Abstract
OBJECTIVES
The aim of this retrospective study was to identify the frequency of recommended metabolic monitoring and follow-up in pediatric patients on second-generation antipsychotic (SGA) medications from a pediatric clinic.
METHODS
A retrospective review of electronic medical records of all patients on antipsychotics from an academic medical center pediatric clinic was conducted. Inclusion criteria required patients to be established members of the pediatric clinic, < 19 years of age, and on ≥ 1 SGA for at least 1 year, regardless of medical diagnosis. Data collection consisted of patient demographic information and frequency of family history, vital signs, and recommended laboratory monitoring.
RESULTS
A total of 67 patients on antipsychotics were identified. After the application of inclusion criteria, 32 patients qualified for review. The average age was 13.5 ± 4 years and gender distribution included 72% males. Only 4 (13%) patients had documented baseline monitoring that included weight, blood pressure, and fasting lipid panel. No patient had a fasting plasma glucose recorded at any point during antipsychotic therapy. Follow-up monitoring decreased over time, with the exception of quarterly weight and annual blood pressure.
CONCLUSIONS
The results of this study highlight the lack of baseline and periodic monitoring that occur when pediatric patients are prescribed antipsychotic medications, putting the patient at risk for adverse events. The marked increase in antipsychotic prescribing and concerns related to their safety emphasize the need for improvement in monitoring of antipsychotic medications. This gap in patient care and safety opens an excellent opportunity for a clinical pharmacy team to provide education and assistance with SGA monitoring for the purpose of providing optimal patient care.
INDEX TERMS: antipsychotics, children, clinic, monitoring
INTRODUCTION
Antipsychotic medication use is highly prevalent in the United States. Since their introduction nearly 50 years ago, these medications have been prescribed to help manage psychiatric conditions for millions of people.1 These agents are increasingly prescribed to youth. Data from the National Ambulatory Medical Care Survey and the National Hospital Ambulatory Care Survey reveal that the overall prescribing frequency of antipsychotics significantly increased from 8.6 per 1000 US children in 1995–1996 to 39.4 per 1000 US children in 2001–2002.1 Conditions for which antipsychotics have been studied in the pediatric population, including schizophrenia, psychosis, Tourette syndrome, autism, and intellectual disabilities/mental retardation, only represent 26% of all antipsychotic prescriptions.1 Non-studied indications for behavioral and affective disorders account for more than half of the antipsychotic prescriptions and reflect a more pronounced increase in off-label use compared to approved indications. Although the use of antipsychotic medications in the pediatric population has increased dramatically over the last decade, limited data are available regarding their safety and efficacy.2,3
In 2003, the Food and Drug Administration (FDA) issued a warning for second-generation antipsychotic (SGA) drugs regarding an increased risk of hyperglycemia and diabetes and required an update on the product labeling to include information about these events.4 The following year, the American Diabetes Association (ADA) and the American Psychiatric Association (APA) released a consensus statement, linking the use of SGAs with an increased risk for diabetes, obesity, and dyslipidemia.5 The panel recommended baseline monitoring of personal and family history (specifically for obesity, diabetes, dyslipidemia, hypertension, or cardiovascular disease), body mass index (BMI), waist circumference, blood pressure, fasting plasma glucose, and fasting lipid profile.5 The patient weight should be reassessed at 4, 8, and 12 weeks after initiating or changing SGA therapy and quarterly thereafter during routine visits.5 Fasting plasma glucose, blood pressure, and lipid levels should be monitored 3 months after initiation of antipsychotic medications.5 Fasting lipid panels may be performed every 5 years in patients within normal ranges or more frequently if clinically indicated.5
Following the announcement of the FDA warning and ADA/APA consensus statement, there was not an increase in baseline laboratory monitoring in patients enrolled in the California, Missouri, and Oregon Medicaid programs.6 The rate of serum glucose and lipid testing in patients prescribed SGAs was 29.5% and 11.4%, respectively.6 In this sample of SGA users, 24.1% were under the age of 19 years.6 Morrato and colleagues6 specifically addressed a high-risk population, Medicaid service recipients, for whom antipsychotic use is very common.7 Furthermore, Haupt et al8 evaluated the prevalence and predictors of lipid and glucose monitoring in commercially insured patients treated with SGA and found calendar years following the 2003 monitoring recommendation guideline period to be a positive predictor of increased metabolic testing.
