Abstract
Background & objective:
Japanese encephalitis (JE) outbreaks are common in Assam, northeastern State of India. Information on resistance in known JE vectors in the affected area is important for effective control measures. This study was undertaken to determine the species abundance of JE vectors endemic to Sibsagar district of Assam, and their susceptibility against DDT and deltamethrin.
Methods:
Adult mosquitoes were collected using CDC light trap and aspirators from human dwellings from 13 endemic villages falling under three Primary Health Centres. Collected mosquitoes were identified and unfed female mosquitoes were used for DDT and deltamethrin sensitivity bioassay. The bioassay was performed following WHO protocol using standard susceptibility test kit. Knockdown time (KDT) was monitored at every 10 minutes intervals, whereas mortalities were recorded 24 h post-exposure. Vector density and resistance status were mapped using geographic information system (GIS) technique.
Results:
A total of 7655 mosquitoes were sampled under three genera, i.e. Anopheles, Culex and Mansonia, and nine species, the JE vector Cx. vishnui group (31.78%) was the most predominant species, followed by Ma. uniformis (16.81%) and Ma. indiana (16.45%). All vector species were suspected to be resistant to DDT and sensitive to deltamethrin, except Ma. indiana, which was suspected to deltamethrin resistant. The KDT50 and KDT95 values of vector mosquitoes for DDT were significantly higher as compared to deltamethrin. The probit model used to estimate KDT50 and KDT95 values did not display normal distribution of percentage knockdown with time for all the vectors tested for DDT and deltamethrin, except for Ma. indiana for deltamethrin assay and Cx. gelidus for the DDT assay.
Interpretation & conclusion:
Differences in insecticide resistance status were observed between insecticides and vector species. The results of this study provided baseline data on insecticide resistance in known JE vectors of Sibsagar, Assam. The maps generated may allow better communication in control operations and comparison of changes in susceptibility status of these vectors over time.
Keywords: DDT, deltamethrin, insecticide resistance, JE vector, knock down time, WHO bioassay
In spite of Japanese encephalitis (JE) emerging as a major health problem in southeast Asian countries, the vector abundance and insecticide susceptibility status of known JE vectors has not been monitored regularly. During the last few decades major JE outbreaks have been reported from different parts of India predominantly in the rural areas1. The JE related mortality, morbidity and disability adjusted life years (DALYs) are alarming posing serious challenge to the concerted control efforts2,3. In the northeastern States of India, the disease appears in sporadic outbreaks with its peak during the rainy season4,5. Population-wise Assam is the largest State among the eight States in northeastern India and plays a significant role in the economy of the region6. JE outbreaks are common and occur at regular intervals in different parts of India7. Dibrugarh is one of the most affected districts of Assam and since 1978 human cases have been regularly reported from this district8. In India, of the 16 mosquito species detected harbouring JE virus, Culex tritaeniorhynchus has been reported as the prominent JE vector1,9. In addition, mosquitoes of Cx. vishnui subgroup have been recognized for many years as important vector associated with JE transmission in India9,10. Information on the population dynamics of mosquitoes, particularly vectors is necessary for implementation of control measures. In any given area common mosquito species occur during epidemics, however, the abundance of any species depends more upon the availability of preferential feeding host, breeding habitats and survival rates and has direct relevance with the disease transmission.
Studies have shown that use of insecticides has reduced vector density during the disease outbreaks11. The JE vectors breeding in agricultural habitats are under tremendous selection pressure of the insecticides sprayed in the fields and, therefore, deserve routine monitoring for their sensitivity status against insecticides used in the control operations12. Vector mosquito resistance to the common insecticides has been reported from many Asian countries, including India12,13,14,15,16,17,18,19. The level of insecticide resistance has been found to vary even in relatively close areas and during the different seasons15,20. These studies suggest that extrapolations of findings from one area to another (even in a small geographical scale) may be inappropriate. Therefore, addressing the insecticide resistance problem among different JE vectors from various endemic areas at micro-level is crucial.
