Abstract
Vitamin D is essential for calcium absorption and for maintaining bone health in the pediatric population. Vitamin D deficiency may develop from nutritional deficiencies, malabsorption, enzyme-inducing medications, and many other etiologies. It may present as hypocalcemia before bone demineralization at periods of increased growth velocity (infancy and adolescence) because the increased calcium demand of the body cannot be met. In children, inadequate concentrations of vitamin D may cause rickets and/or symptomatic hypocalcemia, such as seizures or tetany. In this review, we will discuss the pharmacology behind vitamin D supplementation, laboratory assessments of vitamin D status, current literature concerning vitamin D supplementation, and various supplementation options for the treatment of vitamin D deficiency in the pediatric population.
INDEX TERMS: cholecalciferol, ergocalciferol, pediatric, vitamin D deficiency
INTRODUCTION
Vitamin D plays an essential role in maintaining bone health through regulating calcium concentrations in the body. The development of vitamin D deficiency is associated with deteriorating bone health and in severe cases, hypocalcemia, rickets, and osteomalacia in children and adults.1 Those at greatest risk of vitamin D deficiency include patients with chronic illnesses (e.g., chronic kidney disease [CKD], cystic fibrosis [CF], asthma, and sickle cell disease), dark-pigmented skin, poor nutrition, and infants who are exclusively breastfed.2,3 The primary source of vitamin D is sunlight exposure, which has been limited or blocked extensively for many children over the past 20 years due to the association of skin cancer and ultraviolet rays. Chronic use of certain medications (e.g., glucocorticoids, cytochrome P450 3A4 inducers, anticonvulsants, and anti-retroviral agents) has also been associated with compromised vitamin D concentrations. Given the high rate of bone development early in life, adequate serum concentrations of vitamin D are crucial for the developing child. There has also been a piquing interest in vitamin D in pediatric patients due to the recent epidemiologic reports suggesting that vitamin D may protect against autoimmune disease and play a role in innate immunity.2
VITAMIN D DEFICIENCY
The serum concentration that constitutes vitamin D deficiency is controversial and not well supported by clinical trials, especially in the pediatric population. Deficiency is generally measured by the calcidiol concentration because of its long half-life of 2 to 3 weeks, relatively robust circulating concentration, and resilience to fluctuations in PTH concentrations.4 Table 1 summarizes normal and abnormal serum vitamin D concentrations as classified by the American Academy of Pediatrics (AAP).1,2,5,6 The AAP and the Institute of Medicine (IOM) both define vitamin D insufficiency as calcidiol (25-OH-D) concentrations < 20 ng/mL in the pediatric population.1,7 In contrast, the Endocrine Society and the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines both classify insufficiency as calcidiol concentrations < 30 ng/mL. The Endocrine Society defines deficiency as < 20 ng/mL, and KDOQI defines deficiency as < 15 ng/mL.8,9 The definitions in these last 2 groups are more consistent with the classification system used in adults based on evidence of compromised bone health and elevations in parathyroid hormone (PTH) at calcidiol concentrations up to 32 ng/mL (80 nmol/L) (Table 1).2,10
Table 1.
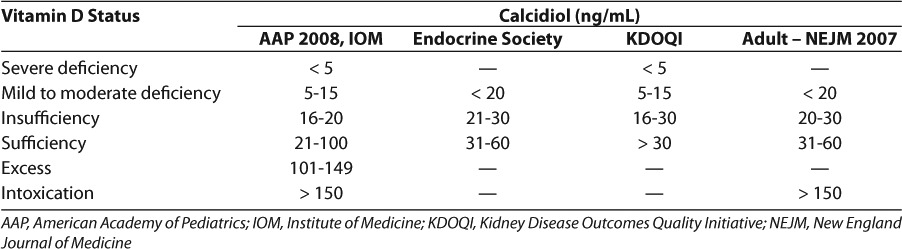
In a vitamin D deficient patient, the intestinal absorption of calcium and phosphorus is decreased. The parathyroid gland recognizes the low serum calcium concentrations and releases PTH to increase the serum calcium back into an adequate range. PTH increases the calcium reabsorption in the kidneys and the excretion of phosphorus, therefore decreasing the risk of complication from an elevated calcium phosphate product (e.g., kidney stones). While this reduction is protecting the body, it is also decreasing bone mineralization at the same time. Over weeks to months, osteomalacia, stunted growth, and rickets may develop.1 Studies have shown that over half of infants, children, and adolescents may be inadequately supplemented.11,12 In 2008, the AAP published a review article with recommended target vitamin D concentrations for healthy infants, children, and adolescents (Table 1).1,9,13
In efforts to achieve and maintain the target vitamin concentrations, the AAP recommends all infants, children, and adolescents should receive a minimum daily intake of 400 international units of vitamin D to prevent rickets and to maintain vitamin D concentrations at > 20 ng/mL (50 nmol/L).1 Term infants should be supplemented with 400 to 800 units daily to account for the insufficient transfer of maternal vitamin D stores and ensure calcidiol concentrations of > 20 ng/mL (50 nmol/L).1 Preterm infants are more likely to be vitamin D deficient since their transplacental transfer from the mother was a shorter duration, hospitalization leading to a negligible amount of UV-mediated vitamin D formation, and possibly lower vitamin D stores due to a lower fat mass.14 To address this population, the AAP published an expert opinion report in 2013 on the calcium and vitamin D requirements of enterally fed preterm infants.14 Although there are no clinical outcome studies in this population, the AAP recommends 200 to 400 units per day of vitamin D supplementation in very low birth weight infants (<1500 g) and 400 units per day of vitamin D supplementation in infants weighing > 1500 g.14 It is reasonable to consider increasing this dose to 1000 units per day in > 1500 g infants, as this is the established upper tolerable intake for healthy full-term infants. The calcidiol concentration goal in the preterm population remains the same as full-term infants (>20 ng/mL).14 In 2010, the IOM issued guidelines that increased the recommended dietary allowance of vitamin D to 600 units daily for healthy children 1 to 18 years of age, which has been echoed by the Endocrine Society.7,9
PHARMACOLOGY
Our bodies obtain vitamin D in 2 different ways. The primary source of vitamin D3 (cholecalciferol) comes from direct synthesis in our skin (>90%). Upon exposure to ultraviolet radiation, 7-dehydrocholesterol in our epidermal cells synthesizes vitamin D3. The remainder of our need is typically obtained from dietary sources in either form, vitamin D3 or vitamin D2 (ergo-calciferol). Both forms undergo hydroxylation in the liver to create the storage form of vitamin D, 25-hydroxy vitamin D (25[OH]-D, calcidiol, or calcifediol). Furthermore, in the kidneys, hydroxylation of calcidiol synthesizes the active metabolite, 1,25-dihydroxyvitamin D (1,25[OH]-D) (calcitriol). This pathway is visually depicted in Figure. Calcitriol is responsible for increasing calcium absorption, bone resorption, and decreasing renal calcium and phosphate excretion to maintain bone health.15 The synthesis of calcitriol is mediated by PTH, serum phosphate concentration, and growth hormone, and may occur in non-renal sites, such as alveolar macrophages and osteoblasts.2,16 Additionally, vitamin D has extraskeletal responsibilities, with vitamin D receptors in the small intestine, colon, osteoblasts, activated T and B lymphocytes, beta islet cells, and major organs (brain, heart, skin, gonads, prostate, breast, and mononuclear cells).2,16 The immunologic effects of vitamin D have stimulated great interest, but studies in these areas are currently limited in pediatric patients.
Figure.