Highlighting children and adolescents, Correll and colleagues9 analyzed the cardiometabolic risk of SGAs during first-time use in the pediatric population. They found that SGA use was associated with a statistically significant weight gain for all agents studied. Specifically, those children given olanzapine experienced a mean increase in weight by 8.54 kg compared to 0.19 kg in the untreated group after 12 weeks of treatment. Increases in other metabolic parameters, such as total cholesterol, glucose, and waist measurement, also were seen. Even with these adverse event risk data in children as well as the recommended monitoring guidelines, a 2007 study showed that most children on SGA agents still did not receive glucose and lipid screening as recommended.10 The aims of our retrospective, pilot study were to identify the frequency of recommended metabolic effect monitoring and follow-up in pediatric patients on antipsychotic medications receiving care from an academic medical center pediatric clinic in the calendar year 2011.
METHODS
This retrospective study was conducted to evaluate the frequency of necessary monitoring and follow-up in pediatric patients on SGAs, regardless of medical diagnosis. Inclusion criteria required patients to be established members (seen within the past year) of the academic medical center pediatric clinic, < 19 years of age, and on ≥ 1 SGA for at least 1 year, regardless of medical diagnosis. Children/adolescents who did not have clinic visit data for the past year or those who did not meet other inclusion criteria were excluded in the study. The cohort of patients reviewed was children/adolescents currently on a SGA during the calendar year of 2011.
Database information was limited. Individual monitoring information was categorical and for analysis only. No protected health information was involved with aggregate reporting. Descriptive statistics were used. This project was reviewed and approved by the institutional review board. Anonymity was maintained throughout the project.
The electronic medical record (EMR) system was queried for patients who met inclusion criteria. Within the EMR, current medications and medication refills were easily found in the medication tab with specific dates representing medication initiation, provider (including external specialists), and when refills were given. If refills were provided by a specialist, the consult reports were used to determine when and what medications were given to the patient. Consult reports were scanned in the electronic chart upon receipt, and the primary care provider (PCP) would comment and sign each report. Consult reports also were reviewed for all data points evaluated in the study (e.g., labs drawn, weight). Consult reports were easily identifiable under the documents tab as a consult report. Within the EMR, the authors also were able to quickly determine when labs were ordered, as well as lab monitoring completion. For this retrospective pilot study, all completed objective data were accessible via the EMR.
Data collected by the retrospective review included medication name (Figure 1) and start date of the medication. Patient demographic information collected included gender, age, height, height percentile, weight, weight percentile, BMI, and BMI percentile. Demographic data recorded and presented in the Table were from the most recent primary care clinic visit. Height, weight, and BMI percentiles were calculated using the Centers for Disease Control and Prevention (CDC) growth charts based on the patient's age and gender.11 Percentiles classified by the CDC growth chart as <1st % were classified as 0 and >99th % as 100 for purposes of this study. Data entries of weight at baseline, 1 month, 2 months, 3 months, and quarterly during SGA therapy were recorded, if available. Other objective information collected included blood pressure (baseline, 3 months, and annually), fasting lipid panel (baseline and 3 months), and fasting plasma glucose (baseline, 3 months, and annually) as displayed in Figure 2. Baseline family histories and annual reviews also were noted. All monitoring data were collected as “yes” or “no.” For example, if the patient had a baseline fasting blood glucose level drawn, the objective result would be “yes” the level was taken. No specific monitoring values were collected. When data points were evaluated at multiple times periods (e.g., baseline and at 6 months), the exact time did not have to occur for inclusion. For example, often a patient would come to the clinic a few weeks before or after the 6-month date, and monitoring completed at that time sufficed for the 6-month evaluation in this study. If a patient was on more than 1 SGA, the start date of the most recently prescribed medication that the patient was on for > 1 year was used to mark the appropriate times for routine monitoring. Baseline values were determined based on when the SGA was started, therefore representing a true baseline. If a patient transferred into the clinic or if the agent was started by a specialist without baseline labs documented in transfer papers or a consult report, the authors counted the baseline to be the first lab draw ordered by the clinic PCP once he or she became aware of the medication. In this case, the baseline would be a clinic baseline value. The “yes” or “no” baseline assessment determined in the above fashion was chosen to find actual rates of appropriate monitoring from a primary care clinic.