In the present study, investigation was carried out to collect information on the prevalence of known Japanese encephalitis vectors and their resistance/sensitivity status against DDT and deltamethrin in the study area. DDT is used for indoor residual spray, while deltamethrin is used for the impregnation of bednets in the region, therefore, these two insecticides were chosen for the susceptibility tests.
Material & Methods
Study area: JE vectors were collected from 13 villages falling under three Primary Health Centres (PHCs, Demow, Geleki and Gaurisagar) of Sibsagar district of Assam during peak JE season (July - August) in 2011. The Sibsagar district (94.25° and 95.25° E, 25.45° and 27.15° N) lies on the north bank of river Brahmaputra and has an elevation of 86.6 m (mean sea level). Villages were selected on the basis of the risk of JE transmission for which the inputs from district health staff engaged in the interventions were taken (5 each under Demow and Geleki PHCs and 3 in Gaurisagar PHC). Climate is humid, subtropical and the temperature varies from 8°C in winters to 35°C in the summers. Hot and humid climate, paddy fields, perennial slow flowing streams, irrigation drains/ditches and duck rearing ponds provide bioclimate of choice for abundance of vector mosquitoes.
Adult mosquito sampling and identification: Adult mosquitoes were collected using 6 volts battery operated CDC miniature light trap (John Hock, USA) hung at two meters from the ground level in human dwellings overnight. Hand held mouth aspirators (John Hock, USA) were used to collect the adult mosquitoes resting on the wall, roof, clothes and other surfaces inside the houses. At least four households were selected for the mosquito collection in each of the study village. Smoking by burning hay and other illuminations were prevented during the mosquito collection. Collected mosquitoes were identified upto species level. Cx. vishnui, Cx. pseudovishnui and Cx. tritaeniorhynchus were clubbed into a group. Preliminary identification of live mosquitoes was carried out morphologically in the glass tubes before conducting the susceptibility assay, which was confirmed after the completion of assay. The unfed female mosquitoes were used for the insecticide sensitivity bioassay so that the tested specimens have physiological status not interfering with the enzymatic activity. The assays were conducted in the batches of 25-35 numbers per test in the field itself. During the collection period, monsoon season was prevailing in the study area with a temperature and humidity ranging from 25 to 35°C and 60 to 95 per cent, respectively.
WHO insecticide bioassay: The bioassay was performed following standard WHO protocol21. DDT (4%), deltamethrin (0.05%) and silicone oil pre-impregnated papers were obtained from vector control research unit, Universiti Sains Malaysia, Malaysia. Mortalities were recorded 24 h post exposure and sensitivity status was graded as per the recommended criteria.
Data analysis and interpretation: Vector density for each PHC (all study sites of each PHC pooled) was expressed in mean ± standard error mean (mean ± SEM) and compared using 1-way analysis of variance (ANOVA), followed by Tukey Kramer test of multiple comparison. Mortality rates recorded were corrected using Schneider-Orelli's formula22. Insecticide sensitivity status of mosquitoes was determined as follow23. corrected mortality rates <80 per cent indicates resistant; corrected mortality rates >98 per cent indicates fully sensitive; and corrected mortality ranging between >80 to <98 per cent indicates suspected resistance that needs to be verified.
Knockdown rates (KDR) among all vector species for each insecticide were compared using repeated ANOVA. Student's t-test was used to compare the KDR of each vector species for both the insecticides. Similarly, KDR at 10 min intervals for both the insecticides were compared using paired Student's t-test. Knockdown time (KDT50 and KDT95) for the mosquito vectors were determined by log-probit method using Log dose probit (Ldp) Line software (Ehabsoft, Egypt). Fitment of probit was assessed using chi-square analysis, whereas the overall significance of the multiple-tests was determined following Bonferroni procedure.
