Abstract
Background & objectives:
Despite major control efforts, malaria remains a major public health problem that still causes high mortality rate worldwide especially in Africa and Asia. Accurate and confirmatory diagnosis before treatment initiation is the only way to control the disease. The present study was undertaken to develop reagents using sandwich ELISA for simultaneous detection of PfHRP2 (Plasmodium falciparum histidine rich protein) and PfLDH (P. falciparum lactate dehydrogenase) antigens in the proven malaria cases.
Methods:
The antibodies were raised against two epitopes of PfHRP2 protein and three unique and unexplored epitopes of PfLDH protein. These antibodies were able to detect PfHRP2 and PfLDH antigens in culture supernatant and parasitized RBC lysate of P. falciparum, respectively up to 50 parasites/μl. The in-house reagents were tested in 200 P. falciparum positive patients residing in Baghpat district of Uttar Pradesh in northern India.
Results:
Microsphere (PLGA) with CpG ODN were used to generate high titre and high affinity antibodies against selected peptides of PfHRP-2 and pLDH antigen in mice and rabbit. The peptide specific peak titre varied from 12,800 - 102,400 with an affinity ranging 0.73 - 3.0 mM. The indigenously developed reagents are able to detect PfHRP2 and PfLDH antigens as low as 75 parasites/μl of blood with a very high sensitivity (96-100%) and specificity (100%).
Interpretation & conclusions:
The study highlight the identification of unique epitopes of PfHRP2 and PfLDH, and the generated antibodies against these antigens were used for quantitative estimation of these two antigens using sandwich ELISA. No corresreactivity with P. vivax infected patients was observed with the sera.
Keywords: Diagnosis, ELISA, histidine rich protein 2, lactate dehydrogenase, malaria, Plasmodium falciparum (Pf)
Malaria is potentially a life-threatening disease, which predominantly occurs in tropical and subtropical regions. It continues to be a major public health problem in endemic countries lacking adequate health care and malaria control programme.
The clinical diagnosis based on symptoms is the most common method used but it shows less specificity due to overlapping of symptoms with other tropical diseases. Microscopic examination of stained blood films is another cornerstone for diagnosis and estimation of parasite load in malaria victims. Although it is cost effective and simple, but it shows poor reproducibility, variable sensitivity and requires skilled operators for accurate diagnosis. Importantly, in remote areas where malaria commonly occurs, it is hard to maintain good quality microscopy and the result based on microscopic examination is sometimes unreliable. In many cases co-infection with Plasmodium falciparum and P. vivax occur, therefore, accurate and prompt parasitological confirmation of malaria infection is essential for effective disease management.
Many of the new technologies for malaria diagnosis incorporate immunochoromatographic procedure, where conjugated monoclonal antibodies are the key reagents. Currently many rapid diagnostic tests (RDTs) are widely used for the diagnosis of malaria. These RDTs are simple lateral-flow immunochromatographic tests that detect parasite specific antigens released from red blood cells. Two of the tests, the ICT Malaria Pf/Pv and ParaSight-F detect histidine rich protein 2 (HRP-2), a protein produced by asexual stages and young gametocyte of P. falciparum1,2. The third test OptiMAL detects Plasmodium lactate dehydrogenase (PLDH), a marker protein for the intraerythrocytic form of the malaria parasite. HRP-2 is an abundant protein produced by all blood stages of P. falciparum3. However, there are certain limitations of these rapid tests, including the decrease in sensitivity4 (70 in parasitaemia <50/μl) and also these tests cannot give quantitative (parasite/μl) results with malaria positive serum samples.
The HRP-2 antigen is expressed by all P. falciparum parasites regardless of knob-phenotype, and can be recovered from culture supernatants as a secreted soluble protein5. HRP-2 can be detected in erythrocytes, serum, plasma, cerebro-spinal fluid and even in urine6,7. Sequencing of the genomic DNA has shown that HRP-2 antigen contains 35 per cent histidine as well as alanine and aspartate (40 and 12%, respectively). It is characterized by the presence of tandem repeats of AHH and AHHAAD. Since HRP-2 antigen is only produced by P. falciparum, these tests cannot be used for the detection of P. vivax or other human malarial parasites. In endemic areas P. falciparum has been reported to lack HRP-2 or HRP-3 or both in positive patients8. In India, P. falciparum lacking PfHRP-2 and the PfHRP-3 gene has so far not been reported. However, the HRP-2 antigen remains detectable for several weeks after parasite clearance which causes false- positive RDT results in the test samples9.
Distribution of P. falciparum isolates lacking PfHRP-2 and PfHRP-3 is a major challenge for RDTs. In these areas parasite specific pLDH is the major target for the detection of malaria. pLDH is an intracellular glycolytic enzyme, which catalyses the oxidation of lactate to pyruvate. In P. falciparum, the coenzyme for LDH is preferably 3-acetyl pyridine adenine dinucleotide, (APAD), whereas the activity of human LDH requires β nicotinamide adenine dinucleotide10 indicating that pLDH could be a good marker following active malarial infections11. pLDH is expressed at high levels during asexual stage or blood-stage in all four malaria parasites12 and also correlated with the number of parasites present in the plasma of infected patients13. pLDH from P. vivax, P. malariae, and P. ovale exhibit 87 per cent sequence identity with pLDH from P. falciparum14. Genetic diversity may be particularly important for PfHRP-2-based RDTs since the antigen consists of a number of alanine and histidine-rich amino acid repeats and varies in size between parasite strains15.
In the present study we developed a simple antigen capture ELISA for detection of P. falciparum specific HRP-2 and LDH in the blood of malaria patients using in-house reagents.