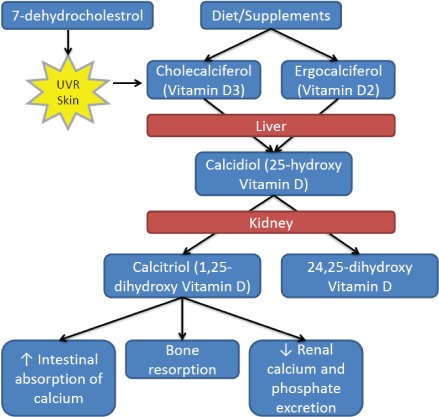
Vitamin D metabolism.87
MEDICATION INDUCED VITAMIN D DEFICIENCY
Metabolism of dietary vitamin D to calcidiol occurs in the liver through the cytochrome P450 enzyme system. Certain classes of medications act on this enzyme system to increase the metabolism of vitamin D and therefore reduce the body's systemic exposure to active vitamin D concentrations. Some anti-epileptic drugs (AEDs) are inducers of the cytochrome P450 system (phenytoin, carbamazepine, oxcarbazepine, phenobarbital, and primidone). Aside from the detrimental bone effects of vitamin D deficiency, rapid decreases in calcium may precipitate a seizure, further complicating the clinical picture (e.g., etiology of seizures). Valproic acid, though it is an inhibitor of the enzyme system, increases bone turnover through increasing osteoclast activity and therefore tilting the balance of bone formation and bone resorption.17,18
Recommendations have been made for all patients on an AED to receive a preventative dose of vitamin D 400 to 2000 units per day.17 Patient characteristics such as baseline calcidiol concentration, polypharmacy, and sun exposure should help guide vitamin D therapy as well. Patients diagnosed with AED-induced osteoporosis may need larger doses of vitamin D replacement therapy to correct biochemical abnormalities (PTH, calcium, and phosphorus).18 Calcidiol concentrations should be monitored (prior to or at the start of AED initiation) and then yearly thereafter. If diagnosed with vitamin D deficiency, initiating therapy with the standard dosing recommendation for children with vitamin D deficiency is acceptable; however, the doses may need to be increased according to the calcidiol concentrations, which should be measured monthly during treatment. Doses of 5000 to 15,000 units per day have been used for AED-induced osteomalacia.17
Rates of vitamin D insufficiency are high in pediatric patients with human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome due to the disease itself and the life-saving highly active antiretroviral therapy (HAART). Rutstein and colleagues19 compared the rates of vitamin D deficiency/insufficiency in children and young adults with HIV to a healthy group. Vitamin D deficiency/insufficiency was present in 36% and 89% of those with HIV (84% on HAART therapy) compared to 15% and 84% of the comparison group, respectively. Protease inhibitors inhibit the cytochrome P450 enzyme system and decrease the production of active vitamin D (calcitriol). Nucleoside reverse transcriptase inhibitors have also been linked to vitamin D deficiency through increased lactate concentrations and not due to cytochrome P450 inhibition. Due to the presence of multiple risk factors for osteoporosis and the high prevalence of deficiency, all patients on HAART should be screened annually for vitamin D deficiency and encouraged to maintain sufficient calcium and vitamin D intake.20
Other drug classes that may affect the absorption, metabolism, or activation of vitamin D include corticosteroids, azole antifungals, and cytochrome P450 3A4 inducers. Although there is no formal recommendation for monitoring, annual monitoring of calcidiol concentrations may be warranted in pediatrics receiving these medications.21
SOURCES OF VITAMIN D
UV Radiation and Cutaneous Cholecalciferol Synthesis
Cutaneous synthesis of vitamin D is a significant source of vitamin D replenishment. The amount of vitamin D synthesized by our skin depends on a number of factors: the age of the individual, the amount of skin exposed, the duration of exposure, geographic-related factors (i.e., latitude, season, time of day, shade, and air pollution), sun block use, and the skin pigment of the individual.1,2 Holick2 estimates exposure of the body in a bathing suit to 1 minimal erythemal dose (MED or the dose of radiation that causes a slight pinkness to the skin 24 hours after exposure) equals about 20,000 units. Thus, exposure of arms and legs to 0.5 MED approximates ingesting 3000 units of vitamin D3. Studies have shown that children, especially infants, may require less sun exposure than adults to produce adequate vitamin D concentrations because of greater surface area to volume ratio and enhanced ability to produce vitamin D than older people.22 A study in 1985 found that 30 minutes of sun exposure for infants in diapers or 2 hours for fully clothed infants without a hat maintained weekly calcidiol concentrations of 11 ng/mL (27.5 nmol/L).23 The AAP recommends that children younger than 6 months be kept out of direct sunlight to reduce the risks of skin cancer.24 Currently, there are no recommendations available to validate the appropriate duration of sun exposure in the pediatric population, and the variability of vitamin D synthesis between individuals would make such a recommendation difficult. The lack of data and the risks associated with prolonged sun exposure suggest food and supplementation as the preferred mode of repleting vitamin D stores.
Dietary Sources of Vitamin D
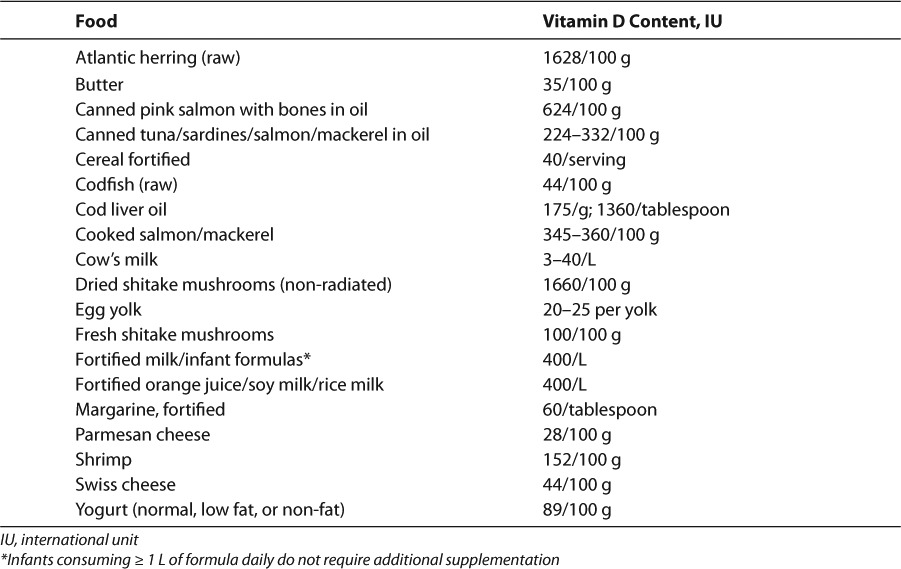
There are many natural food sources of vitamin D2 and vitamin D3, including oily fish (e.g., salmon, mackerel), cod liver oil, organ meats, and egg yolks (Table 2). However, these products are not particularly kid-friendly and routine adequate intake may be difficult. In the United States (US), there are fortified food options, including infant formula, milk, and orange juice, to help meet needs. Also, all infant formulas sold in the US contain at least 400 units/L of vitamin D.25
Table 2.