Mapping of vectors and insecticide resistance: Geographical information of the 13 study sites was recorded using global positioning system (Oregon-550, Garmin USA), onto which the densities of nine vector species were marked. DDT and deltamethrin susceptibility of six JE vectors was determined collectively for each PHC. The maps were prepared using Arc GIS version 10 computer programme (Esri, USA).
Results
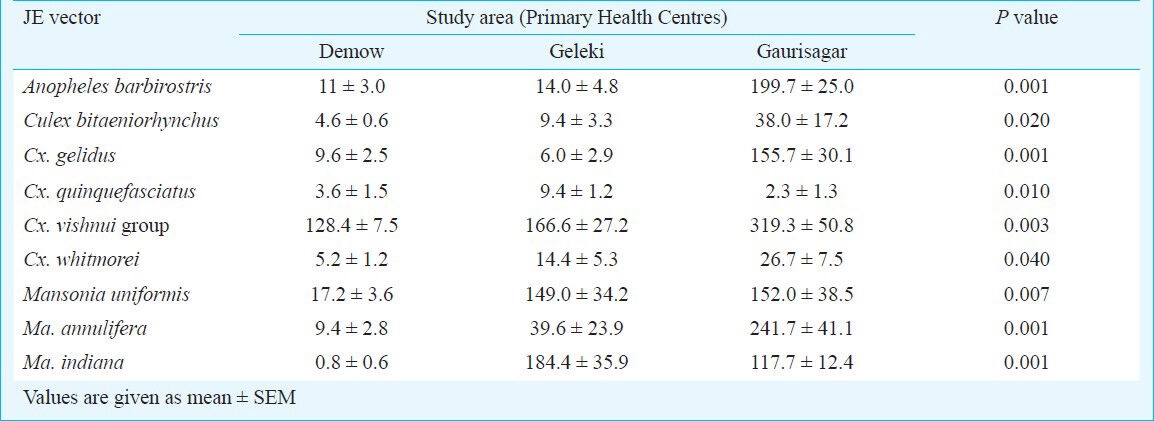
Vector abundance: Using light traps and aspirators (latter for indoor resting collection), a total of 9650 mosquitoes belonging to five genera, viz., Anopheles (7 species), Culex (6 species), Mansonia (3 species), Coquillettidia (1 species) and Armigeres (1 species) were collected during the study. Of all these, 7655 corresponding to three genera (Anopheles, Culex and Mansonia) and nine species were among the known JE vectors reported in the Indian subcontinent. Prevalence and distribution of JE vectors (mean ± SEM) is shown in Table I. Overall vector density was significantly high in the Gaurisagar PHC as compared to the other two Primary Health Centres (P=0.02). Similarly, the vector density was significantly different among the study villages of each PHC (P<0.0001 for all the three Primary Health Centres). Cx. vishnui group (31.78%) was the most predominant species of all collected vector species (all data pooled) followed by Ma. uniformis (16.81%) and Ma. indiana (16.45%) (P<0.0001). In Demow PHC, Cx. vishnui group density was significantly higher than the other two vectors (P<0.0001), whereas in Geleki PHC, the density of these three vectors was similar. In Gaurisagar PHC, unlike the other two Primary Health Centres, An. barbirostris and Ma. annulifera were widely distributed and their densities were similar to Cx. vishnui group mosquito (Table I).
Table I.