Material & Methods
The study was conducted in the Department of Biochemistry, All India Institute of Medical Sciences (AIIMS), New Delhi, India, and the study protocol was approved by the Ethics Committee on animal experimentation, AIIMS.
Identification and synthesis of peptides and adjuvant: The protein sequence for PfLDH (PBD id PF3D7_1324900) and PvLDH (PVX_116630) were obtained from the PlasmoDB version 9.2 malaria database (www.plasmodb.org). DNAstar and Bcelpred software (www.IMTECH Bioinformatics) were used for the selection of peptides on the basis of physiochemical properties such as antigenicity, hydrophilicity, hydrophobicity, flexibility/mobility, exposed surface antigenicity and β turns. Structural data for epitope location were drawn by PYMOL [DeLano WL (2002) The PYMOL Molecular Graphics System on world wide web http://www.pymol.org)]. Alignments were performed by EMBOSS Stretcher protein alignment EMBL-EBI software (www.ebi.ac.uk).
Two peptides with tandem repeat sequence from the PfHRP-2 protein, and three unique peptide sequences from LDH protein were selected. The sequences were AHH (AHHAAD)4 (HRP-2 peptide I), (AHHAA)4 (HRP-2 peptide II) and (LFDIVKNMPHGKALDTSHT)2 (LDH peptide I), (TNVMAYSNCKVSGSNTYDDLA)2 (LDH peptide II), (VVLGANGVEQVIELQLNSEEKA)2 (LDH peptide III).
All peptides were synthesized by solid phase peptide synthesis using F-moc chemistry16. After analyzing all the above peptide sequences of Pf LDH by BLAST (http://blast.ncbi.nlm.nih.gov), no sequence similarity with the other vertebrate LDH including humans was observed. The purity of each peptide was assessed by amino acid analysis and HPLC and after purification these were found to be >95 per cent pure. The CpG oligodeoxynucleotide (ODN) 1826 of B class (also known as K type) with a nuclease resistant phosphorothioate backbone was used as an adjuvant to enhance antibody response. It was procured commercially from Coley pharmaceuticals (Wellesley, USA).
Preparation of CpG ODN microparticles (Microspheres): Poly (poly-DL-lactide-co-glycolide) PLGA microparticles entrapping peptide antigens with CpG ODN [peptide: CpG, (6:1) w/w] were prepared using water in oil-in water emulsion and solvent evaporation method17. The percentage entrapment for different peptides was in the range of 50-60 per cent as determined by BCA (bicinchoninic protein assay)18. The size distribution of microsphere was determined by laser diffraction (Malvern Instrument, UK) and the size was between 5-10 μm. Microsphere morphology was also studied by scanning electron microscopy (Philips, CM 10, USA). The percentage entrapment of CpG ODN was determined by extracting CpG ODN from microparticle in 10mM TE (Tris-EDTA) buffer, pH 8.3 as per protocol19.
Animals, immunization and serum collection: Six to eight week old inbred BALB/c (H-2d) mice and three months old rabbit were procured from the Experimental Animal Facility, AIIMS, New Delhi, India. A group of six mice were immunized subcutaneously at the foot-pad with PfHRP-2 peptide I and PfLDH peptides [regions I (33-51aa), II (51-71 aa) and III (280-306aa)], respectively in microsphere containing CpG ODN (5 μg) with primary dose of 30 μg on day 0 followed by a booster dose of 20 μg with 2.5 μg CpG ODN on days 32 and 45. Likewise, four rabbits were also immunized subcutaneously at multiple site with PfHRP-2 peptide II with primary dose of 100 μg with 20 μg CpG ODN on day 0 followed by a booster dose of 50 μg with 10 μg CpG ODN on days 32 and 45. The animals were provided with food and water ad libitum. Serum was collected on days 15, 28, 42, 60 and 90.
ELISA: The peptide specific antibody and peak titres were estimated using standard ELISA protocol. Briefly, 100 ng/100 μl of the peptide was coated per well and incubated with serum sample (mice/rabbit serum) at a standardized dilution (1:100 v/v) for serum IgG levels as well as two-fold serial dilution for measurement of peak antibody levels as per our reported protocol20. The end point titres were expressed as the reciprocal of the highest serum dilution giving an absorbance = pre-immune serum +4SD.
Purification and biotinylation of antibodies raised against synthetic peptides of PfHRP-2 and PfLDH protein: Initially albumin was removed from serum by ammonium sulphate (40-50% sodium) precipitation or “salting out” procedure21 and subsequently, Sepharose Protein A column was used to further purify the enriched IgG fraction. The pooled fractions were dialyzed against 0.01 M PBS (pH 7.4). The dialysate was concentrated and the IgG amount was determined using the BCA method18.
Biotinylation of the purified antibodies was done using N-hydroxysuccinimide ester of biotin (Sigma Immuno Probe Biotinylation reagents, USA) according to the manufacture's protocol Further, biotinylated antibodies were purified by gel filtration procedure (G-25 column). The extent of biotinylation and the ratio of biotin to antibody was determined by the avidin-HABA (4’-Hydroxazobenzene-2-carboxylic-acid) assay22, 2-3 biotin molecules were found to be linked per molecules of IgG.
Binding affinity of the antipeptide antibodies (KD): The affinity of antibodies raised against different peptides in mice was determined by measuring the dissociation constant (KD)23. In brief, immunized serum from mice/rabbit at 1 : 200 dilutions was incubated with different concentrations of the peptide (0.1-10 nM) for 15 h at 20°C so as to attain antigen-antibody equilibrium. The antigen-antibody complexes were transferred onto the wells of the microtitre plates previously coated with the respective peptide (500 ng/well). The plates were incubated for 90 min at 37°C. After washing 3 times with PBS-T, goat anti-mouse/rabbit IgG HRPO (horse radish peroxidase) conjugate was (1:1000) added (100 μl /well) and incubated for 1 h at 37°C. Dissociation constants were calculated using regression analysis and a simplification of the mathematical equation of Scatchard and Klotz24.
