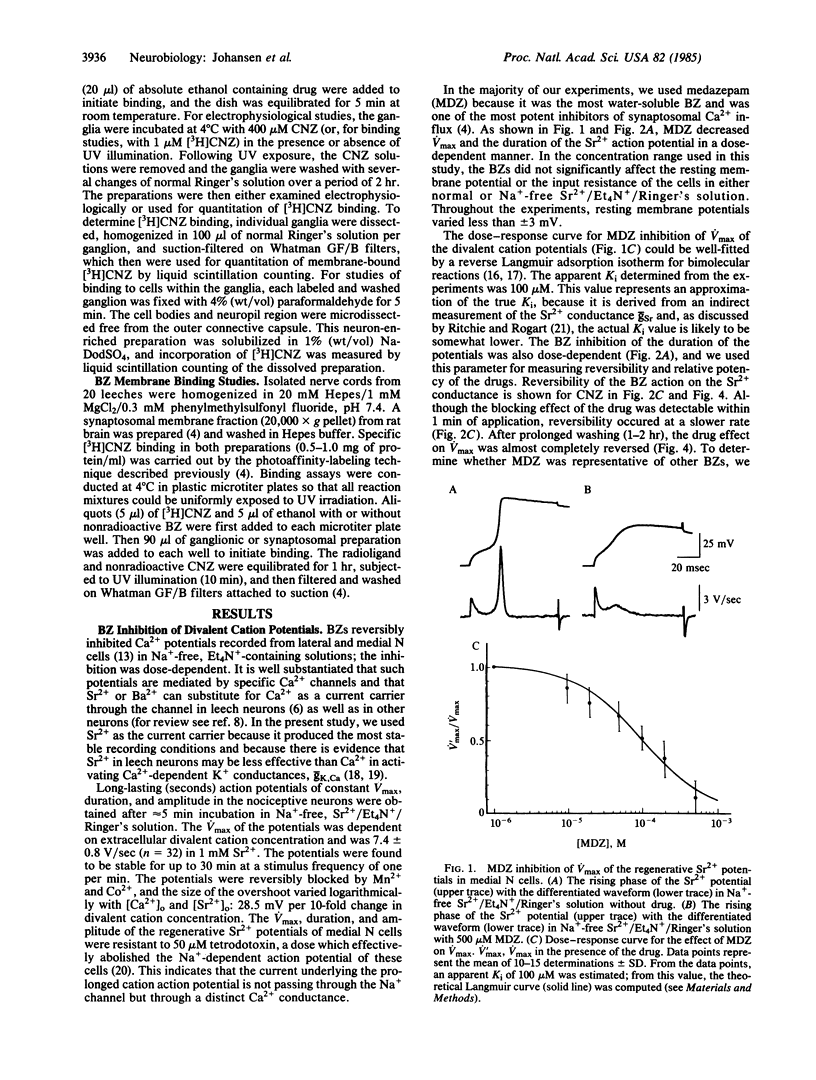
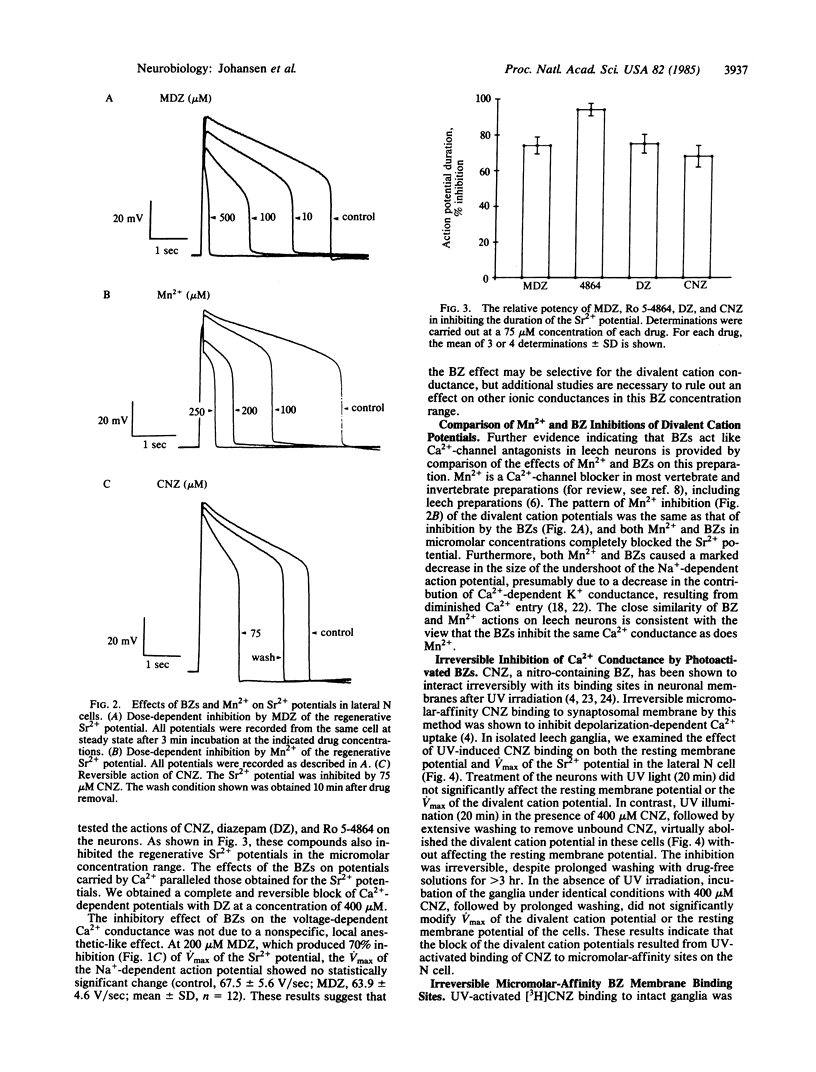
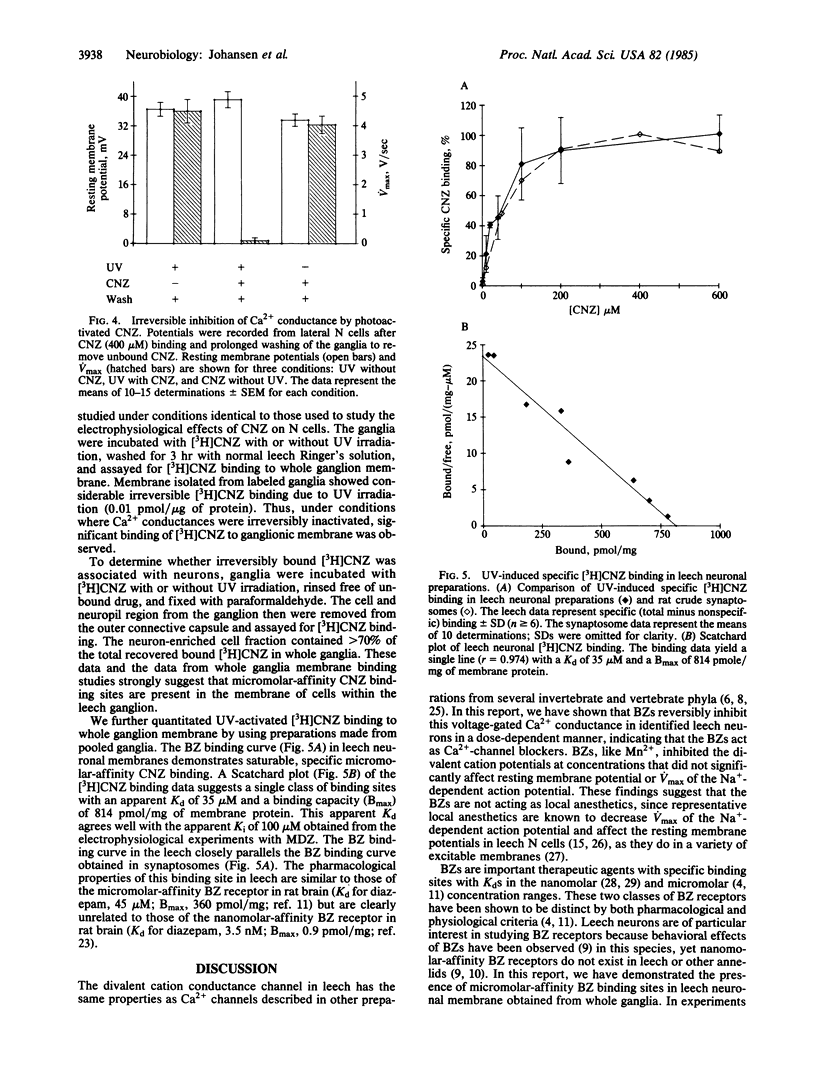
Abstract
Benzodiazepines (BZs) in micromolar concentrations inhibit Mn2+- and Co2+-sensitive regenerative divalent cation potentials, which are revealed in the presence of tetraethylammonium ion, in leech nociceptive neurons (N cells). This BZ effect is reversible and dose-dependent. The BZs, like Mn2+ and Co2+, inhibit the maximum rate of depolarization (Vmax) and duration of divalent cation potentials at concentrations that do not significantly affect resting membrane potential or Vmax of the Na+-dependent action potential. Ultraviolet-induced BZ binding to micromolar-affinity sites in ganglia and isolated cells irreversibly blocks Ca2+ conductance in neurons without significantly affecting resting membrane potentials. BZ binding studies with leech neuronal membrane show saturable, specific binding in the micromolar concentration range that was similar to BZ binding to synaptosomal membrane fractions. The apparent Kd obtained from the micromolar-affinity BZ binding curve for leech ganglionic membrane preparations agrees well with the apparent Ki estimated from the dose-response curve measuring BZ inhibition of Vmax of the divalent cation potentials. These findings indicate that BZs act like Ca2+-channel antagonists in intact neuronal preparations and are consistent with the hypothesis that BZ binding to micromolar-affinity receptors modulates voltage-gated Ca2+ channels.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowling A. C., DeLorenzo R. J. Micromolar affinity benzodiazepine receptors: identification and characterization in central nervous system. Science. 1982 Jun 11;216(4551):1247–1250. doi: 10.1126/science.6281893. [DOI] [PubMed] [Google Scholar]