Summary of known JE vectors collected in the study area
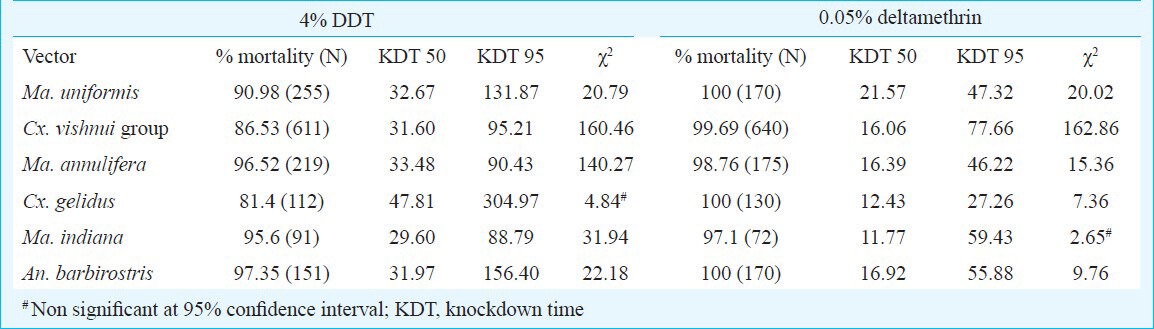
WHO insecticide bioassay: The results of susceptibility status of six potential JE vectors to the diagnostic dosages of DDT and deltamethrin are shown in (Table II). The mortality, after 24 h recovery period was <98 per cent for DDT in all the tested vector mosquitoes, which shows that resistance is suspected in the vector population. The highest mortality to DDT (97.35%) was recorded in An. barbirostris, whereas lowest (81.4%) was recorded in Cx. gelidus mosquito. On the other hand, mortality after 24 h recovery was >98 per cent for deltamethrin in the vector mosquitoes, except in Ma. indiana, whereas only 97.1 per cent mortality could be achieved. For DDT, KDT50 and KDT95 values (47.81 and 304.97 min, respectively) were highest in Cx. gelidus, whereas lowest (29.60 and 88.79 min respectively) in Ma. indiana. For deltamethrin, highest 50 and 95% knockdown values were recorded in Ma. uniformis (21.57 min) and Cx. vishnui group (77.66 min), respectively, and lowest were found in Cx. vishnui group (16.06 min) and Cx. gelidus (27.26 min) mosquitoes, respectively. The KDT50 and KDT95 values of vector mosquitoes (data pooled) for deltamethrin was significantly less as compared to the DDT (P<0.00 for KDT50 and P=0.03; for KDT95). Similarly, the knockdown rates at 10 min intervals were significantly different between both the insecticides (P=0.02). Probit model used to estimate KDT50 and KDT95 values did not display normal distribution of percentage knockdown with time for all the vectors tested for DDT and deltamethrin, except for Ma. indiana for deltamethrin assay and Cx. gelidus for the DDT assay. The KDT50 and KDT95 values of DDT for all the vector mosquitoes were increased by factors ranging 1.5-3.8 (in KDT50) and 1.2-11.2 (in KDT95) as compared to deltamethrin.
Table II.
Percentage mortality, KDT50 and KDT95 values (minutes) of known JE vectors for DDT and deltamethrin
Vector density and insecticide resistance mapping: Mosquito density of JE vectors was determined using pie method in all the study sites. The density of each vector has been shown using different colours. To depict the resistance status of deltamethrin and DDT for different vectors, the vector density of study sites were aggregated at PHC level and the resistance status was shown using green colour for sensitive and yellow colour for suspected resistance in the vector mosquitoes (Fig.). The map shows that all the vector species tested, except Ma. indiana, were sensitive to deltamethrin, whereas Ma. indiana showed suspected resistance status. On the other hand, all the species showed suspected resistance status for DDT.
Fig.
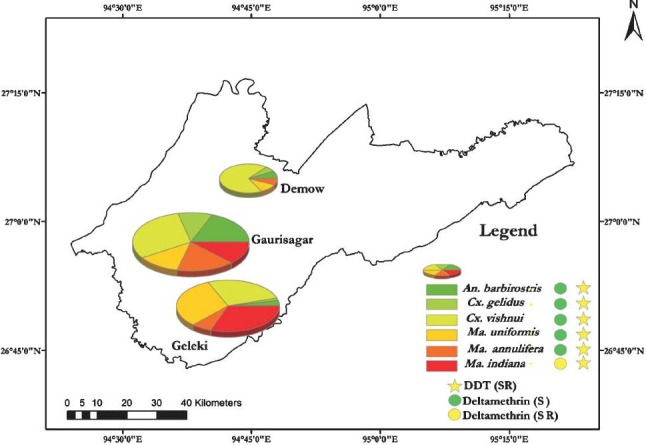
Density and resistance status of major known JE vectors at PHC level. SR, suspected resistance; S, sensitive.