Vitamin D Content of Foods88
Vitamin D in Breast Milk
Breast milk contains very little vitamin D, an average of 22 units/L (range 15 to 50 units/L) in a vitamin D-sufficient mother.26 Recent studies suggest that maternal intake of higher than recommended doses of vitamin D (4000 to 6400 units daily) may achieve vitamin D concentrations in breast milk to provide sufficient vitamin D supplementation for breastfeeding infants. However, this approach is not recommended.27,28 Due to the low vitamin D concentrations found in breast milk, the newest recommendation for exclusively breastfed infants is to provide a supplement of 400 units per day (increased from 200 units per day).1
Vitamin D Formulations
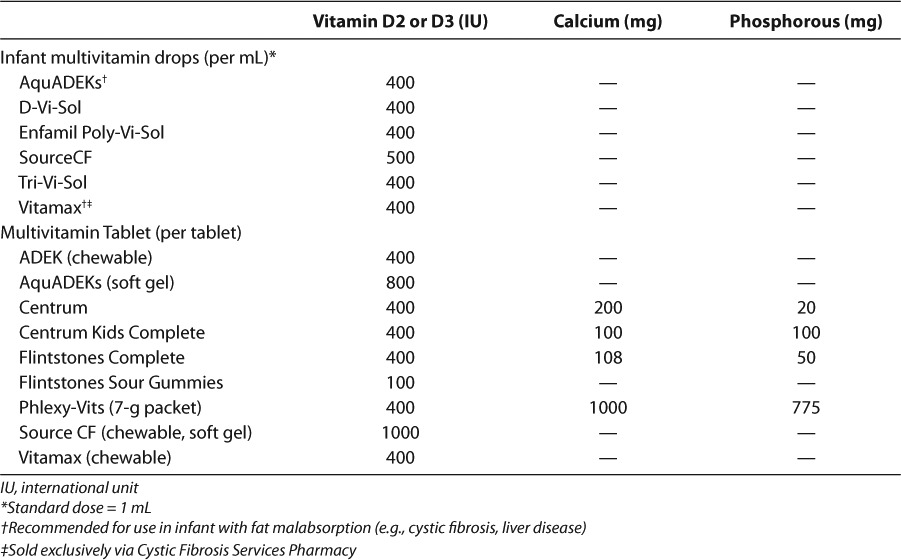
Vitamin D is available commercially as ergo-calciferol, cholecalciferol, and calcitriol. Ergocalciferol and cholecalciferol, once thought to be equipotent, may increase vitamin D stores to varying degrees. Recent evidence suggests that cholecalciferol increases calcidiol concentrations two- to threefold more than ergocalciferol.29,30 The formulations available in the US are summarized in Table 3 and the vitamin D content of commonly used pediatric multivitamins in Table 4. Despite the evidence suggesting the pharmacodynamic differences between cholecalciferol and ergocalciferol, most guidelines do not have a preference between the 2 products.1,7,9 However, the KDOQI and Cystic Fibrosis Foundation (CFF) guidelines prefer vitamin D2 due to safety data in animals.8,31,32 There are no direct comparisons of the 2 formulations and in general, calcitriol does not have a role in repleting vitamin D stores.
Table 3.
Available Formulations of Vitamin D89
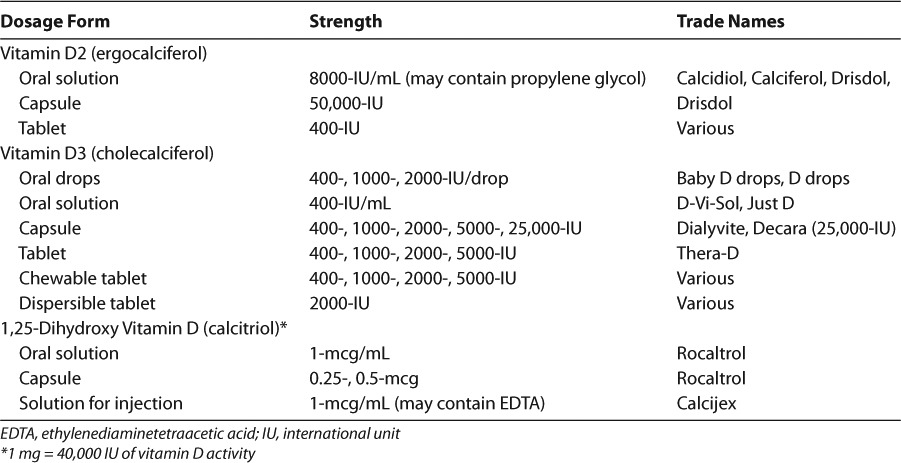
Table 4.
Vitamin D, Calcium, and Phosphorous Content of Common Multivitamins90
VITAMIN D SUPPLEMENTATION IN CHRONIC DISEASE
Vitamin D Deficiency Rickets
Severe vitamin D deficiency can lead to symptomatic hypocalcemia, which can result in seizures, osteomalacia, or rickets. Rickets involves bone demineralization that occurs in areas adjacent to the growth plate.1 The exact prevalence of rickets is unknown. However, case reports and case series of documented rickets suggest this problem still exists today.1 Rickets may be caused by reasons other than nutritional vitamin D deficiency (e.g., calcium and phosphorus deficiency, inherited forms of hypophosphatemic rickets, and vitamin D receptor mutations); however, these etiologies will not be discussed in this review.
Dosing
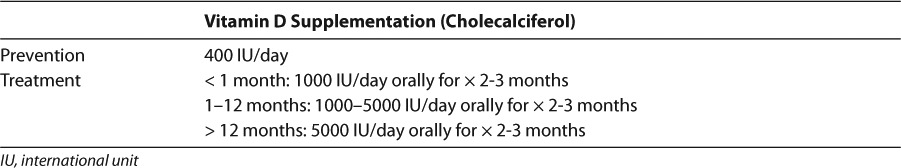
For the treatment of vitamin D deficiency rickets, the AAP recommends an initial 2- to 3-month regimen of “high-dose” vitamin D therapy of 1000 units daily in neonates, 1000 to 5000 units daily in infants 1 to 12 months old, and 5000 units daily in patients over 12 months old.1 These recommendations are summarized in Table 5. Although radiologic evidence of healing occurs within 2 to 4 weeks of treatment, large dose treatment (of either vitamin D3 or D2) should be continued for 2 to 3 months.1 After sufficient calcidiol concentrations are achieved, a maintenance dose of 400 units of vitamin D daily is recommended in all age groups.1 Larger maintenance doses (800 units per day) may be considered in the following at-risk populations: premature infants, dark-skinned infants and children, children who reside in areas of limited sun exposure (>37.5° latitude), obese patients (due to fat sequestration of vitamin D), and those on medications known to compromise vitamin D concentrations discussed in this review.1,9,33
Table 5.
Vitamin D Dosing for Prevention and Treatment of Nutritional Vitamin D Deficiency in Children1
In patients where daily compliance is a concern, an alternative dosing strategy can be utilized for the treatment of vitamin D deficiency, known as “stoss therapy,” from the German word stossen, meaning “to push.” For patients over 1 month of age, 100,000 to 600,000 units of vitamin D can be given orally as a single dose, followed by maintenance doses.34,35 When instituting this approach, liquid formulations (e.g., Drisdol) should be avoided to prevent potential propylene glycol toxicity.35 Calcitriol is also not preferred for stoss therapy as it has a short half-life and does not build up vitamin D body stores. Strategies to safely institute stoss therapy include crushing 25,000 units or 50,000 units tablets or softening 50,000 units gel capsules in water and blending in foods, such as applesauce.35 Stoss therapy has been successfully implemented using intramuscular formulations as well; however, this option will not be explored since this product is no longer available in the US.
Evidence
Evidence in infants, children, and adolescents are sparse concerning what dose corrects vitamin D deficiency rickets. Current recommendations have been made based on expert opinion.1,22 There is, however, published evidence on the safety and efficacy of stoss therapy in children with clinical and biochemical evidence of vitamin D deficiency.34–37 Shah et al35 administered 300,000 or 600,000 units of vitamin D2 orally (100,000 units every 2 weeks) to 42 patients with vitamin D deficiency rickets between 5 and 109 months of age. At 14 days postadministration, radiographic evaluations confirmed the efficacy of this regimen. However, routine use of stoss therapy has overwhelming risk of hypercalcemia; 34% of infants who received 600,000 units of vitamin D every 3 to 5 months during the first one and a half years of life reported hypercalcemia.38 A study involving Turkish children and adolescents 12 to 17 years old showed intake of < 100 units of vitamin D was inadequate, resulting in calcidiol concentrations < 11 ng/mL.39 The 2003 AAP guideline recommendations were based on the premise that 200 units daily of vitamin D would achieve calcidiol concentrations > 11 ng/mL to prevent rickets. Since then, more studies have shown rickets can manifest in patients with calcidiol concentrations up to 20 ng/mL.40,41 In the past, doses of cod liver oil equal to 400 units of vitamin D daily achieved calcidiol concentrations > 20 ng/mL without concerning adverse effects.42,43 Based on this evidence, most guidelines recommend at least 400 units of vitamin D daily.1,7,9 Clinical trials are still needed to exactly determine the dose of vitamin D to achieve optimal calcidiol concentrations as well as the calcidiol concentration required to prevent bone demineralization and rickets in the pediatric population.