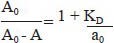
Where A0 is the absorbance without free antigen, A is the absorbance with free antigen and a0 is the total amount of antigen added to the reaction mixture.
Cultivation of P. falciparum and preparation of P. falciparum lysate: P. falciparum isolates (Indian isolates FDL-B, FDL-NG, FSH-4 and FSH-11) were maintained in in vitro cultures using O+ RBCs and AB+ serum25. Antigen was prepared from cultures enriched with late trophozoite and schizoints. Parasites were freed by saponin lysis and soluble extract was obtained after sonication at 14 μA for 90 sec. Each batch of culture was monitored for parasitaemia by microscopy, then parasites were harvested and culture supernatants were aspirated. Parasitized RBCs were washed thrice with PBS, and both the pellet and supernatant were stored at -20°C.
Study area and study population: A cross-sectional survey was conducted in the villages of the Baghpat district of Uttar Pradesh (UP), in northern India, from August 2010 to November 2011. This area is endemic for malaria having seasonal transmission of both P. vivax and P. falciparum. Early and prolonged monsoons are responsible for intensive transmission for both the species. All the patients were recruited after taking written informed consent from them. Overall 2050 individuals were screened for malaria infection. Giemsa stained thick and thin peripheral blood smears of each patient were examined by trained microscopist. The malaria positive samples were provided to us by Dr Sukla Biswas, NIMR, New Delhi after taking the ethical clearence from NIMR, New Delhi. All these samples were collected with the help of Doctor on duty of the primary health centre, Bhagphat, U.P. In addition, finger-prick blood samples of malaria positive and healthy subjects were tested by immunochromatography based rapid diagnostic test (RDT) kit, FalciVax, rapid test for Pv/Pf (Zephyr Biomedicals, Verna, Goa, India). Parasite density was estimated by counting the number of parasites per 200 leukocytes and the counts were converted to number of parasites/μl blood taking 8000 leukocytes/μl as a standard mean. More than 100 microscopic thick smear fields were checked before declaring a slide negative.
Two hundred confirmed malaria patients from the above samples were included in the study. Blood samples from patients with uncomplicated P. falciparum infection were collected by finger-prick in heparinized tubes. In a similar way, blood samples were also collected from 50 cases of P. vivax infected malaria and 50 healthy individuals to serve as a negative control. Patients diagnosed with malaria were treated with recommended antimalarials as per National Malaria Eradication Programme. All the samples were transported at 4°C to NIMR, New Delhi.
Development of PfHRP2 and PfLDH antigen assay design: For detecting the PfHRP2 antigen levels in P. falciparum positive patients, an ELISA was designed in which the plates were coated overnight at 4°C with 2.5 μg/100 μl of purified anti-PfHRP2 peptide I antibody raised in mice. After blocking with 5 per cent BSA (bovine serum albumin), 100 μl of RBC lysates of the blood samples from P. falciparum positive patients were added in 1:100 dilutions in each well and incubated at 37°C for 1 h. Plates were washed with PBS-Tween-20 and then 2.5 μg/100μl of purified biotinylated rabbit anti-PfHRP2 peptide II antibody was added and incubated at 37°C for 1h. For detecting parasite LDH antigen levels in the same patients, an ELISA was designed in which plates were coated with 2.5 μg of purified anti-PfLDH peptide III antibody in coating buffer and kept overnight. After blocking with 5 per cent BSA, 100 μl of RBC lysates of the blood samples from P. falciparum positive patients were added in 1:100 dilution in each well and incubated at 37°C for 1 h. Plates were washed with PBS-Tween-20 and then a cocktail of 2.5 μg of purified biotinylated anti-PfLDH peptide I and anti-PfLDH peptide II antibody was added and incubated at 37°C for 1 h. The bound antigen-antibody complex was detected using streptavidin-HRPO (1:1000 dilutions). Colour was developed using ortho pherylene diamine (OPD) as a chromogen and the absorbance was read at 492 nm. Similar ELISA was done with Pf culture supernatant at different dilutions (neat, 1:2, 1:4, 1:8, 1:16, 1:32 and 1:64) or parasitized RBC lysate at various parasitaemia levels (ranging from 250,000-50 parasites/μl) to develop a standard curve. RBC lysate and culture supernatant from non-infected blood samples of normal volunteers were used as control.
Statistical analysis: The data analysis was done with the help of Stata/IC (version 12.1) Stata Corporation College, Stations, Texas, USA. ELISA result was analyzed by Rank-Sum test (non-parametric approach) to compare the values of HRP-2 and LDH antigen between cases and control and comparison between two sets of ELISA and microscopy was done by Spearman Rank correlation coefficient. Antibody titres of different peptides from different bleeds were determined by the Friedman test separately for each group and group variability was analyzed by Kruskal-Wallis test (data not shown). The slope of the lines (KD values) was calculated by regression analysis.
Results
Selection of peptides for generating high titre antibody in animal models: Three regions from pLDH were identified that showed amino acid residues differed between P. falciparum and P. vivax (Fig. 1). All three selected regions showing four to six amino acid difference were thought to be sufficient to generate specific antibodies against each selected LDH peptide and also able to differentiate one malaria LDH from another malaria species.
Fig. 1.

