as well as at (clincialtrials.gov) (NCT00934245).
Results
Of the 5533 FARI cases identified, 4796 patients were available for collection of nasopharyngeal samples and influenza viruses were identified in 822 (17%). Of these, 443 (54%) were identified as influenza A (H1N1) pdm09, 373 (45%) as influenza B virus and six were other infections [3 influenza A H3N2, 3 co-infections with influenza A (H1N1) pdm09 and influenza B]. The analysis was restricted to cases with singleton infection with either influenza A (H1N1) pdm09 or influenza B viruses (total 816 cases).
Demographic and clinical features of patients included in the analysis are shown in Table I. A large proportion of patients were <5 yr old in influenza B infection group as compared to the patients with influenza A(H1N1) pdm09 infection (27 vs. 16%, P<0.05). The mean age was lower for patients with influenza B (16.4 yr) than influenza A (H1N1) pdm09 infection (18.7 yr; P=0.04). No gender differences were seen among the patients infected with influenza A (H1N1) pdm09 and influenza B viruses (51% male in both groups). Overall M/F ratio was 1.03 (CI- 0.89-1.17), but M/F ratios of 1.2 (CI 0.92-1.5) and 1.4 (CI 1.1-1.7) were observed among patients under 18 yr age with influenza A (H1N1) pdm09 and influenza B virus infection, respectively, while these ratios were 0.84 (CI 0.59-1.07) and 0.54 (CI 0.34-0.74), respectively among patients >18 yr age. Twelve individuals were ≥65 yr and among them four tested positive for influenza A (H1N1) pdm09 virus.
Table I.
Demographic and clinical features of patients with febrile acute respiratory infection positive for influenza A (H1N1) pdm09 or influenza B viruses

The most common symptoms among patients detected with influenza A(H1N1) pdm09 were nasal discharge/runny nose (95%) and cough (94%) and the distribution of these symptoms among patients infected with influenza B was similar (Table I). Bi-variate analysis showed chills/rigours (data only collected in those >5 yr of age), sore throat (all ages) and headache (all ages) to be significantly (P<0.05) more prevalent among individuals infected with influenza A (H1N1) pdm09 virus than with those infected with influenza B. Measured temperature of 37.8°C or greater was noted in 5.5 per cent cases of all influenza positives. Gastrointestinal symptoms (i.e. abdominal pains, vomiting and/or diarrhoea) were infrequent and were not associated with influenza A (H1N1) pdm09 infection (Table I). Among the cases, six children presented as pneumonia [4 influenza B and 2 influenza A (H1N1) pdm09] and one child as severe pneumonia (influenza B).
As there were differences in age distribution of patients with influenza A (H1N1) pdm09 and influenza B infection, their clinical features were analysed by age-groups (Table II). Among children <5 yr, there were no significant differences in clinical presentation of influenza B and influenza A (H1N1) pdm09 infections. In the 5-18 yr age group, presence of chills/rigours (OR 4.0; CI 2.2, 7.40), sore throat (OR 6.8; CI 2.3, 27.3) and headache (OR 2.0; CI 1.3, 3.3) was significantly associated with influenza A (H1N1) pdm09 infection. In cases ≥18 yr of age, presence of chills/rigours (OR 2.4; CI 1.4, 4.0) and headache (OR 1.7, CI 1.0, 2.7) was significantly associated with influenza A (H1N1) pdm09 infection.
Table II.
Comparison of symptoms of individuals infected with influenza A (H1N1) pdm09 and influenza B viruses by age group
Discussion
The present study was unique because it was conducted in a sub-tropical area, performed at the community level data for collection, and included a large sample size. Additionally, the study period (November 2009 - August 2010) included both the periods of circulation of pandemic and influenza B virus.