Vitamin D Deficiency in CKD
Epidemiologic studies suggest that patients with CKD are at an increased risk for vitamin D deficiency due to reduced sun exposure, lower intake of foods rich in vitamin D, and increased melanin content of the skin observed in this population.8,44,45 In a cohort of children with CKD from 2005 to 2006, the prevalence of vitamin D deficiency was 39% (n=88) with the mean 25(OH) D concentration of 21.8 ng/mL.46 Additionally, these patients exhibit physiologic challenges that increase risks for deficiency, including decreased endogenous production, decreased intestinal absorption, decreased enzyme activity to form functional vitamin D in the kidneys, and in those with proteinuria, increased urinary loss of calcidiol, and vitamin D-binding protein.32,47–50 In patients with CKD, vitamin D supplementation appears to have benefit in preventing or reducing hyperparathyroidism that occurs as a part of renal osteodystrophy to repair bone and mineral disturbances.32 The recommendations we will explore concerning vitamin D supplementation in pediatric patients with CKD were developed based on data observed in the adult population. However, since the publication of the KDOQI guidelines, more information is available in the literature about vitamin D deficiency in pediatric patients with CKD.
Dosing
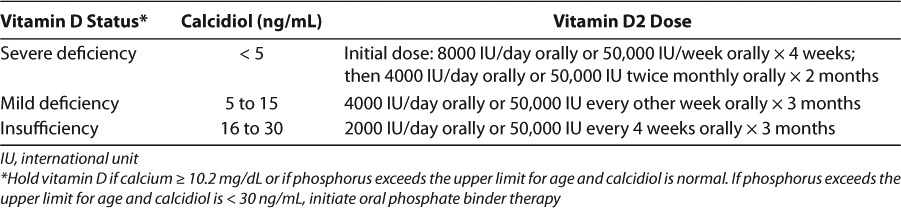
Table 6 summarizes the recommendations in the pediatric KDOQI guidelines for patients with vitamin D insufficiency or deficiency.8 Patients with calcidiol concentrations > 30 ng/mL are indicated for larger initial doses of vitamin D than those with adequate calcidiol concentrations. Of note, the guidelines prefer vitamin D2 as the supplement of choice over vitamin D3 due to safety data in animals.8,31,51 However, vitamin D3 is noted as an acceptable alternative. Calcidiol concentrations should be measured at 3 months of therapy, to assess the need for further treatment, and annually, once concentrations are adequate.8 Additionally, serum corrected calcium concentrations and phosphorous concentrations should be assessed at 1 month and every 3 months.8 If total serum corrected calcium exceeds 10.2 mg/dL or if serum phosphate exceeds the upper limit for age and calcidiol concentrations are normal, vitamin D may be discontinued. Otherwise, once calcidiol concentrations are deemed adequate, maintenance doses of vitamin D2 (400 units daily) should be resumed.7,8 For non-compliant patients, vitamin D can be administered as a single oral dose of 50,000 units monthly.35,52
Table 6.
Recommendations for Vitamin D Supplementation in Children with CKD Stages 2 to 48
Evidence
The prevalence of vitamin D insufficiency or deficiency in the pediatric population with CKD varies in recent literature from 39% to 77%.46,53 Risk factors for more advanced deficiency include advanced CKD, non-Caucasian ethnicity, overweight or obesity, and lack of sun exposure. 46,53 In a retrospective, single center study of 57 children (mean age 11 years) with CKD (stages 2 through 4), vitamin D2 was used for 12 weeks at doses recommended in the KDOQI guidelines to successfully replete vitamin D stores.54 Of note in this study, PTH concentrations decreased from 122 to 80 ng/mL after treatment. In a study involving adults with CKD, administration of vitamin D2 increased calcidiol concentrations from 17 to 27 ng/mL (p<0.05) and decreased PTH concentrations from 231 to 192 pg/mL (p<0.05) after 6 months.55 In Zisman et al,56 52 adult patients with CKD (stage 3 or 4), vitamin D deficiency, and hyperparathyroidism observed normalization of calcidiol concentrations (p<0.05) and decrease in PTH concentrations from 13.1% to 2.0% (non-significant p-value) with vitamin D2 supplementation. A prospective trial in pediatric patients with moderate CKD showed increased mean growth velocity into the normal range after 1 year of vitamin D therapy, which continued in the subsequent 2 years of treatment.57
Calcitriol
In vitamin D deficiency, calcitriol is not recommended as initial therapy or for routine use because of its short half-life and inability to increase vitamin D stores. Doses are limited because of its rapid onset and risk of hypercalcemia. However, calcitriol has utility in children with CKD stages 2 to 5 for the treatment of secondary hyperparathyroidism. 58 Additionally, it can be used as an adjunct to calcium supplementation for patients with severe vitamin D deficiency with severe symptomatic hypocalcemia, including seizure and tetany.58 As kidney function continues to decline, the enzyme activity of 1-alpha hydroxylase decreases and therefore, calcitriol preparations may be needed rather than vitamin D2 or D3 preparations.
Vitamin D Deficiency in CF
With the increase in life expectancy from 2 to 36 years in the last 40 years, bone disease has transpired as a common complication in patients with CF with low bone mineral density observed in 50% to 75% of patients.59 There are a myriad of contributory risk factors including malnutrition, vitamin D deficiency due to malabsorption from pancreatic insufficiency, inadequate absorption of calcium, physical inactivity, altered sex hormone production, chronic lung infection with elevated level of bone-active cytokines, and glucocorticoid use in this population. Maintaining optimal vitamin D stores in this population is especially important because severe bone disease may exclude these individuals from being qualified for lung transplantation. Guidelines from the CFF's Consensus Conference on Bone Health recommend that vitamin D2 supplementation be given to maintain calcidiol concentrations ≥ 30 ng/mL.59 However, a more recent study published in 2011 suggests that 35 ng/mL is the more appropriate cut off, where PTH is < 50 pg/mL and bone resorption and fracture risk is decreased.60
Dosing
In CF patients with insufficient calcidiol concentrations, doses up to 50,000 units of vitamin D2 daily for several months may be necessary for initial treatment.61 For maintenance therapy, the CFF guidelines recommend at least 400 units and 800 units of vitamin D2 daily for infants and patients over 1 year of age, respectively.62 However, as supported by the literature, these doses have been found not to sustain calcidiol concentrations in this population and therefore, doses should be titrated to obtain calcidiol concentrations > 30 to 35 ng/mL. Dosing recommendations for children younger than 5 years old are vitamin D2 12,000 units biweekly, and 50,000 units weekly or biweekly of vitamin D2 for those 5 years and older.59 Very high dosing strategies such as 700,000 units of vitamin D2 over 14 days have been safely administered to a pediatric CF population with successful adequate calcidiol concentration.63 If high dose vitamin D2 is inadequate, more polar vitamin D analogs, calcitriol, or phototherapy may be reasonable alternatives.59 Of note, the treatment doses are recommended in addition to the daily recommended maintenance therapy these patients are receiving.62
Evidence
Given that the majority (60%) of the 60,000 patients with CF in North America and Europe are under the age of 18, studies concerning vitamin D status in patients with CF often involve pediatric patients.59 In a retrospective chart review of 147 concentrations from 97 pediatric individuals with calcidiol concentrations < 30 ng/mL, 50,000 units of vitamin D2 daily for 28 days resulted in approximately half achieving concentrations > 30 ng/mL.61 This initial regimen was more successful than vitamin D 250,000 units 1, 2, or 3 times a week for 8 weeks in pediatric patients.64 Long-term follow-up (6 to 18 months posttreatment) in 39 patients showed 48% of those who achieved sufficient calcidiol concentrations became insufficient on maintenance doses of 400 to 800 units of vitamin D2.61 In a 2011 trial of adult patients with CF, patients with calcidiol concentrations < 30 ng/mL were given 50,000 units of vitamin D2 daily for 30 days followed by maintenance doses of vitamin D3 800 to 1000 units daily. After 30 days of treatment, serum calcidiol increased from 15.1 to 48.7 ng/mL (p<0.05) without any concerning side effects. However, adequate concentrations were not sustained on maintenance doses. The mean serum calcidiol dropped to 18.9 ng/mL (p<0.05), and 50% of treated patients became vitamin D insufficient within 1 year.60 In a study of 20 adolescent and adult patients with CF, administration of 800 units daily of vitamin D was inadequate for 40% of patients after 4 to 10 weeks of therapy.65 In another study of exclusively adult CF patients, administration of vitamin D3 (>400 units daily) increased calcidiol concentrations in 92% of patients; however, normalized calcidiol concentrations were achieved in only 17% of patients and no assessment on the most appropriate dose was made.66 In a study conducted by Kelly et al,67 95% of adult CF patients required 1800 units of vitamin D2 daily to achieve calcidiol concentrations above 25 ng/mL. Although supplementation with calcitriol does not replete vitamin D stores, it may be an option for CF patients unresponsive to vitamin D2 and D3 to manage consequences of vitamin D deficiency. Brown et al68 reported that calcitriol (0.5 mcg daily for 14 days) increased the fractional absorption of calcium (p<0.05) and lowered PTH (p<0.03) in 10 adults with CF.