Figure 1.
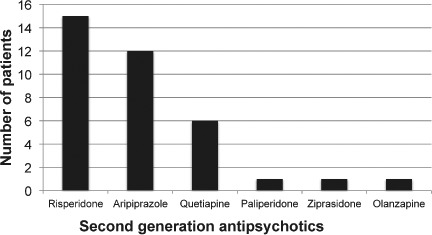
Antipsychotics used by patients.
Table.
Patient Characteristics (n=32)
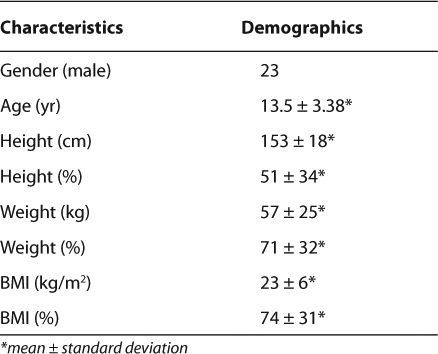
Figure 2.
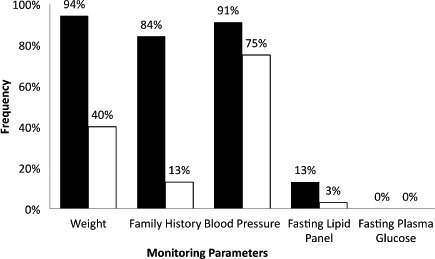
Frequency of second-generation antipsychotic monitoring. Routine monitoring (i.e., quarterly, every 3 months, or annually) recommendations differ among monitoring parameters.
An important note in this retrospective study was that the EMR and clinic system in analysis were not a closed system. Patients were able to see, and were often seen by, outside specialists. However, in data collection and review, the authors paid special and careful attention to all the consult notes to determine if monitoring was completed. Monitoring was counted as complete if it was documented in any form in the patient's electronic chart (e.g., consult note, pediatrician note, or laboratory data). If the clinic provider signed-off on the consult note where monitoring documentation was provided, this counted as recommended monitoring for those tests completed by the outside entity. Baseline characteristics of the study sample were compiled and organized in a Microsoft Excel 2010 database (Microsoft Corp., Redmond, WA). Univariate descriptive statistics were used in analysis.
RESULTS
A total of 67 patients were identified by the EMR query as eligible for chart review; however, 14 were excluded due to no data for a complete year/not being a patient in the pediatric clinic for the past year. In addition, 21 others were not on an SGA for a minimum of 1 year. A total of 32 patients were included in the study after the application of inclusion criteria. Four patients were on 2 concurrent SGA agents at the time of analysis. All participants who met inclusion criteria started their SGA after March 2004, and 89% of the SGA agents were started in 2007 or later. The majority of SGAs in the study were started in 2010. These start dates all represent the post-2003 SGA monitoring guideline publication period.
The Table lists the demographic distribution of the study sample. The average age for the study population was 13.5 ± 3 years and consisted of 9 females (28%) and 23 males (72%). The average BMI percentile was 74%; however, due to 1 outlier child with a BMI percentile < 1%, the range was 0% to 100%. The most common medication used was risperidone followed by aripiprazole (Figure 1). Figure 2 represents frequency of SGA monitoring. A baseline weight was taken in 94% of patients, but monthly monitoring decreased to 15 patients (47%) after the first month, 11 patients (34%) after the second month, and 8 patients (25%) after the third month. The decrease in weight monitoring was more often associated with patients not seen around the recommended monitoring points rather than weight measurements not taken at the appointment. Family history was recorded at baseline for 27 patients (84%) and updated annually for 13 patients (41%). Blood pressure values were taken in 29 patients (91%) at baseline, 11 patients (34%) at 3 months, and 24 patients (75%) annually. In addition, a fasting lipid panel was measured in only 13% of the patients at baseline and 1 patient (3%) 3 months later. A fasting plasma glucose level was never recorded for all 32 patients, but a baseline random glucose reading was taken in 4 patients (13%).