- Braestrup C., Squires R. F. Specific benzodiazepine receptors in rat brain characterized by high-affinity (3H)diazepam binding. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3805–3809. doi: 10.1073/pnas.74.9.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradetti R., Moroni F., Pepeu G. Pharmacological effects of benzodiazepines in the leech; benzodiazepine and GABA receptors and GABA level. Pharmacol Res Commun. 1980 Jun;12(6):581–585. doi: 10.1016/s0031-6989(80)80144-5. [DOI] [PubMed] [Google Scholar]
- Cuervo L. A., Adelman W. J., Jr Equilibrium and kinetic properties of the interaction between tetrodotoxin and the excitable membrane of the squid giant axon. J Gen Physiol. 1970 Mar;55(3):309–335. doi: 10.1085/jgp.55.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLorenzo R. J. The calmodulin hypothesis of neurotransmission. Cell Calcium. 1981 Aug;2(4):365–385. doi: 10.1016/0143-4160(81)90026-9. [DOI] [PubMed] [Google Scholar]
- Ferrendelli J. A., Daniels-McQueen S. Comparative actions of phenytoin and other anticonvulsant drugs on potassium- and veratridine-stimulated calcium uptake in synaptosomes. J Pharmacol Exp Ther. 1982 Jan;220(1):29–34. [PubMed] [Google Scholar]
- Greenblatt D. J., Woo E., Allen M. D., Orsulak P. J., Shader R. I. Rapid recovery from massive diazepam overdose. JAMA. 1978 Oct 20;240(17):1872–1874. [PubMed] [Google Scholar]
- Hagiwara S., Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Jansen J. K., Nicholls J. G. Conductance changes, an electrogenic pump and the hyperpolarization of leech neurones following impulses. J Physiol. 1973 Mar;229(3):635–655. doi: 10.1113/jphysiol.1973.sp010158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen J., Hockfield S., McKay R. D. Distribution and morphology of nociceptive cells in the CNS of three species of leeches. J Comp Neurol. 1984 Jun 20;226(2):263–273. doi: 10.1002/cne.902260210. [DOI] [PubMed] [Google Scholar]
- Johansen J., Yang J., Kleinhaus A. L. Actions of procaine on specific nociceptive cells in leech central nervous system. J Neurosci. 1984 May;4(5):1253–1261. doi: 10.1523/JNEUROSCI.04-05-01253.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao C. Y., Walker S. E. Active groups of saxitoxin and tetrodotoxin as deduced from actions of saxitoxin analogues on frog muscle and squid axon. J Physiol. 