EMBOSS stretcher alignment of Plasmidium lactate dehydrogenase amino acid sequences. The unique plasmodial epitope differentiating PfLDH and PvLDH appear below the highlighted region. Accession numbers are PfLDH: PlasmoDBid: PF3D7_1324900), PVLDH: PlasmoDBid: PVX_116630.
Surface localization of the three selected peptides was necessary to evaluate the accessibility of raising antipeptide antibody for native antigen (pLDH protein). Surface localization of the peptides was by a PYMOL software program (Fig. 2) illustrated that all peptides were found to be located on the surface of the native protein.
Fig. 2.
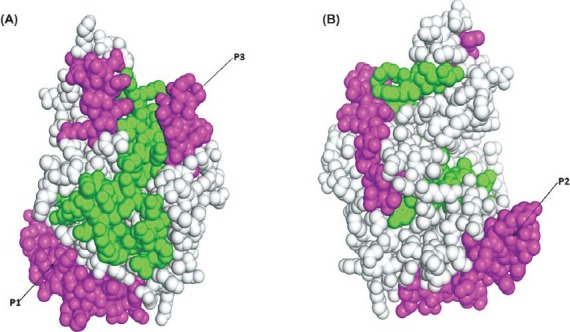
Schematic diagram showing the localization of P. falciparum specific LDH epitope in the pLDH protein at (A) 0° rotation and (B) 180° rotation along the Y axis (PDB Id: 2A94). The peptide localization of all Pf LDH peptide was indicated in magenta. The Figure was drawn by PyMOL [DeLano WL (2002) The PyMOL molecular graphic system on world wide Web: http://www.pymol.org, for this study.
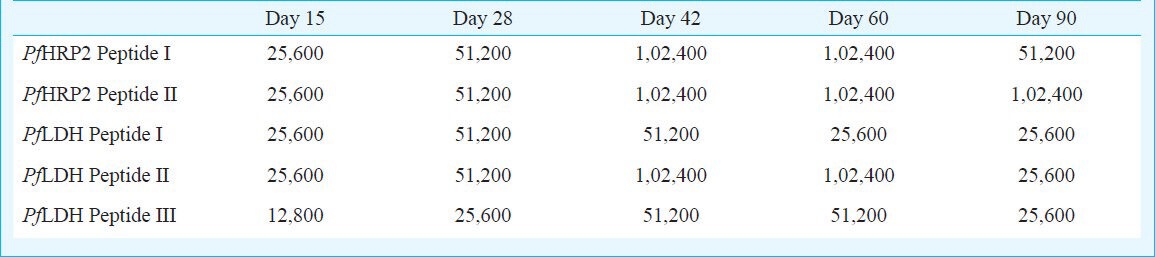
Serum antipeptide humoral response in microparticle formulation of P. falciparum LDH and HRP-2 antigens: The antibody responses to all the five peptides raised in mice and rabbits were measured as a proportion of peak antibody titres. Peptides entrapped in PLGA microspheres with the CpG ODN generated peptide specific high antibody levels in all the five bleeds and the levels were maintained till 90 days post-immunization. IgG peak titre for PfHRP-2 peptide I ranged 51,200 - 1, 02,400 on days 28 and 42 (Table I). Mice/rabbits immunized with PfLDH peptide I and PfHRP-2 peptide II also showed peak antibody levels 51,200 on days 28 and 42 and the titres fell to 25,600 on day 90. Similarly for PfLDH peptide II antisera, IgG peak titres were in the range of 51,200 - 1,02,400 on days 28 and 42, then gradually declined by day 90 to 25,600. For PfLDH peptide III antisera, IgG peak titres were in the range 25,600 - 51,200 on days 28 and 42 which persisted up to day 90 (Table I).
Table I.
End point titres (in thousands) of antibodies raised against the peptides of PfHRP2 and PfLDH antigens
Dissociation constant (KD) of PfHRP-2 peptide antisera and PfLDH peptides antisera: The KD value of anti-PfHRP-2 peptide I antisera (0.73 nM) was lower than anti-PfHRP-2 peptide II antisera (1.5 nM) and the KD value of anti-PfLDH peptide II antisera (1.2 nM) was also lower as compared to the anti-PfLDH peptide I and III antisera (1.4 and 3.0 nM). Thus all the peptides generated high titre and high affinity antibodies.
Quantification of PfHRP-2 antigen in P. falciparum culture supernatant or parasitized RBC lysates using PfHRP-2-based ELISA: The PfHRP2 assay allowed the detection of HRP-2 antigen in the culture supernatant at dilution (neat, 1:2, 1:4, 1:8, 1:16, 1:32 and 1:64) of all the four isolates of P. falciparum (Fig. 3) and the parasitized RBC lysate (250,000-50 parasites/μl) (Fig. 4A). The lower limit of PfHRP-2 antigen detection (50 parasites/μl) in parasitized RBC lysate was better than the detection limit by light microscopy which gave 100 per cent sensitivity when the number of parasites/ μl of blood was >50. The number of parasites/μl blood was found to be positively correlated with PfLDH antigenaemia (r=0.863, P<0.001) and in the infected RBC (IRBC) lysates (r=0.839, P<0.001). The assay was repeated thrice to confirm reproducibility.
Fig. 3.