DISCUSSION
The use of SGAs has markedly increased partly due to the lower risk of extrapyramidal symptoms (EPSs) at clinically effective doses compared to first-generation antipsychotics. However, SGAs have been associated with dramatic weight gain, type 2 diabetes mellitus, dyslipidemia, and cardiovascular disease.2,5 Cohen and colleagues12 assessed the short-term adverse effects of 6 commonly used SGAs in children and adolescents and found that secondary effects occurred with significant frequency in each drug. The adverse effects analyzed included increases in weight, glucose, cholesterol, triglycerides, prolactin, sedation, and EPS. These data highlight the importance and need for proper medication monitoring.
Our study showed that of the 32 patients eligible, 27 (84%) patients had a family history recorded at baseline. However, only 13% of patients had baseline monitoring that included weight, blood pressure, and fasting lipid panel. No patients had a fasting plasma glucose recorded at any point during SGA therapy. Follow-up monitoring decreased over time, with the exception of quarterly weight and annual blood pressure.
The minimal amount of monitoring noted in this study in addition to other research revealing the potential adverse effects of SGAs in the pediatric population illustrate the importance of improving antipsychotic management. Andrade et al2 found a potentially fourfold increased rate of diabetes in children on SGA therapy in comparison with those who were not on antipsychotic drug therapy. None of the patients included in this study had a fasting plasma glucose checked at any time while receiving therapy. A baseline random glucose reading was taken in 4 patients and possibly negated the need for a fasting measurement, but this comprises a small percentage of patients. Moreover, SGAs have been linked to changes in lipid values in the pediatric population.13 This was another parameter rarely assessed in this study sample. Though additional research is required to confirm the magnitude of the risk for diabetes and dyslipidemia, the importance of glucose and lipid monitoring should not be overlooked due to the complications associated with these abnormalities.
This retrospective, pilot study has several limitations. The monitoring protocol created by the ADA and the APA for SGAs was not intended specifically for the pediatric population. Thus, monitoring parameters that differ from those recorded in this study may be more appropriate for patients younger than 18 years. This should not discount the fact that these adverse events have been reported in all ages. Monitoring should still be completed, especially for glucose and lipid levels, 2 categories that were rarely checked in the study sample. Waist circumference, another monitoring parameter listed in the guidelines, was not recorded in the EMR and could not be assessed. In addition, the size of the study sample was small, affecting the generalizability of the results to the population.
Being a retrospective review, it was not possible to rule out multiple courses or historical use of other SGAs. Furthermore, assessing compliance retrospectively presents a challenge. The authors made every effort to only include patients with active prescriptions who were currently taking the SGA as prescribed. Even though the authors were only collecting if baseline values were completed (yes or no), it was possible the baseline drawn at the clinic was not the true baseline for the medication. For example, a patient could have been started on an SGA by an outside provider with no baseline monitoring recorded and not seen for 3 months by the PCP. At that time monitoring was started and counted as baseline for purposes of this study. Lastly, this class of medication is often either started and managed by a specialist or recommended by the specialist with the PCP to manage. However, it is imperative that the PCP identify and monitor these agents or obtain appropriate records to ensure the patient is appropriately monitored. In this study, documentation and acknowledgment by a specialist for appropriate monitoring was identified and included. Lack of communication between specialist and PCP was identified in this study as a possible barrier to proper monitoring and presents as an avenue for future research.
CONCLUSIONS
The results of this study highlight the lack of baseline and periodic monitoring that occur when pediatric patients are prescribed antipsychotic medications, putting the patient at risk for adverse events. The marked increase in antipsychotic prescribing and concerns related to their safety emphasize the need for improvement in monitoring of antipsychotic medications. The complexity of SGA monitoring also can lead to potential adverse consequences. Adherence with monitoring recommendations for this class of medications may increase the likelihood of proper medication management and patient safety. This gap in patient care and safety opens an excellent opportunity for a clinical pharmacy team to provide education and assistance with SGA monitoring for the purpose of providing optimal patient care.