1982 Feb;323:619–637. doi: 10.1113/jphysiol.1982.sp014095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhaus A. L. Divalent cations and the action potential of leech Retzius cells. Pflugers Arch. 1976 May 12;363(2):97–104. doi: 10.1007/BF01062276. [DOI] [PubMed] [Google Scholar]
- Kleinhaus A. L., Prichard J. W. Calcium dependent action potentials produced in leech Retzius cells by tetraethylammonium chloride. J Physiol. 1975 Mar;246(2):351–369. doi: 10.1113/jphysiol.1975.sp010894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhaus A. L., Prichard J. W. Close relation between TEA responses and Ca-dependent membrane phenomena of four identified leech neurones. J Physiol. 1977 Aug;270(1):181–194. doi: 10.1113/jphysiol.1977.sp011945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhaus A. L., Prichard J. W. Differential action of tetrodotoxin on identified leech neurons. Comp Biochem Physiol C. 1983;74(1):211–218. doi: 10.1016/0742-8413(83)90176-7. [DOI] [PubMed] [Google Scholar]
- Kleinhaus A. L., Prichard J. W. Interaction of divalent cations and barbiturates on four identified leech neurons. Comp Biochem Physiol C. 1979;63C(2):351–357. doi: 10.1016/0306-4492(79)90085-6. [DOI] [PubMed] [Google Scholar]
- Kleinhaus A. L. Segregation of leech neurones by the effect of sparteine on action potential duration. J Physiol. 1980 Feb;299:309–321. doi: 10.1113/jphysiol.1980.sp013126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie S. W., Friedman M. B., Coleman R. R. Effects of chlordiazepoxide on depolarization-induced calcium influx into synaptosomes. Biochem Pharmacol. 1980 Sep 15;29(18):2439–2443. doi: 10.1016/0006-2952(80)90347-0. [DOI] [PubMed] [Google Scholar]
- Lister R. G., File S. E., Greenblatt D. J. The behavioural effects of lorazepam are poorly related to its concentration in the brain. Life Sci. 1983 Apr 25;32(17):2033–2040. doi: 10.1016/0024-3205(83)90055-3. [DOI] [PubMed] [Google Scholar]