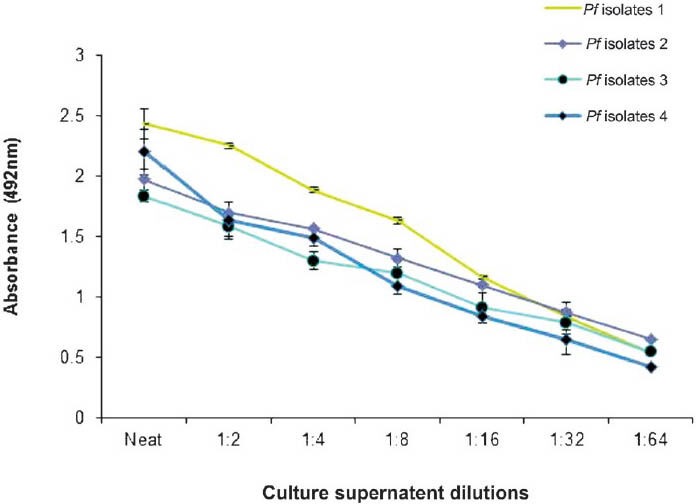
Quantification of PfHRP2 antigen in culture supernatants of four different isolates of P. falciparum (Isolate 1 FDL-B, Isolate 2 FDL-NG, Isolate 3 FSH-4 and Isolate 4 FSH-11). Values are mean + SD of 3 observations.
Fig. 4.

Quantification of PfHRP2 antigen (A) and PfLDH antigen (B) in parasitized RBC lysates of four different isolates of P. falciparum (Isolate 1 FDL-B, Isolate 2 FDL-NG, Isolate 3 FSH-4 and Isolate 4 FSH-11). Values are mean + SD of 3 observations.
Quantification of LDH antigen in P. falciparum culture supernatant or IRBC lysates using PfLDH-based ELISA: The PfLDH assay allowed the detection of Pf LDH antigen in the parasitized RBC lysate at parasitaemia levels ranging 250,000-50 parasites/μl (Fig. 4B). The lower limit of PfLDH antigen detection (50 parasites/μl) in parasitized RBC lysate was better than the detection limit by light microscopy which gave 100 per cent sensitivity when the number of parasites/ μl of blood was ≥50. The number of parasites/μl blood were found to be positively correlated with PfLDH antigenaemia (r=0.831, P<0.001) in the infected RBC lysates. We did not perceive any detectable amount of pLDH in culture supernatants. The assay was repeated thrice for reproducibility.
Detection of Pf HRP-2 antigen in blood of malaria patients by PfHRP-2 assay: Two hundred malaria positive serum samples confirmed by microscopy and RDT were studied to evaluate the newly developed antigen capture assay for PfHRP-2 and PfLDH. The results are presented in Table II. In this study, at 95% CI the specificity of PfHRP-2 assays for diagnosis of P. falciparum parasites were 100 per cent (91.1-100%) and sensitivity was in the range 96 per cent (77.7-99.8%) to 100 per cent (96.4-100%) when the parasitaemia was 0.0015 to 0.015 per cent, respectively. The per cent positive predictive values (PPV%) were found to be 74.7 to 100 per cent, the negative predictive values % (NPV%) were found to be 91.1 to 100 per cent and area under curve (AUC) was found to be 1.00 when parasite/μl >10,000-150. However, PPV% was calculated to be 82.8-100 per cent, NPV% was 98.04 per cent (88.2-99.2%) when parasite/μl >75-150 and AUC was found to be 0.98. This assay was able to detect the PfHRP-2 antigen in the blood of all the subjects with parasitaemia level ranging 75 to 250, 000 parasites/μl blood which corresponds to 0.0015 to 5 per cent parasitaemia (Fig. 5A). When the association between number of parasites/μl blood and HRP-2 antigenemia was measured, the level of parasiteamia were found to be positively correlated with PfHRP2 antigenemia (r=0.862, P<0.001). When all 200 patients of proven malaria infection were divided into different groups based on parasite/μl blood, the association between mean parasitemia levels in different groups and the corresponding mean PfHRP-2 antigenemia was also positively correlated (r=0.979, P<0.001). As the parasitemia level increased, the PfHRP-2 concentration was found to be increased (Fig. 5A, 5B). The indigenously developed antibodies were able to detect PfHRP-2 in these blood samples at parasitaemia of approximately 75 parasites/μl (Table II). Fifty of P. vivax positive cases and 50 healthy, smear negative individuals served as control and had undetectable levels of PfHRP antigen. Cut-off for positivity was calculated as ≥mean + 4SD of negative control. A sample is given an absorbance (Cut-off) ≥ 0.20 was considered positive for PfHRP-2 antigen.
Table II.
Performance of PfHRP2- and PfLDH- based ELISA on P. falciparum confirmed serum samples of patients from Baghpat district of Uttar Pradesh

Fig. 5.
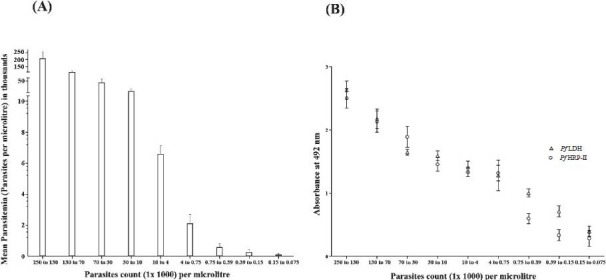
(A) Association between mean parasitaemia and parasite count in different groups of P. falciparum positive patients. Parasitaemia is expressed as parasites/μl and data expressed as mean +4 SD. (B) Relationship between parasite count and mean PfHRP2 (O) and mean PfLDH antigen concentration (Δ) in P. falciparum positive. Parasitemia is expressed as parasites/μl and data is expressed as mean + 4 SD.