Vitamin D Deficiency in Sickle Cell Disease
Pain crisis is a hallmark of sickle cell disease. The symptoms of pain crisis are thought to be somewhat similar to the symptoms that one would experience with vitamin D deficiency. For example, in both conditions, pain is characterized by an aching and dull pain. The location of the pain can be limited to the extremities and lower spine. It can be exacerbated by increased activities and exertion.2,69–71 Because of these similarities, studies have looked at the prevalence of vitamin D deficiency in the sickle cell population. In fact, in 1 recent study that was performed in Madrid, Spain, 56% of children with sickle cell had concentrations of vitamin D < 20 ng/mL and 18% of them had concentrations < 11 ng/mL.72 The ranges of prevalence from other studies, however, were as high as 65% to 100%.73–75 Supplement of vitamin D may help alleviate the pain experienced by patients with sickle cell disease and improve their overall bone health.
Evidence
Evidence of vitamin D supplementation in children and adolescents with sickle cell disease are limited. In 1 case report, a 16-year-old female with homozygous SS disease presented with chronic pain involving many parts of her body, which included the lower extremities, left shoulder, and neck.76 Her pain was not alleviated by ibuprofen, pregabalin, amitriptyline, or various opioids (totaled about 40 mg equivalents of morphine daily). A detailed metabolic workup was performed, and she was found to have a vitamin D concentration of < 7.9 ng/mL. Because of this finding, she was started on cholecalciferol 50,000 units orally twice a week for 8 weeks. At the end of this course of therapy, her vitamin D concentration had jumped up to 47 ng/mL and was switched to cholecalciferol 50,000 units once weekly. By week 14, her concentration was at 30 ng/mL, and she had complete alleviation of all her pain symptoms and her bone mass density increased by 11% in 2 years.
Because of the success found in the previous case report, the same investigator performed a randomized, double blind pilot study in 2012, in which subjects (n=46; 13.2 ± 3.1 years) with sickle cell disease were given either high dose cholecalciferol (40,000 to 100,000 units weekly) or placebo for 6 weeks.77 Approximately 53% and 83% of the subjects were initially found to have vitamin D insufficiency and deficiency, respectively. The treatment group was found to have fewer pain days per week, higher quality-of-life scores, and higher serum 25-hydroxyvitamin D concentrations. The authors suggested that a larger study with longer duration will need to be performed to validate this result. In fact, at the hospital where one of the authors of this review article works, he also had successes in using cholecalciferol 50,000 units orally twice a week in 2 pediatric patients with sickle cell disease, and their pain scores were greatly reduced.
Even with theses success stories, numerous questions still remain about the use of vitamin D supplementation in sickle cell disease, such as 1) what is the optimal dose of cholecalciferol, 2) what is the duration of therapy, 3) what are the long-term side effects of such a large dose therapy in the pediatric population, 4) does it work for all forms of sickle cell disease, and 5) will this therapy work for patients without vitamin D deficiency?
Vitamin D Deficiency in Asthma
Asthma is a common diagnosis found in the pediatric population. Scientists hypothesize that the increased prevalence of asthma may be in part due to the rise of vitamin D deficiency in the pediatric population.78 Maternal intake of vitamin D during pregnancy may also play a role in the children's risk of having wheezing symptoms.79 In fact, some studies have described an association between vitamin D deficiency and asthma, while one has not.80–82 We will look at the evidence on the association between vitamin D deficiency and asthma and the need of vitamin D supplementation in patients with this clinical condition.
Evidence
Limited data exist on vitamin D concentrations in children with asthma. A case control study was performed at a pediatric allergy and immunology clinic in Qatar.81 The aim of the study was to describe the association between asthma and vitamin D in children and to look at the difference in vitamin D concentrations in asthmatic children (7.0 ± 3.8 years) and control (8.4 ± 3.6 years). In this study, vitamin D deficiency was found to be more prevalent in asthmatics than controls. The mean value of vitamin D was 17.5 ± 11 ng/mL in the group with asthma and 20.8 ± 10.0 ng/mL in the controlled group. Elevated serum immunoglobulin E was observed in patients with lower vitamin D concentrations.13
In another cross-sectional study, serum 25-hydroxyvitamin D3 concentrations were compared between the group with asthma (n=50) and the healthy group (n=50).80 The age of the subjects ranged from 6 to 18 years. The results of this study showed that vitamin D concentrations had direct correlations with both the forced expiratory volume/forced vital capacity ( FEV1/FVC) ratio and the predicted FEV1 (p=0.024 and p=0.026, respectively), meaning that the less the vitamin D concentrations, the more significantly increased odds of the subjects' asthmatic state. However, the state of vitamin D deficiency was not associated with the duration of disease, number of hospitalization, and the eosinophil counts.80
On the other hand, one retrospective, case-control study did not find an association between asthma severity and serum 25-hydroxyvitamin D concentrations.82 In this study, 263 subjects with asthma were compared to 284 normal subjects (ages: 2 to 19 years). Their asthma symptoms were assessed and serum vitamin D concentrations were obtained. No significant difference in vitamin D concentrations was found between the asthmatic group and the controlled group, and the severity of asthma symptoms was not correlated with the vitamin D concentrations.82
Oral or intravenous corticosteroids are often used as a regimen for patients with asthma exacerbation. If the patients' asthma is not well-controlled, they may potentially be exposed to repeated courses of corticosteroids. Long-term or repeated course of corticosteroids is known to cause vitamin D deficiency.83 One may wonder is the decrease in serum vitamin D concentrations in children with asthma due to the disease itself or the use of corticosteroids. To answer part of this question, a retrospective review was performed in 100 asthmatic children looking at the patients' characteristics and their vitamin D concentrations. 84 This study showed that the total steroid dose, the use of oral steroids, and the use of inhaled steroids were associated with an inverse correlation with their vitamin D concentrations (p=0.001, p=0.02, and p=0.0475, respectively).17 There may be a never ending cycle in which poor control of asthma will lead to the use of inhaled and oral corticosteroid, which in turn may cause a reduction in vitamin D concentrations, which in turn may worsen the patients' asthmatic state.
The next question that one would ask is: does vitamin D supplementation improve the clinical course of asthma? The addition of vitamin D supplementation was evaluated in a study of subjects with steroid-resistant asthma.85 After exposing a small amount of vitamin D (5 × 10−7 M) to cultures of CD4+ regulatory T cells, the secretion of IL-10 was greatly increased in this steroid-resistant group and was comparable to the concentrations seen in the controlled group. Similarly, in an experimental model of asthmatic patients, the addition of vitamin D helped decrease the dose of dexamethasone by 10-fold.86 The authors of this study postulated that vitamin D supplementation may increase the anti-inflammatory property of corticosteroid in asthmatic patients by enhancing the glucocorticoid-induced mitogen-activated protein kinase phosphatase-1 expression.86
Before starting every asthmatic patient on vitamin D supplementation, larger studies need to be performed to evaluate the efficacy of this regimen in improving the clinical course of asthma and reducing the need of steroid use in asthmatic patients. Also, studies need to look at the optimal dose and duration of use for this clinical condition.