- Möhler H., Battersby M. K., Richards J. G. Benzodiazepine receptor protein identified and visualized in brain tissue by a photoaffinity label. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1666–1670. doi: 10.1073/pnas.77.3.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls J. G., Baylor D. A. Specific modalities and receptive fields of sensory neurons in CNS of the leech. J Neurophysiol. 1968 Sep;31(5):740–756. doi: 10.1152/jn.1968.31.5.740. [DOI] [PubMed] [Google Scholar]
- Nielsen M., Braestrup C., Squires R. F. Evidence for a late evolutionary appearance of brain-specific benzodiazepine receptors: an investigation of 18 vertebrate and 5 invertebrate species. Brain Res. 1978 Feb 10;141(2):342–346. doi: 10.1016/0006-8993(78)90203-2. [DOI] [PubMed] [Google Scholar]
- Reuter H. Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature. 1983 Feb 17;301(5901):569–574. doi: 10.1038/301569a0. [DOI] [PubMed] [Google Scholar]
- Ritchie J. M., Rogart R. B. The binding of saxitoxin and tetrodotoxin to excitable tissue. Rev Physiol Biochem Pharmacol. 1977;79:1–50. doi: 10.1007/BFb0037088. [DOI] [PubMed] [Google Scholar]
- Taft W. C., DeLorenzo R. J. Micromolar-affinity benzodiazepine receptors regulate voltage-sensitive calcium channels in nerve terminal preparations. Proc Natl Acad Sci U S A. 1984 May;81(10):3118–3122. doi: 10.1073/pnas.81.10.3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallman J. F., Paul S. M., Skolnick P., Gallager D. W. Receptors for the age of anxiety: pharmacology of the benzodiazepines. Science. 1980 Jan 18;207(4428):274–281. doi: 10.1126/science.6101294. [DOI] [PubMed] [Google Scholar]
- Thomas J. W., Tallman J. F. Characterization of photoaffinity labeling of benzodiazepine binding sites. J Biol Chem. 1981 Oct 10;256(19):9838–9842. [PubMed] [Google Scholar]
- Yang J., Johansen J., Kleinhaus A. L. Procaine actions on tetrodotoxin sensitive and insensitive leech neurons. Brain Res. 1984 Jun 8;302(2):297–304. doi: 10.1016/0006-8993(84)90243-9. [DOI] [PubMed] [Google Scholar]