Detection of Pf LDH antigen in blood of malaria patients by Pf LDH assay: Blood samples from 200 malaria patients were also examined for PfLDH and compared with microscopy and RDT. The specificity of PfLDH assays for diagnosis of P. falciparum parasites was 100 per cent (91.1-100%) and sensitivity was 100 per cent (83.4-100%) at 95% CI. The PPV% values were found to be 83.4-100 per cent, the NPV% values were found to be 91.1 to 100 per cent and AUC was 1.00 when parasite/μl >75 which corresponded to >0.0015 per cent parasitaemia. The developed in-house assay detected PfLDH antigen in the blood of all the subjects with parasitaemia level ranging from 75 to 250,000 parasites/μl blood, which corresponded to 0.0015 to 5 per cent parasitaemia (Fig. 5A). When the association between number of parasites/μl and PfLDH antigenaemia was measured, the level of parasitaemia were found to be positively correlated with PfLDH antigenaemia (r=0.878, P<0.001) (Fig. 5B). The associations between mean parasitaemia levels in different groups of patients as described above and the corresponding mean PfLDH antigenaemia were also found positively correlated (r=0.972, P<0.001). As the parasitaemia level increased, the PfLDH concentration in the blood was also found to be increased in these patients (Fig. 5B). Thus, the indigenously developed antibodies detected PfLDH in these patient samples at parasitaemia of approximately 75 parasites/μl of blood. A sample is given an absorbance (Cut-off) ≥0.13 was considered positive for PfLDH antigen detection. The developed reagents were also tested with 50 samples of P.vivax positive serum. It did not show any reactivity with the P.vivax samples. The assay showed undetectable levels of PfLDH antigen in 50 healthy and smear negative controls.
Discussion
In the present study, the performance of antibodies raised against two epitopes for PfHRP-2 and three different epitopes for PfLDH antigens was assessed for their use as immunoreagents for development of ELISA-based diagnostic assay to assess the antigen load in a large number of Pf infected serum samples. All the selected regions were surface exposed to the native protein and hence generated antibodies against these peptides and thus was able to differentiate between P. falciparum and P. vivax.
The use of the core characteristics of Plasmodium HRP-2 and LDH antigens for detection of malaria, for estimation of parasite biomass5,26, in RDT27 and in antigen-antibody assay has been reported28. This feature was exploited in the designing of first immunodiagnostic assay for P. falciparum- specific HRP-229 and later the second generation immunochromatographic based rapid diagnostic tests30 were developed for P. falciparum. Studies on sequence variability among PfLDH isoforms from different strains showed low diversity, which suggested that the antigen conserved its sequence31. The characteristics of the core technology for PfLDH have been outlined elsewhere13; these studies used dipstick and ELISA to evaluate the characteristic of PfLDH. It was shown that PfLDH dipstick had a threshold effect of approximately 200 parasites/μl of blood. Levels of PfLDH were more consistent with peripheral parasitaemia than PfHRP-2.
Thus, we have selected peptides from two regions of HRP-2 and three unique and unexplored region of pLDH based on the structural, peak antibody titres and affinity values for different antibodies, the antisera of PfHRP-2 peptide I and PfLDH peptide III (278-300 amino acid regions) were used as capture antibody and the antisera of PfHRP-2 peptide II and a cocktail mixture of PfLDH peptide I (33-51 amino acid region) and PfLDH peptide II (51-71 amino acid regions) were used as detecting antibody in the enzyme immunoassay. Also to enhance the immunogenicity, the microencapsulated peptides were co-entrapped with CpG oligodeoxynucleotide as an adjuvant. It is well known that CpG adjuvant is more potent when used in conjunction with the delivery system largely as a consequence of its improved delivery into the endosome of antigen presenting cell (APC), where it can more easily interact with TLR9 (Toll like receptor 9)32. Hence in this study, it was observed that when the length of peptide sequences of PfHRP-2 and PfLDH proteins was increased by tandem repeat and CpG was combined with the microsphere delivery, the dissociation constant of antisera was considerably reduced thereby indicating that antibodies have high affinity, which helped to detect low parasitaemia levels up to ~75 parasites/μl blood in P. falciparum positive serum. The antibodies developed against Plasmodium lactate dehydrogenase were used in the differential diagnosis of P.falciparum and P.vivax infection. The lower limits of detection for PfHRP-2 and PfLDH were comparable to those reported for other rapid diagnostic tests33. Also, PfLDH and PfHRP-2 levels detected in the present study can be used to complement microscopy in the target population. Microscopy only detects circulating infected RBC and is not an accurate measure of parasite burden, particularly in P. falciparum infections where mature stages are known to be sequestered in various tissues. It is now widely accepted that PfLDH levels reflect current infection whereas PfHRP-2 levels indicate both past and current infection9. Thus concurrent measurement of these two antigens by ELISA provides a better evaluation of parasite burden especially in areas, where malaria is endemic30. There are a few RDT kits commercially available for the diagnosis of P. falciparum specific HRP-2 and LDH antigen27. The overall sensitivity reported was 88.7 per cent with blood parasite count <100 parasite/μl and increased from 94.3 to 99.3 per cent when the blood parasite count was >100 -10000 parasite/μl with a specificity of 97.5 per cent34. However, in the present study certain unique epitopes of PfHRP-2 and PfLDH were identified and used for diagnosis of malaria with 96 and 100 per cent sensitivity and 100 per cent specificity when the parasite count was >75 parasite/μl blood. Our data also provided a standardized protocol for the measurement of both PfLDH and PfHRP-2 antigens in the same patient sample using separate capture or detecting antibodies specific to these antigens.
In conclusion, both the antigen capture assays were comparable to each other for detection of P. falciparum infection at low levels of parasitaemia. The sensitivity limit (0.0015% parasitaemia) of PfHRP-2 and PfLDH assays was comparable to those achieved by microscopic examination of thick blood smears or other RDTs.