Discussion
The present study was undertaken in three most affected Primary Health Centres of Sibsagar district of Assam. Of the nine known JE vectors, Cx. bitaeniorhynchus, Cx. quinquefasciatus and Cx. whitmorei were collected in very low number, throughout the study period, and the remaining six vectors were widely distributed and collected in large number. Cx. vishnui group was most abundant species among all the three Primary Health Centres. The known Indian JE vector density was high in Gaurisagar as compared to the other two Primary Health Centres. The population dynamics of JE vectors largely depends upon rice cultivation, water bodies, temperature and humidity in the rural areas24. The Gaurisagar area has many water bodies, duck rearing ponds and large area under paddy cultivation adjacent to the domiciliary surroundings. As a result, the JE vector mosquito density was comparatively high in the Gaurisagar. Cx. vishnui and Cx. gelidus have been reported to be most prevalent JE vector in many parts of Assam4. In many parts of India and Southeast Asian countries, Cx. tritaeniorhynchus has been recorded in abundance and incriminated as major vector JE vector1. Population density of Cx. vishnui group was substantially high among all the sampling villages during peak JE period, this species is most likely to be a potential JE vector in the area.
In the study area, DDT is used for indoor residual spray regularly and deltamethrin-treated bednets were recently provided free of cost in limited numbers to the villagers. However, during the study only 80-90 per cent of households were found using treated bednets and many of them were not regularly re-treating their bednets. The results obtained in the present study showed that all the six known JE vector mosquitoes were not completely susceptible to DDT. The mortality obtained at 24 h post-exposure of DDT was >80 per cent and <98 per cent, suggesting further verification data are required in the study area. Similar results have been obtained in the other parts of northeast India, where DDT was tested on Anopheles mosquito14,15. In all the PHCs studied, the tested mosquitoes were fully susceptible to deltamethrin, except Ma. indiana, for which verification data are needed. The high KDT50 and KDT95 values of DDT as compared to deltamethrin compare well with the fact that DDT has been in use for many years, which have led to the development of resistance among the mosquito species in various parts of Assam15,25. Further, the agriculture expansion is also a major factor contributing to resistance development in vectors in the region4,14. The KDT50 values of deltamethrin observed in the present study were higher than observed elsewhere15. The knockdown values are largely associated with the level of use of an insecticide, which would lead to possible resistance in the mosquito species20. It was interesting to note that in Ma. indiana, the KDT50 and KDT95 values of DDT were lower than other mosquitoes, suggesting that these were more knockdown sensitive to DDT. The knockdown resistance mechanism is one of the most important forms of resistance, which occurs when insecticide detoxifying enzyme level is elevated in the mosquito15,26. The insecticide resistance is a complex mechanism and depends upon different physiological conditions of insect25. It has been shown that use of different insecticides may accelerate cross-resistance among insects27.
GIS mapping has been used before to find out the malaria hot spots at health subcentre level28,29. The maps generated in the present study may be effective in communicating the main findings with the local health authority at implementation level and planning an effective vector control strategy.
In conclusion, the present study confirmed the development of low level DDT resistance in all known JE vectors prevalent in the study area, therefore, control programmes employing DDT as residual insecticide may not achieve satisfactory results. Deltamethrin used in impregnation of bednets could be useful at individual household level. More areas are needed to be scanned and mapped for the insecticide resistance status of major JE vectors.
Acknowledgment
Authors acknowledge Dr Akhil Hazarika, Joint Director Health of Sibsagar district for providing necessary support during the study.