CONCLUSION
Vitamin D insufficiency is a common problem in pediatrics, especially those who have chronic illness, and who are malnourished, limited geographically to the amount of sun exposure, as well as those with darker skin, and on chronic medications. The accelerated rate of bone development during a child's life suggests that adequate concentrations of vitamin D are an important issue in this population. Although more research is needed concerning the goals of vitamin D therapy and dosing in this population, there are helpful evidence-based guidelines to direct therapy for rickets, CKD, and CF. More research is needed to evaluate the efficacy of vitamin D supplementations for pediatric patients with asthma and sickle cell disease. In patients with growth delays or reasons to suspect deficiency, calcidiol concentrations should be evaluated to assess the need for supplementation.
ABBREVIATIONS
- AAP
American Academy of Pediatrics
- AEDs
anti-epileptic drugs
- CF
cystic fibrosis
- CFF
Cystic Fibrosis Foundation
- CKD
chronic kidney disease
- HAART
highly active antiretroviral therapy
- HIV
human immunodeficiency virus
- IOM
Institute of Medicine
- IU
international units
- KDOQI
Kidney Disease Outcomes Quality Initiative
- NEJM
New England Journal of Medicine
- PTH
parathyroid hormone
- US
United States
Footnotes
DISCLOSURE The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.Misra M, Pacaud D, Petryk A et al. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122(2):398–417. doi: 10.1542/peds.2007-1894. [DOI] [PubMed] [Google Scholar]
- 2.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 3.Zhou C, Assem M, Tay JC et al. Steroid and xenobiotic receptor and vitamin D receptor crosstalk mediates CYP24 expression and drug-induced osteomalacia. J Clin Invest. 2006;116(6):1703–1712. doi: 10.1172/JCI27793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fraser DR. Vitamin D. Lancet. 1995;345(8942):104–107. doi: 10.1016/s0140-6736(95)90067-5. [DOI] [PubMed] [Google Scholar]
- 5.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80(suppl 6):1689S–1696S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 6.Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19(2):73–78. doi: 10.1016/j.annepidem.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ross AC, Taylor CL, Yaktine AL, Del Valle HB, editors. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: The National Academies Press; 2013. eds. Institute of Medicine of the National Academies. [PubMed] [Google Scholar]
- 8.National Kidney Foundation, Inc. Guideline 8. Prevention and treatment of vitamin D insufficiency and vitamin D deficiency in CKD patients. KDOQI clinical practic guidelines for bone metabolism and disease in children with chronic kidney disease. 2005 http://www.kidney.org/professionals/kdoqi/guidelines_pedbone/guide8.htm. Accessed August 30, 2013. [Google Scholar]
- 9.Holick MF, Binkley NC, Bischoff-Ferrari HA et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 10.Chapuy MC, Preziosi P, Maamer M et al. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int. 1997;7(5):439–443. doi: 10.1007/s001980050030. [DOI] [PubMed] [Google Scholar]
- 11.Abdul-Razzak KK, Ajlony MJ, Khoursheed AM et al. Vitamin D deficiency among healthy infants and toddlers: a prospective study from Irbid, Jordan. Pediatr Int. 2011;53(6):839–845. doi: 10.1111/j.1442-200X.2011.03388.x. [DOI] [PubMed] [Google Scholar]
- 12.Gordon CM, Feldman HA, Sinclair L et al. Prevalence of vitamin D deficiency among healthy infants and toddlers. Arch Pediatr Adolesc Med. 2008;162(6):505–512. doi: 10.1001/archpedi.162.6.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koutkia P, Chen TC, Holick MF. Vitamin D intoxication associated with an over-the-counter supplement. N Engl J Med. 2001;345(1):66–67. doi: 10.1056/NEJM200107053450115. [DOI] [PubMed] [Google Scholar]
- 14.Abrams SA, Committee on Nutrition Calcium and vitamin d requirements of enterally fed preterm infants. Pediatrics. 2013;131(5):e1676–e1683. doi: 10.1542/peds.2013-0420. [DOI] [PubMed] [Google Scholar]
- 15.Levine MA, Zapalowski C, Kappy MS. Disorders of calcium, phosphate, parathyroid hormone and vitamin D metabolism. In: Kappy MS, Allen DB, Geffner ME, editors. Principles and Practice of Pediatric Endocrinology. Springfield, IL: Charles C. Thomas Publisher, LTD; 2005. pp. 716–719. In. eds. [Google Scholar]
- 16.Adams JS, Hewison M. Update in vitamin D. J Clin Endocrinol Metab. 2010;95(2):471–478. doi: 10.1210/jc.2009-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drezner MK. Treatment of anticonvulsant drug-induced bone disease. Epilepsy Behav. 2004;5(suppl 2):S41–S47. doi: 10.1016/j.yebeh.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 18.Verrotti A, Coppola G, Parisi P et al. Bone and calcium metabolism and antiepileptic drugs. Clin Neurol Neurosurg. 2010;112(1):1–10. doi: 10.1016/j.clineuro.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 19.Rutstein R, Downes A, Zemel B et al. Vitamin D status in children and young adults with perinatally acquired HIV infection. Clinical Nutrition. 2011;30(5):624–628. doi: 10.1016/j.clnu.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Panel on Antiretroviral Therapy and Medication Management of HIV-Infected Children. Guidelines for the use of antiretroviral agents in pediatric HIV infection. 2011 http://aidsinfo.nih.gov/contentfiles/lvguidelines/pediatricguidelines.pdf. Accessed March 29, 2012. [Google Scholar]
- 21.Weldon D. The effects of corticosteroids on bone growth and bone density. Ann Allergy Asthma Immunol. 2009;103(1):3–11. doi: 10.1016/S1081-1206(10)60135-4. quiz 11–13, 50. [DOI] [PubMed] [Google Scholar]
- 22.Munns C, Zacharin MR, Rodda CP et al. Prevention and treatment of infant and childhood vitamin D deficiency in Australia and New Zealand: a consensus statement. Med J Aust. 2006;185(5):268–272. doi: 10.5694/j.1326-5377.2006.tb00558.x. [DOI] [PubMed] [Google Scholar]
- 23.Specker BL, Valanis B, Hertzberg V et al. Sunshine exposure and serum 25-hydroxyvitamin D concentrations in exclusively breast-fed infants. J Pediatr. 1985;107(3):372–376. doi: 10.1016/s0022-3476(85)80509-6. [DOI] [PubMed] [Google Scholar]
- 24.American Academy of Pediatrics, Committee on Environmental Health. Ultraviolet light: a hazard to children. Pediatrics. 1999;104(2 pt 1):328–333. [PubMed] [Google Scholar]
- 25.Tsang RC, Zlotkin SH, Nichols BL Nutrition During Infancy: Principles and Practice. 2nd ed. Cincinnati, OH: Digital Education Publishing; 1997. [Google Scholar]
- 26.Leerbeck E, Sondergaard H. The total content of vitamin D in human milk and cow's milk. Br J Nutr. 1980;44(1):7–12. doi: 10.1079/bjn19800004. [DOI] [PubMed] [Google Scholar]