Acknowledgment
Authors acknowledge the Indian Council of Medical Research (ICMR), New Delhi for providing financial assistance, and thank Dr Manoj Kumar and the Department of Biomedical Informatics Centre (ICMR), Department of Biophysics, AIIMS, New Delhi for their help in PYMOL study and localization of pLDH peptides on pLDH protein. Authors also thank doctor(s) of Primary Health Centre at Bhagpat district of Uttar Pradesh for collection of blood samples.
References
- 1.Tjitra E, Suprianto S, Dyer M, Currie BJ, Anstey NM. Field evaluation of the ICT Malaria P.f/P.v immunochromatographic test for detection of Plasmodium falciparum and Plasmodium vivax in patients with a presumptive clinical diagnosis of malaria in eastern Indonesia. J Clin Microbiol. 1999;37:2412–7. doi: 10.1128/jcm.37.8.2412-2417.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banchongaksorn T, Yomokgul P, Panyim S, Rooney W, Vickers P. A field trial of the ParaSight-F test for the diagnosis of Plasmodium falciparum infection. Trans R Soc Trop Med Hyg. 1996;90:244–5. doi: 10.1016/s0035-9203(96)90231-x. [DOI] [PubMed] [Google Scholar]
- 3.Howard RJ, Uni S, Aikawa M, Aley SB, Leech JH, Lew AM, et al. Secretion of a malarial Histidine-rich protein (Pf HRP II) from Plasmodium falciparum-infected erythrocytes. J Cell Biol. 1986;103:1269–77. doi: 10.1083/jcb.103.4.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moody A, Angela HC, Elisabeth G, Peter C. Performance of the OptiMal malaria antigen capture dipstick for malaria diagnosis and treatment monitoring at the Hospital for Tropical Diseases, London. Br J Haematol. 2000;109:891–4. doi: 10.1046/j.1365-2141.2000.01974.x. [DOI] [PubMed] [Google Scholar]
- 5.Dondorp AM, Desakorn V, Pongtavornpinyo W, Sahassananda D, Silamut K, Chotivanich K, et al. Estimation of the total parasite biomass in acute falciparum malaria from plasma PfHRP2. PLoS Med. 2005;2:e204. doi: 10.1371/journal.pmed.0020204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kifude CM, Rajasekariah HG, Sullivan DJ, Jr, Stewart VA, Angov E, Martin SK, et al. Enzyme-linked immunosorbent assay for detection of Plasmodium falciparum histidine-rich protein 2 in blood, plasma, and serum. Clin Vaccine Immunol. 2008;15:1012–8. doi: 10.1128/CVI.00385-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Genton B, Paget S, Beck HP, Gibson N, Alpers MP. Diagnosis of Plasmodium falciparum infection using ParaSight (R)-F test in blood and urine of Papua New Guinean children. Southeast Asian J Trop Med Public Health. 1998;29:35–40. [PubMed] [Google Scholar]
- 8.Gamboa D, Ho MF, Bendezu J, Torres K, Chiodini PL, Barnwell JW, et al. A large proportion of P. falciparum isolates in the Amazon region of Peru lack pfhrp2 and pfhrp3: implications for malaria Rapid Diagnostic Tests. PLoS One. 2010;25(5) 1:e8091. doi: 10.1371/journal.pone.0008091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iqbal J, Siddique A, Jameel M, Hira PR. Persistent histidine- rich protein 2, parasite lactate dehydrogenase, and panmalarial antigen reactivity after clearance of Plasmodium falciparum monoinfection. J Clin Microbiol. 2004;4:4237–41. doi: 10.1128/JCM.42.9.4237-4241.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vander Jagt DL, Hunsaker LA, Campos NM, Baack BR. D-lactate production in erythrocytes infected with Plasmodium falciparum. Mol Biochem Parasitol. 1990;42:277–84. doi: 10.1016/0166-6851(90)90171-h. [DOI] [PubMed] [Google Scholar]
- 11.Makler MT, Hinrichs DJ. Measurement of the lactate dehydrogenase activity of Plasmodium falciparum as an assessment of parasitemia. Am J Trop Med Hyg. 1993;48:205–10. doi: 10.4269/ajtmh.1993.48.205. [DOI] [PubMed] [Google Scholar]
- 12.Sherman IW. Biochemistry of Plasmodium (malarial parasites) Microbiol Rev. 1979;43:453–95. doi: 10.1128/mr.43.4.453-495.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piper R, Lebras J, Wentworth L, Hunt-Cooke A, Houzé S, Chiodini P, et al. Immunocapture diagnostic assays for malaria using Plasmodium lactate dehydrogenase (pLDH) Am J Trop Med Hyg. 1999;60:109–18. doi: 10.4269/ajtmh.1999.60.109. [DOI] [PubMed] [Google Scholar]
- 14.Brown WM, Yowell CA, Hoard A, Vander Jagt TA, Hunsaker LA, Deck LM, et al. Comparative structural analysis and kinetic properties of lactate dehydrogenases from the four species of human malarial parasites. Biochemistry. 2004;43:6219–29. doi: 10.1021/bi049892w. [DOI] [PubMed] [Google Scholar]
- 15.Rock EP, Marsh K, Saul AJ, Wellems TE, Taylor DW, Maloy WL, et al. Comparative analysis of the Plasmodium falciparum histidine-rich proteins HRP-I, HRP-II and HRP-III in malaria parasites of diverse origin. Parasitology. 1987;95:209–27. doi: 10.1017/s0031182000057681. [DOI] [PubMed] [Google Scholar]