References
- 1.Kanojia PC. Ecological study on mosquito vectors of Japanese encephalitis virus in Bellary district, Karnataka. Indian J Med Res. 2007;126:152–7. [PubMed] [Google Scholar]
- 2.Reuben R, Gajanana A. Japanese encephalitis in India. Indian. J Pediatr. 1997;64:243–5. doi: 10.1007/BF02752458. [DOI] [PubMed] [Google Scholar]
- 3.Gajanana A, Rajendra R, Samuel P, Thanmozhi V, Tsai TF, Kimura-Kuroda J, Reuben R. Japanese encephalitis in South Arcot district, Tamil Nadu: A three year longitudinal study of vector abundance and infection frequency. J Med Entomol. 1997;34:651–9. doi: 10.1093/jmedent/34.6.651. [DOI] [PubMed] [Google Scholar]
- 4.Phukan AC, Bora PK, Mahanta J. Japanese encephalitis in Assam, northeast India. South East Asian J Trop Med Public Health. 2004;35:618–22. [PubMed] [Google Scholar]
- 5.Khan SA, Dutta P, Khan AM, Topno R, Choudhury P, Borah J, et al. Japanese encephalitis epidemiology in Arunachal Pradesh, a hill state in northeast India. Asian Pacific J Trop Dis. 2011;1:119–22. [Google Scholar]
- 6.Rabha B, Goswami D, Dhiman S, Das NG, Talukdar PK, Nath MJ, et al. A cross-sectional investigation of malaria epidemiology among seven tea estates in Assam, India. J Parasit Dis. 2012;36:1–6. doi: 10.1007/s12639-011-0070-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saxena V, Dhole TN. Preventive strategies for frequent outbreaks of Japanese encephalitis in Northern India. J Biosci. 2008;33:505–14. doi: 10.1007/s12038-008-0069-9. [DOI] [PubMed] [Google Scholar]
- 8.Singh A, Saxena SK, Srivastava AK, Mathur A. Japanese encephalitis: A persistent threat. Proc Natl Acad Sci. 2012;82:55–68. [Google Scholar]
- 9.Samuel PP, Hiriyan J, Gajanana A. Japanese encephalitis virus infection in mosquitoes and its epidemiological implications. ICMR Bull. 2000;30:37–42. [Google Scholar]
- 10.Murty US, Satya Kumar DVR, Sriram K, Rao KM, Singh TG, Arunachalam M, et al. Seasonal prevalence of Culex vishnui group, the major vector of Japanese encephalitis virus in an endemic district of Andhra Pradesh, India. J Am Mosq Control Assoc. 2002;18:290–3. [PubMed] [Google Scholar]
- 11.Srivastava VK, Singh A, Thapar BR. Field evaluation of malathion fogging against Japanese encephalitis vector, Culex tritaeniorhynchus. J Vector Borne Dis. 2008;45:249–50. [PubMed] [Google Scholar]
- 12.Karunaratne SHPP, Hemingway J. Insecticide resistance spectra and resistance mechanisms in populations of Japanese encephalitis vector mosquitoes, Culex tritaeniorhynchus and Cx. gelidus, in Sri Lanka. Med Vet Entomol. 2000;14:430–6. doi: 10.1046/j.1365-2915.2000.00252.x. [DOI] [PubMed] [Google Scholar]
- 13.Karunaratne SHPP, Vaughan A, Paton MG, Hemingway J. Amplification of a serine esterase gene is involved in insecticide resistance in Sri Lankan Culex tritaeniorhynchus. Insect Mol Biol. 1998;7:307–15. doi: 10.1046/j.1365-2583.1998.740307.x. [DOI] [PubMed] [Google Scholar]
- 14.Dhiman S, Gopalakrishnan R, Goswami D, Baruah I, Singh L. Malaria epidemiology along Indo-Bangladesh border in Tripura state, India. Southeast Asian J Trop Med Public Health. 2010;41:1279–89. [PubMed] [Google Scholar]