The comparison of clinical features among patients infected with influenza A (H1N1) pdm09 virus and those with influenza B virus showed that in older age groups, certain clinical symptoms were more common with pandemic influenza A (H1N1) pdm09 virus infection. In a study from Nicaragua with community-based surveillance, the authors have reported that children with influenza A (H1N1) pdm09 virus infection were more likely to present with the symptoms of lower respiratory infection (sore throat, wheezing, rhonchi, crepitations, pneumonia) and gastrointestinal symptoms (nausea, and loss of appetite) than children with seasonal influenza (H3N2 and B) infection20. Another study from Singapore conducted among military camps reports clinical differences between influenza A (H1N1) pdm09 virus and seasonal influenza (H3N2 and B)21. While these studies identified suspect influenza cases from among those presenting to outpatient clinics and camp clinic with symptoms, in the present study we have identified them through active household surveillance. Thus, the patients with milder illnesses were also included who were not likely to have sought medical care. A study from Singapore reported that runny nose and dyspnoea were more common in seasonal influenza virus infection while cough and sore throat were more common in influenza A (H1N1) pdm09 infection14. Another study from Gujarat, India, conducted among hospitalized cases reported similar proportion of respiratory symptoms (cough, nasal discharge, sore throat) among patients with influenza A (H1N1) pdm09 and seasonal influenza virus infections16. While several studies have reported more gastrointestinal symptoms (vomiting, diarrhoea, nausea, loss of appetite) in influenza A (H1N1) pdm0918,20, we did not find any significant difference in the occurrence of these symptoms among our study groups. A possible explanation for these observed differences could be that our case enrollment from the community yielded a sample of mostly non-severe cases, while most previous studies were hospital/clinic based.
Our finding were comparable to the results of a study from Singapore that demonstrated significant differences among pandemic and seasonal groups in likelihood of fever, cough, rhinorrhoea and dyspnoea in patients identified from primary care facilities and hospitals14. Likewise, a hospital-based study in Philadelphia, Pennsylvania, found cough (98 vs 83%), myalgias (71 vs 46%) and pleuritic chest pain (45 vs 15%) significantly more common in pandemic compared to seasonal influenza cases13. Comparison of the clinical manifestations among influenza A (H1N1) pdm09 and influenza B positives revealed age-specific differences. Chills/rigours, sore-throat and headache in age 5-18 group and chills/rigours in ≥18 yr age group were more common with influenza A (H1N1) pdm09 infection when compared to influenza B infection. A similar finding has been reported from Nicaragua with sore throat being significantly associated with influenza A (H1N1) pdm09 virus infection among 2-14 yr aged patients20. Direct comparisons become difficult since most previous studies have used a comparison group comprising predominantly of patients with seasonal influenza A virus infections. Our study finding of different M/F ratios for cases <18 and >18 yr age groups was comparable with a report from Japan30.
The limitations of the study include a possibility of measurement errors. The symptoms of headache, sore throat, or myalgia may not be easily or completely ascertained in children, especially in those under 5 yr. In the absence of the patient, the information for screening criteria was collected by proxy, which could result in under-identification of cases. However, this is unlikely to have affected comparisons across identified patients. The clinical presentation of children included in the present analysis may also have been altered in case they had received killed (northern hemisphere) trivalent influenza vaccine in November-December 2009 as a part of the trial. This vaccine did not include the influenza A (H1N1) pdm09 virus but influenza B virus was one of the components. Since we did not observe any major difference in the clinical findings between those infected with influenza B or pandemic influenza, that effect of influenza vaccination may likely be minimal.
Despite these limitations, our findings mostly complement the studies done in community and health care facility settings. Fever being an essential criterion in FARI, all cases had a history of fever but only 5.5 per cent had measured temperature of ≥37.8 °C. Possible explanations could be the time delay between onset of fever and clinical examination and the use of antipyretics. The most commonly used screening definition in influenza surveillance, influenza-like illnesses (ILI), is defined as “a temperature of ≥37.8°C, oral or equivalent, and cough and/or sore throat, in the absence of a known cause other than influenza”31,32. Among the components of ILI definition, only sore throat was found to be more common among pandemic than seasonal influenza cases, and only among those 5-18 yr old. As temperature measurement at home is not common in rural populations such as ours, definitions of ILI which require a documented fever may be insensitive. Inclusion of history of fever in addition to its measurement in the ILI definition may improve its sensitivity.
In conclusion, there were minor differences in the clinical presentation of patients with influenza A (H1N1) pdm09 and influenza B infections in the studied rural Indian community, and the importance of these for case identification and management needs further exploration.
Acknowledgment
Authors thank the residents of the study villages, the field and laboratory staff for their commitment, and the CDC Influenza Division personnel for their constructive comments during the pre-submission peer-review process. Anthony Mounts and Joshua Mott participated in initial planning for this study. The study was supported by cooperative agreements U01IP000177 from the Centers for Disease Control and Prevention, Atlanta, USA.