- 27.Basile LA, Taylor SN, Wagner CL et al. The effect of high-dose vitamin D supplementation on serum vitamin D levels and milk calcium concentration in lactating women and their infants. Breastfeed Med. 2006;1(1):27–35. doi: 10.1089/bfm.2006.1.27. [DOI] [PubMed] [Google Scholar]
- 28.Wagner CL, Greer FR. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122(5):1142–1152. doi: 10.1542/peds.2008-1862. [DOI] [PubMed] [Google Scholar]
- 29.Armas LA, Hollis BW, Heaney RP. Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab. 2004;89(11):5387–5391. doi: 10.1210/jc.2004-0360. [DOI] [PubMed] [Google Scholar]
- 30.Heaney RP, Recker RR, Grote J et al. Vitamin D(3) is more potent than vitamin D(2) in humans. J Clin Endocrinol Metab. 2011;96(3):E447–E452. doi: 10.1210/jc.2010-2230. [DOI] [PubMed] [Google Scholar]
- 31.Coburn JW, Tan AU, Jr, Levine BS et al. 1 alpha-Hydroxy-vitamin D2: a new look at an “old” compound. Nephrol Dial Transplant. 1996;11(suppl 3):153–157. doi: 10.1093/ndt/11.supp3.153. [DOI] [PubMed] [Google Scholar]
- 32.Langman CB, Brooks ER. Renal osteodystrophy in children: a systemic disease associated with cardiovascular manifestations. Growth Horm IGF Res. 2006;16(suppl A):S79–S83. doi: 10.1016/j.ghir.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Wortsman J, Matsuoka LY, Chen TC et al. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72(3):690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 34.Hochberg Z, Bereket A, Davenport M et al. Consensus development for the supplementation of vitamin D in childhood and adolescence. Horm Res. 2002;58(1):39–51. doi: 10.1159/000063214. [DOI] [PubMed] [Google Scholar]
- 35.Shah BR, Finberg L. Single-day therapy for nutritional vitamin D-deficiency rickets: a preferred method. J Pediatr. 1994;125(3):487–490. doi: 10.1016/s0022-3476(05)83303-7. [DOI] [PubMed] [Google Scholar]
- 36.Harrison HE, Harrison HC. Disorders of calcium and phosphate metabolism in childhood and adolescence. Major Probl Clin Pediatr. 1979;20:1–314. [PubMed] [Google Scholar]
- 37.Lubani MM, al-Shab TS, al-Saleh QA et al. Vitamin-D-deficiency rickets in Kuwait: the prevalence of a preventable disease. Ann Trop Paediatr. 1989;9(3):134–139. doi: 10.1080/02724936.1989.11748616. [DOI] [PubMed] [Google Scholar]
- 38.Markestad T, Hesse V, Siebenhuner M et al. Intermittent high-dose vitamin D prophylaxis during infancy: effect on vitamin D metabolites, calcium, and phosphorus. Am J Clin Nutr. 1987;46(4):652–658. doi: 10.1093/ajcn/46.4.652. [DOI] [PubMed] [Google Scholar]
- 39.Gultekin A, Ozalp I, Hasanoglu A et al. Serum-25-hydroxycholecalciferol levels in children and adolescents. Turk J Pediatr. 1987;29(3):155–162. [PubMed] [Google Scholar]
- 40.Kreiter SR, Schwartz RP, Kirkman HN, Jr et al. Nutritional rickets in African American breast-fed infants. J Pediatr. 2000;137(2):153–157. doi: 10.1067/mpd.2000.109009. [DOI] [PubMed] [Google Scholar]
- 41.Spence JT, Serwint JR. Secondary prevention of vitamin D-deficiency rickets. Pediatrics. 2004;113(1 pt 1):e70–e72. doi: 10.1542/peds.113.1.e70. [DOI] [PubMed] [Google Scholar]
- 42.Park EA. The therapy of rickets. JAMA. 1940;115(5):370–379. [Google Scholar]
- 43.Rajakumar K, Thomas SB. Reemerging nutritional rickets: a historical perspective. Arch Pediatr Adolesc Med. 2005;159(4):335–341. doi: 10.1001/archpedi.159.4.335. [DOI] [PubMed] [Google Scholar]
- 44.Clemens TL, Adams JS, Henderson SL et al. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet. 1982;1(8263):74–76. doi: 10.1016/s0140-6736(82)90214-8. [DOI] [PubMed] [Google Scholar]
- 45.Thomas MK, Lloyd-Jones DM, Thadhani RI et al. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998;338(12):777–783. doi: 10.1056/NEJM199803193381201. [DOI] [PubMed] [Google Scholar]
- 46.Ali FN, Arguelles LM, Langman CB et al. Vitamin D deficiency in children with chronic kidney disease: uncovering an epidemic. Pediatrics. 2009;123(3):791–796. doi: 10.1542/peds.2008-0634. [DOI] [PubMed] [Google Scholar]
- 47.Holick MF. Vitamin D and the kidney. Kidney Int. 1987;32(6):912–929. doi: 10.1038/ki.1987.295. [DOI] [PubMed] [Google Scholar]
- 48.Koenig KG, Lindberg JS, Zerwekh JE et al. Free and total 1,25-dihydroxyvitamin D levels in subjects with renal disease. Kidney Int. 1992;41(1):161–165. doi: 10.1038/ki.1992.22. [DOI] [PubMed] [Google Scholar]
- 49.Saha H. Calcium and vitamin D homeostasis in patients with heavy proteinuria. Clin Nephrol. 1994;41(5):290–296. [PubMed] [Google Scholar]
- 50.Shimada T, Hasegawa H, Yamazaki Y et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19(3):429–435. doi: 10.1359/JBMR.0301264. [DOI] [PubMed] [Google Scholar]
- 51.Harrington DD, Page EH. Acute vitamin D3 toxicosis in horses: case reports and experimental studies of the comparative toxicity of vitamins D2 and D3. J Am Vet Med Assoc. 1983;182(12):1358–1369. [PubMed] [Google Scholar]
- 52.Kruse K. Pathophysiology of calcium metabolism in children with vitamin D-deficiency rickets. J Pediatr. 1995;126(5 pt 1):736–741. doi: 10.1016/s0022-3476(95)70401-9. [DOI] [PubMed] [Google Scholar]
- 53.Seeherunvong W, Abitbol CL, Chandar J et al. Vitamin D insufficiency and deficiency in children with early chronic kidney disease. J Pediatr. 2009;154(6):906–911. doi: 10.1016/j.jpeds.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 54.Menon S, Valentini RP, Hidalgo G et al. Vitamin D insufficiency and hyperparathyroidism in children with chronic kidney disease. Pediatr Nephrol. 2008;23(10):1831–1836. doi: 10.1007/s00467-008-0842-x. [DOI] [PubMed] [Google Scholar]
- 55.Al-Aly Z, Qazi RA, Gonzalez EA et al. Changes in serum 25-hydroxyvitamin D and plasma intact PTH levels following treatment with ergocalciferol in patients with CKD. Am J Kidney Dis. 2007;50(1):59–68. doi: 10.1053/j.ajkd.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 56.Zisman AL, Hristova M, Ho LT et al. Impact of ergocalciferol treatment of vitamin D deficiency on serum parathyroid hormone concentrations in chronic kidney disease. Am J Nephrol. 2007;27(1):36–43. doi: 10.1159/000098561. [DOI] [PubMed] [Google Scholar]
- 57.Langman CB, Mazur AT, Baron R et al. 25-hydroxyvitamin D3 (calcifediol) therapy of juvenile renal osteodystrophy: beneficial effect on linear growth velocity. J Pediatr. 1982;100(5):815–820. doi: 10.1016/s0022-3476(82)80602-1. [DOI] [PubMed] [Google Scholar]
- 58.Root AW, Diamone FB. Disorders of mineral homeostasis in the newborn, infant, child, and adolescent. In: Sperling MA, editor. Pediatric Endocrinology. 3rd ed. Philadephia, PA: Saunders Elsevier; 2008. p. 699. In. ed. [Google Scholar]
- 59.Aris RM, Merkel PA, Bachrach LK et al. Guide to bone health and disease in cystic fibrosis. J Clin Endocrinol Metab. 2005;90(3):1888–1896. doi: 10.1210/jc.2004-1629. [DOI] [PubMed] [Google Scholar]
- 60.West NE, Lechtzin N, Merlo CA et al. Appropriate goal level for 25-hydroxyvitamin D in cystic fibrosis. Chest. 2011;140(2):469–474. doi: 10.1378/chest.10-2114. [DOI] [PubMed] [Google Scholar]