- 16.Fields GB, Noble RL. Solid phase peptide synthesis utilizing F-moc amino acids. Int J Peptide Protein Res. 1990;35:161–214. doi: 10.1111/j.1399-3011.1990.tb00939.x. [DOI] [PubMed] [Google Scholar]
- 17.Karal-Yilmaz O, Serhatli M, Baysal K, Baysal BM. Preparation and in vitro characterization of vascular endothelial growth factor (VEGF)-loaded poly(D,L-lactic-co-glycolic acid) microspheres using a double emulsion/solvent evaporation technique. J Microencapsul. 2011;28:46–54. doi: 10.3109/02652048.2010.523795. [DOI] [PubMed] [Google Scholar]
- 18.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 19.Barman SP, Lunsford L, Chambers P, Hedley ML. Two methods for quantifying DNA extracted from poly (lactide-co-glycolide) microspheres. J Controlled Release. 2000;69:337–44. doi: 10.1016/s0168-3659(00)00308-4. [DOI] [PubMed] [Google Scholar]
- 20.Tomar D, Biswas S, Tripathi V, Rao DN. Development of diagnostic reagents: raising antibodies against synthetic peptides of PfHRP-2 and LDH using microsphere delivery. Immunobiology. 2006;211:797–805. doi: 10.1016/j.imbio.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Grodzki AC, Berenstein E. Antibody purification: ammonium sulfate fractionation or gel filtration. In: Oliver C, Jamur MC, editors. Immunocytochemical methods and protocols. Methods in molecular biology. 3rd ed. Vol. 588. New York: Springer Book Archives; 2010. pp. 15–26. [DOI] [PubMed] [Google Scholar]
- 22.Wilchek M, Bayer EA. Biotin-containing reagents. Methods Enzymol. 1990;184:123–38. doi: 10.1016/0076-6879(90)84267-k. [DOI] [PubMed] [Google Scholar]
- 23.Friguet B, Chaffotte AF, Djavadi-Ohaniance L, Goldberg ME. Measurements of the true affinity constant in solution of antigen-antibody complexes by enzyme-linked immunosorbent assay. J Immunol Methods. 1985;77:305–19. doi: 10.1016/0022-1759(85)90044-4. [DOI] [PubMed] [Google Scholar]
- 24.Karush F. The affinity of antibody: Range, variability and the role of multivalence. In: Good RA, Day SB, editors. Comprehensive immunology. Vol. 5. U.K: Springer Link Publisher; 1978. pp. 85–116. [Google Scholar]
- 25.Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193:673–75. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 26.Martin SK, Rajasekariah GH, Awinda G, Waitumbi J, Kifude C. Unified parasite lactate dehydrogenase and histidine-rich protein ELISA for quantification of Plasmodium falciparum. Am J Trop Med Hyg. 2009;80:516–22. [PubMed] [Google Scholar]
- 27.Maltha J, Gillet P, Bottieau E, Cnops L, van Esbroeck M, Jacobs J, et al. Evaluation of a rapid diagnostic test (CareStartTM Malaria HRP-2/pLDH(Pf/pan)Combo Test) for the diagnosis of malaria in a reference setting. Malaria J. 2010. [accessed on December 13, 2010]. p. 9. Available from: http://www.malariajournal.com/ [DOI] [PMC free article] [PubMed]
- 28.Taylor DW, Voller A. The development and validation of a simple antigen detection ELISA for Plasmodium falciparum malaria. Trans R Soc Trop Med Hyg. 1993;87:29–31. doi: 10.1016/0035-9203(93)90409-j. [DOI] [PubMed] [Google Scholar]
- 29.Doderer C, Heschung A, Guntz P, Cazenave JP, Hansmann Y, Senegas A, et al. A new ELISA kit which uses a combination of Plasmodium falciparum extract and recombinant Plasmodium vivax antigens as an alternative to IFAT for detection of malaria antibodies. Malaria J. 2007. [accessed on June 19, 2008]. p. 6. Available from: http://www.malariajournal.com/content/6/1/19 . [DOI] [PMC free article] [PubMed]
- 30.Singh N, Valecha N, Sharma VP. Malaria diagnosis by field workers using an immunochromatographic test. Trans R Soc Trop Med Hyg. 1997;91:396–7. doi: 10.1016/s0035-9203(97)90254-6. [DOI] [PubMed] [Google Scholar]
- 31.Talman AM, Duval L, Legrand E, Hubert V, Yen S, Bell D, et al. Evaluation of the intra- and inter-specific genetic variability of Plasmodium lactate dehydrogenase. Malaria J. 2007. [accessed on October 28, 2008]. Available from: http://www.malariajournal.com/content/6/1/140 . [DOI] [PMC free article] [PubMed]
- 32.Suwarti S, Yamazaki T, Svetlana C, Hanagata N. Recognition of CpG oligodeoxynucleotides by human Toll-like receptor 9 and subsequent cytokine induction. Biochem Biophys Res Commun. 2013;430:1234–9. doi: 10.1016/j.bbrc.2012.12.068. [DOI] [PubMed] [Google Scholar]
- 33.Iqbal J, Khalid N, Hira PR. Comparison of two commercial assays with expert microscopy for confirmation of symptomatically diagnosed malaria. J Clin Microbiol. 2002;40:4675–8. doi: 10.1128/JCM.40.12.4675-4678.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murray CK, Bennett JW. Rapid diagnosis of malaria. Interdiscip Perspect Infect Dis. 2009. [accessed on October 12, 2009]. Available from http://www.ncbi.nlm.nih.gov/pmc/articles/pmc2696022 . [DOI] [PMC free article] [PubMed]