References
- 1.Standardization of terminology of the pandemic A (H1N1) 2009 virus. Wkly Epidemiol Rec. 2011;86:480. [PubMed] [Google Scholar]
- 2.Outbreak of Swine-Origin Influenza A (H1N1) Virus Infection - Mexico, March - April. 2009. [accessed on February 22, 2011]. Available from: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5817a5.htm . [PubMed]
- 3.Potdar VA, Chadha MS, Jadhav SM, Mullick J, Cherian SS, Mishra AC. Genetic characterization of the influenza A pandemic (H1N1) 2009 virus isolates from India. PLoS One. 2010;5:e9693. doi: 10.1371/journal.pone.0009693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mishra AC, Chadha MS, Choudhary ML, Potdar VA. Pandemic influenza (H1N1) 2009 is associated with severe disease in India. PLoS One. 2010;5:7. doi: 10.1371/journal.pone.0010540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mukherjee A, Roy T, Agarwal AS, Sarkar M, Lal R, Chakrabarti S, et al. Prevalence and epidemiology of pandemic H1N1 strains in hospitals of Eastern India. J Public Health Epidemiol. 2010;2:171–4. [Google Scholar]
- 6.Campbell A, Rodin R, Kropp R, Mao Y, Hong Z, Vachon J, et al. Risk of severe outcomes among patients admitted to hospital with pandemic (H1N1) influenza. Can Med Assoc J. 2010;182:349–55. doi: 10.1503/cmaj.091823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Looker C, Carville K, Grant K, Kelly H. Influenza A (H1N1) in Victoria, Australia: A community case series and analysis of household transmission. PLoS One. 2010;5:e13702. doi: 10.1371/journal.pone.0013702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldberg L, Greenberg D, Zelcer I, Slanovic L, Shemer-Avni Y, Nativ R, et al. Epidemiologic, clinical, laboratory, and therapeutic characteristics of influenza A/H1N1 in Moslem Bedouin and Jewish children hospitalized in Southern Israel during 2009. Pediatr Infect Dis J. 2011;30:530–3. doi: 10.1097/INF.0b013e31821810ff. [DOI] [PubMed] [Google Scholar]
- 9.Poeppl W, Hell M, Herkner H, Stoiser B, Fritsche G, Schurz-Bamieh N, et al. Clinical aspects of 2009 pandemic influenza A (H1N1) virus infection in Austria. Infection. 2011;39:341–52. doi: 10.1007/s15010-011-0121-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.H1N1 in post-pandemic period: Director-General's opening statement at virtual press conference. World Health Organization (WHO) 2010. [accessed on September 6, 2011]. Available from: http://www.who.int/mediacentre/news/statements/2010/h1n1_vpc_20100810/en/index.html .
- 11.Tamma P, Turnbull A, Milstone A, Cosgrove S, Valsamakis A, Budd A, et al. Clinical outcomes of seasonal influenza and pandemic influenza A (H1N1) in pediatric inpatients. BMC Pediatrics. 2010;10:72. doi: 10.1186/1471-2431-10-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindblade KA, Arvelo W, Gray J, Estevez A, Frenkel G, Reyes L, et al. A comparison of the epidemiology and clinical presentation of seasonal influenza A and 2009 pandemic influenza A (H1N1) in Guatemala. PLoS One. 2010;5(12):11. doi: 10.1371/journal.pone.0015826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shiley KT, Nadolski G, Mickus T, Fishman NO, Lautenbach E. Differences in the epidemiological characteristics and clinical outcomes of pandemic (H1N1) 2009 influenza, compared with seasonal influenza. Infect Control Hosp Epidemiol. 2010;31:676–82. doi: 10.1086/653204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang JW-T, Tambyah PA, Lai FYL, Lee HK, Lee CK, Loh TP, et al. Differing symptom patterns in early pandemic vs seasonal influenza infections. Arch Intern Med. 2010;170:861–7. doi: 10.1001/archinternmed.2010.108. [DOI] [PubMed] [Google Scholar]
- 15.Kelly HA, Grant KA, Williams S, Fielding J, Smith D. Epidemiological characteristics of pandemic influenza H1N1 2009 and seasonal influenza infection. Med J Aust. 2009;191:146–9. doi: 10.5694/j.1326-5377.2009.tb02723.x. [DOI] [PubMed] [Google Scholar]
- 16.Chudasama RK, Patel UV, Verma PB. Hospitalizations associated with 2009 influenza A (H1N1) and seasonal influenza in Saurashtra region, India. J Infect Dev Ctries. 2010;4:834–41. doi: 10.3855/jidc.1049. [DOI] [PubMed] [Google Scholar]