- 15.Sarkar M, Bhattacharyya IK, Borkotoki A, Goswami D, Rabha B, Baruah I, et al. Insecticide resistance and detoxifying enzyme activity in the principal bancroftian filariasis vector, Culex quinquefasciatus in north-eastern India. Med Vet Entomol. 2009;23:122–31. doi: 10.1111/j.1365-2915.2009.00805.x. [DOI] [PubMed] [Google Scholar]
- 16.Bhattacharyya DR, Handique R, Prakash A, Dutta P, Mahanta J, Srivastava VK. Insecticide susceptibility status of potential vectors of Japanese encephalitis in Dibrugarh district, Assam. J Commun Dis. 1996;28:67–9. [PubMed] [Google Scholar]
- 17.Khan SA, Dutta P, Narain K, Srivastava VK. Studies on day-time resting habits of JE Vector mosquitoes in upper Assam with a note on insecticide susceptibility status. J Commun Dis. 1997;29:367–70. [PubMed] [Google Scholar]
- 18.Kulkarni SM, Naik PS. Susceptibility status of Culex tritaeniorhynchus Giles, 1901, to insecticides in the State of Goa. Indian J Med Res. 1991;93:179. [PubMed] [Google Scholar]
- 19.Kulkarni SM, Geevarghese G, Geoge PJ. Susceptibility status of five species of Japanese encephalitis vectors to insecticides from Kolar district, Karnataka. Indian J Med Res. 1992;95:297–300. [PubMed] [Google Scholar]
- 20.Kamau L, Vulule JM. Status of insecticide susceptibility in Anopheles arabiensis from Mwea rice irrigation scheme, Central Kenya. Malaria J. 2006;5:46. doi: 10.1186/1475-2875-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Criteria and meaning of tests for determining susceptibility or resistance of insects to insecticides. WHO Tech Rep Ser. 1963;265:135–8. [Google Scholar]
- 22.Puntener W. 2nd ed. Basal, Switzerland: Ciba-Geigy Limited; 1981. Manual for field trials in plant protection. [Google Scholar]
- 23.Vector resistance to insecticide, 15th Report of the WHO Expert Committee on Vector Biology and Control. WHO Tech Rep Ser. 1992;818:1–61. [PubMed] [Google Scholar]
- 24.Khan SA, Narian K, Handique R, Dutta P, Mahanta J, Satyanarayana K, et al. Role of some environmental factors in modulating seasonal abundance of potential Japanese encephalitis vectors in Assam, India. Southeast Asian J Trop Med Public Health. 1996;27:382–91. [PubMed] [Google Scholar]
- 25.Sarkar M, Borkotoki A, Baruah I, Bhattacharyya IK, Srivastava RB. Molecular analysis of knock down resistance (kdr) mutation and distribution of kdr genotypes in a wild population of Culex quinquefasciatus from India. Trop Med Int Health. 2009;14:1097–104. doi: 10.1111/j.1365-3156.2009.02323.x. [DOI] [PubMed] [Google Scholar]
- 26.Roberts DR, Andre RG. Insecticide resistant issues in vector-borne disease control. Am J Trop Med Hyg. 1994;50:21–34. doi: 10.4269/ajtmh.1994.50.21. [DOI] [PubMed] [Google Scholar]
- 27.Bielza P, Espinosa PJ, Quinto V, Abellan J, Contreras J. Synergism studies with binary mixtures of pyrethroid, carbamate and organophosphate insecticides on Frankliniella occidentalis (Pergande) Pest Manag Sci. 2007;63:84–9. doi: 10.1002/ps.1328. [DOI] [PubMed] [Google Scholar]
- 28.Yadav K, Nath MJ, Talukdar PK, Saikia PK, Baruah I, Singh L. Malaria risk areas of Udalguri district of Assam, India: a GIS-based study. Int J Geogr Inf Sci. 2012;26:123–31. [Google Scholar]
- 29.Nath MJ, Bora A, Talukdar PK, Das NG, Dhiman S, Baruah I, et al. A longitudinal study of malaria associated with deforestation in Sonitpur district of Assam, India. Geocarto Int. 2012;27:79–88. [Google Scholar]