ACKNOWLEDGMENTS
Dr. Michelle Condren for her support and review of this project. Ramos LV, Honey BL, Brahm NC. Evaluation of second-generation antipsychotic monitoring in an outpatient pediatric clinic. 15th Annual Meeting of the College of Psychiatric and Neurologic Pharmacists (CPNP). Tampa FL. April 29 – 30, 2012.
ABBREVIATIONS
- ADA
American Diabetes Association
- APA
American Psychiatric Association
- BMI
body mass index
- CDC
Centers for Disease Control and Prevention
- EMR
electronic medical record
- EPS
extrapyramidal symptom
- PCP
primary care provider
- SGA
second generation antipsychotic
Footnotes
DISCLOSURE The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.Cooper WO, Arbogast PG, Ding H et al. Trends in prescribing of antipsychotic medications for US children. Ambul Pediatr. 2006;6(2):79–83. doi: 10.1016/j.ambp.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Andrade SE, Lo JC, Roblin D et al. Antipsychotic medication use among children and risk of diabetes mellitus. Pediatrics. 2011;128(6):1135–1141. doi: 10.1542/peds.2011-0855. [DOI] [PubMed] [Google Scholar]
- 3.Pringsheim T, Panagiotopoulos C, Davidson J et al. Evidence-based recommendations for monitoring safety of second generation antipsychotics in children and youth. J Can Acad Child Adolesc Psychiatry. 2011;20(3):218–233. [PMC free article] [PubMed] [Google Scholar]
- 4.Rosack J. FDA to require diabetes warning on antipsychotics. Psychiatric News. 2003;38(20):1–27. [Google Scholar]
- 5.Consensus statement from American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, and North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596–601. doi: 10.2337/diacare.27.2.596. [DOI] [PubMed] [Google Scholar]
- 6.Morrato EH, Druss B, Hartung DM et al. Metabolic testing rates in 3 state Medicaid programs after FDA warnings and ADA/APA recommendations for second-generation antipsychotic drugs. Arch Gen Psychiatry. 2010;67(1):17–24. doi: 10.1001/archgenpsychiatry.2009.179. [DOI] [PubMed] [Google Scholar]
- 7.Zuvekas SH. Prescription drugs and the changing patterns of treatment for mental disorders, 1996–2001. Health Aff. 2005;24(1):195–205. doi: 10.1377/hlthaff.24.1.195. [DOI] [PubMed] [Google Scholar]
- 8.Haupt DW, Rosenblatt LC, Kim E et al. Prevalence and predictors of lipid and glusoce monitoring in commercially insured patients treated with second-generation antipsychotic agents. Am J Psychiatry. 2009;166(3):345–353. doi: 10.1176/appi.ajp.2008.08030383. [DOI] [PubMed] [Google Scholar]
- 9.Correll CU, Manu P, Olshanskiy V et al. Cardiometabolic risk of second-generation antipsychotic medications during first time use in children and adolescents. JAMA. 2009;302(16):1765–1773. doi: 10.1001/jama.2009.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morrato EH, Nicol GE, Maahs D et al. Metabolic screening in children receiving antipsychotic drug treatment. Arch Pediatr Adolesc Med. 2010;164(4):344–351. doi: 10.1001/archpediatrics.2010.48. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention, National Center for Health Statistics. Growth charts. http://www.cdc.gov/growthcharts/cdc_charts.htm. Accessed August 13, 2012. [Google Scholar]
- 12.Cohen D, Bonnot O, Bodeau N et al. Adverse effects of second-generation antipsychotics in children and adolescents: a Bayesian meta-analysis. J Clin Psychopharmacol. 2012;32(3):309–316. doi: 10.1097/JCP.0b013e3182549259. [DOI] [PubMed] [Google Scholar]
- 13.Correll CU, Harris J, Figen V et al. Antipsychotic drug administration does not correlate with prolonged rate-corrected QT interval in children and adolescents: results from a nested case-control study. J Child Adolesc Psychopharmacol. 2011;21(4):365–368. doi: 10.1089/cap.2011.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]