- 17.Ong AK, Chen MI, Lin L, Tan AS, Nwe NW, Barkham T, et al. Improving the clinical diagnosis of influenza - a comparative analysis of new influenza A (H1N1) cases. PLoS One. 2009;4:e8453. doi: 10.1371/journal.pone.0008453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang Y-S, van Hal SJ, Spencer PM, Gosbell IB, Collett PW. Comparison of adult patients hospitalised with pandemic (H1N1) 2009 influenza and seasonal influenza during the “Protect” phase of the pandemic response. Med J Aust. 2010;192:90–3. doi: 10.5694/j.1326-5377.2010.tb03426.x. [DOI] [PubMed] [Google Scholar]
- 19.Cowling BJ, Chan KH, Fang VJ, Lau LLH, So HC, Fung ROP, et al. Comparative epidemiology of pandemic and seasonal influenza A in households. N Engl J Med. 2010;362:2175–84. doi: 10.1056/NEJMoa0911530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gordon A, Saborío S, Videa E, López R, Kuan G, Balmaseda A, et al. Clinical attack rate and presentation of pandemic H1N1 influenza versus seasonal influenza A and B in a pediatric cohort in Nicaragua. Clin Infect Dis. 2010;50:1462–7. doi: 10.1086/652647. [DOI] [PubMed] [Google Scholar]
- 21.Yap J, Tan CH, Cook AR, Loh JP, Tambyah PA, Tan BH, et al. Differing clinical characteristics between influenza strains among young healthy adults in the tropics. BMC Infect Dis. 2012;12:12. doi: 10.1186/1471-2334-12-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khuwaja S, Mgbere O, Awosika-Olumo A, Momin F, Ngo K. Using sentinel surveillance system to monitor seasonal and novel H1N1 influenza infection in Houston, Texas: Outcome Analysis of 2008-2009 Flu Season. J Community Health. 2011;36:857–63. doi: 10.1007/s10900-011-9386-2. [DOI] [PubMed] [Google Scholar]
- 23.Adhikari BR, Shakya G, Upadhyay BP, Prakash Kc K, Shrestha SD, Dhungana GR. Outbreak of pandemic influenza A/H1N1 2009 in Nepal. Virol J. 2011;8:133–41. doi: 10.1186/1743-422X-8-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aguirre E, Papenburg J, Ouakki M, Fontela PS, Guimont C, De Serres G, et al. Comparison of pandemic and seasonal influenza in the pediatric emergency department. Pediatr Infect Dis J. 2011;30:633–9. doi: 10.1097/INF.0b013e3182103d54. [DOI] [PubMed] [Google Scholar]
- 25.Carcione D, Giele C, Dowse GK, Mak DB, Goggin L, Kwan K, et al. Comparison of pandemic (H1N1) 2009 and seasonal influenza, Western Australia, 2009. Emerg Infect Dis. 2010;16:1388–95. doi: 10.3201/eid1609.100076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sullender W, Fowler KB, Krishnan A, Gupta V, Moulton LH, Lafond K, et al. Design and initiation of a study to assess the direct and indirect effects of influenza vaccine given to children in rural India. Vaccine. 2012;30:5235–9. doi: 10.1016/j.vaccine.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shu B, Wu K-H, Emery S, Villanueva J, Johnson R, Guthrie E, et al. Design and performance of the CDC real-time reverse transcriptase PCR swine flu panel for detection of 2009 A (H1N1) pandemic influenza virus. J Clin Microbiol. 2011;49:2614–9. doi: 10.1128/JCM.02636-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.CDC protocol of real-time RTPCR for swine influenza A (H1N1) (2009) Available from: http://www.who.int/csr/resources/publications/swineflu/CDCrealtimeRTPCRprotocol_20090428.pdf .
- 29.WHO information for molecular diagnosis of influenza virus in humans-update, WHO. August. 2011. Available from : http://www.who.int/influenza/resources/documents/molecular_diagnosis_influenza_virus_humans_update_201108.pdf .
- 30.Eshima N, Tokumaru O, Hara S, Bacal K, Korematsu S, Tabata M, et al. Sex- and age-related differences in morbidity rates of 2009 pandemic influenza A H1N1 virus of swine origin in Japan. PLoS One. 2011;6(4):e19409. doi: 10.1371/journal.pone.0019409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Update: Influenza Activity - United States, August 30, 2009 - March 27, 2010, and Composition of the 2010-11 Influenza Vaccine. [accessed on May 3, 2011]. Available from: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5914a3.htm?s_cid=mm5914a3_e . [PubMed]
- 32.WHO; 2010. WHO. Human infection with pandemic (H1N1) 2009 virus: updated interim WHO guidance on global surveillance. Available from: http://www.who.int/csr/disease/swineflu/guidance/surveillance/WHO_case_definition_swine_flu _2009_04_29.pdf . [Google Scholar]