- 61.Green DM, Leonard AR, Paranjape SM et al. Transient effectiveness of vitamin D2 therapy in pediatric cystic fibrosis patients. J Cyst Fibros. 2010;9(2):143–149. doi: 10.1016/j.jcf.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 62.Borowitz D, Robinson KA, Rosenfeld M et al. Cystic Fibrosis Foundation evidence-based guidelines for management of infants with cystic fibrosis. J Pediatr. 2009;155(suppl 6):S73–S93. doi: 10.1016/j.jpeds.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boas SR, Hageman JR, Ho LT et al. Very high-dose ergocalciferol is effective for correcting vitamin D deficiency in children and young adults with cystic fibrosis. J Cyst Fibros. 2009;8(4):270–272. doi: 10.1016/j.jcf.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 64.Green D, Carson K, Leonard A et al. Current treatment recommendations for correcting vitamin D deficiency in pediatric patients with cystic fibrosis are inadequate. J Pediatr. 2008;153(4):554–559. doi: 10.1016/j.jpeds.2008.04.058. [DOI] [PubMed] [Google Scholar]
- 65.Hanly JG, McKenna MJ, Quigley C et al. Hypovitaminosis D and response to supplementation in older patients with cystic fibrosis. Q J Med. 1985;56(219):377–385. [PubMed] [Google Scholar]
- 66.Stephenson A, Brotherwood M, Robert R et al. Cholecalciferol significantly increases 25-hydroxyvitamin D concentrations in adults with cystic fibrosis. Am J Clin Nutr. 2007;85(5):1307–1311. doi: 10.1093/ajcn/85.5.1307. [DOI] [PubMed] [Google Scholar]
- 67.Kelly EM, Pencharz P, Tullis E. Effect of vitamin D supplementation on low serum 25-hydroxyvitamin D in adults with cystic fibrosis [Abstract] Pediatr Pulmonol. 2002;34(S24):344. [Google Scholar]
- 68.Brown SA, Ontjes DA, Lester GE et al. Short-term calcitriol administration improves calcium homeostasis in adults with cystic fibrosis. Osteoporos Int. 2003;14(5):442–449. doi: 10.1007/s00198-002-1331-x. [DOI] [PubMed] [Google Scholar]
- 69.Gloth FM, III, Greenough WB., III. Vitamin D deficiency as a contributor to multiple forms of chronic pain. Mayo Clin Proc. 2004;79(5):696, 699. doi: 10.1016/S0025-6196(11)62303-3. author reply 699. [DOI] [PubMed] [Google Scholar]
- 70.Lotfi A, Abdel-Nasser AM, Hamdy A et al. Hypovitaminosis D in female patients with chronic low back pain. Clin Rheumatol. 2007;26(11):1895–1901. doi: 10.1007/s10067-007-0603-4. [DOI] [PubMed] [Google Scholar]
- 71.Straube S, Andrew Moore R, Derry S et al. Vitamin D and chronic pain. Pain. 2009;141(1–2):10–13. doi: 10.1016/j.pain.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 72.Garrido C, Cela E, Belendez C et al. Status of vitamin D in children with sickle cell disease living in Madrid, Spain. Eur J Pediatr. 2012;171(12):1793–1798. doi: 10.1007/s00431-012-1817-2. [DOI] [PubMed] [Google Scholar]
- 73.Buison AM, Kawchak DA, Schall J et al. Low vitamin D status in children with sickle cell disease. J Pediatr. 2004;145(5):622–627. doi: 10.1016/j.jpeds.2004.06.055. [DOI] [PubMed] [Google Scholar]
- 74.Chapelon E, Garabedian M, Brousse V et al. Osteopenia and vitamin D deficiency in children with sickle cell disease. Eur J Haematol. 2009;83(6):572–578. doi: 10.1111/j.1600-0609.2009.01333.x. [DOI] [PubMed] [Google Scholar]
- 75.Rovner AJ, Stallings VA, Kawchak DA et al. High risk of vitamin D deficiency in children with sickle cell disease. J Am Diet Assoc. 2008;108(9):1512–1516. doi: 10.1016/j.jada.2008.06.433. [DOI] [PubMed] [Google Scholar]
- 76.Osunkwo I. Complete resolution of sickle cell chronic pain with high dose vitamin D therapy: a case report and review of the literature. J Pediatr Hematol Oncol. 2011;33(7):549–551. doi: 10.1097/MPH.0b013e31821ed3ea. [DOI] [PubMed] [Google Scholar]
- 77.Osunkwo I, Ziegler TR, Alvarez J et al. High dose vitamin D therapy for chronic pain in children and adolescents with sickle cell disease: results of a randomized double blind pilot study. Br J Haematol. 2012;159(2):211–215. doi: 10.1111/bjh.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Litonjua AA, Weiss ST. Is vitamin D deficiency to blame for the asthma epidemic? J Allergy Clin Immunol. 2007;120(5):1031–1035. doi: 10.1016/j.jaci.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 79.Camargo CA, Jr, Rifas-Shiman SL, Litonjua AA et al. Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 y of age. Am J Clin Nutr. 2007;85(3):788–795. doi: 10.1093/ajcn/85.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alyasin S, Momen T, Kashef S et al. The relationship between serum 25 hydroxy vitamin d levels and asthma in children. Allergy Asthma Immunol Res. 2011;3(4):251–255. doi: 10.4168/aair.2011.3.4.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ehlayel MS, Bener A, Sabbah A. Is high prevalence of vitamin D deficiency evidence for asthma and allergy risks? Eur Ann Allergy Clin Immunol. 2011;43(3):81–88. [PubMed] [Google Scholar]
- 82.Menon J, Maranda L, Nwosu BU. Serum 25-hydroxyvitamin D levels do not correlate with asthma severity in a case-controlled study of children and adolescents. J Pediatr Endocrinol Metab. 2012;25(7–8):673–679. doi: 10.1515/jpem-2012-0143. [DOI] [PubMed] [Google Scholar]
- 83.Rousseau-Nepton I, Lang B, Rodd C. Long-term bone health in glucocorticoid-treated children with rheumatic diseases. Curr Rheumatol Rep. 2013;15(3):315. doi: 10.1007/s11926-012-0315-x. [DOI] [PubMed] [Google Scholar]
- 84.Searing DA, Zhang Y, Murphy JR et al. Decreased serum vitamin D levels in children with asthma are associated with increased corticosteroid use. J Allergy Clin Immunol. 2010;125(5):995–1000. doi: 10.1016/j.jaci.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xystrakis E, Kusumakar S, Boswell S et al. Reversing the defective induction of IL-10-secreting regulatory T cells in glucocorticoid-resistant asthma patients. J Clin Invest. 2006;116(1):146–155. doi: 10.1172/JCI21759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang Y, Goleva E, Leung DY. Vitamin D enhances glucocorticoid-induced mitogen-activated protein kinase phosphatase-1 (MKP-1) expression and their anti-proliferative effect in peripheral blood mononuclear cells. J Allergy Clin Immunol. 2009;123(2):S121. [Google Scholar]
- 87.Holick MF. Vitamin D and bone health. J Nutr. 1996;126(suppl 4):1159S–1164S. doi: 10.1093/jn/126.suppl_4.1159S. [DOI] [PubMed] [Google Scholar]
- 88.US Department of Agriculture. Provisional table on the vitamin D content of foods. March 1999 www.nal.usda.gov. Accessed March 29, 2012. [Google Scholar]
- 89.Wolters Kluwer Health, Inc. Vitamin D formulations: drug facts and comparisons. 2005 http://www.factsandcomparisons.com Accessed March 29, 2012. [Google Scholar]
- 90.American Academy of Pediatrics, Committee on Nutrition. Pediatric Nutrition Handbook. 6th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2008. [Google Scholar]


