Abstract

Microbial fuel cells (MFCs) are a promising technology for energy-efficient domestic wastewater treatment, but the effluent quality has typically not been sufficient for discharge without further treatment. A two-stage laboratory-scale combined treatment process, consisting of microbial fuel cells and an anaerobic fluidized bed membrane bioreactor (MFC-AFMBR), was examined here to produce high quality effluent with minimal energy demands. The combined system was operated continuously for 50 days at room temperature (∼25 °C) with domestic wastewater having a total chemical oxygen demand (tCOD) of 210 ± 11 mg/L. At a combined hydraulic retention time (HRT) for both processes of 9 h, the effluent tCOD was reduced to 16 ± 3 mg/L (92.5% removal), and there was nearly complete removal of total suspended solids (TSS; from 45 ± 10 mg/L to <1 mg/L). The AFMBR was operated at a constant high permeate flux of 16 L/m2/h over 50 days, without the need or use of any membrane cleaning or backwashing. Total electrical energy required for the operation of the MFC-AFMBR system was 0.0186 kWh/m3, which was slightly less than the electrical energy produced by the MFCs (0.0197 kWh/m3). The energy in the methane produced in the AFMBR was comparatively negligible (0.005 kWh/m3). These results show that a combined MFC-AFMBR system could be used to effectively treat domestic primary effluent at ambient temperatures, producing high effluent quality with low energy requirements.
Introduction
Growing concerns over the large energy requirements needed for effective wastewater treatment has stimulated interest in the use of wastewater as a source of renewable energy.1 Microbial fuel cells (MFCs) are being developed as a sustainable energy technology, as they can directly produce electricity from wastewater allowing for energy recovery to offset the costs of wastewater treatment.2,3 In an air-cathode MFC, organic matter in wastewater is oxidized by microorganisms, and electrons discharged to the anode travel through an external circuit to the cathode where they combine with oxygen, forming water.4,5 Passive transfer of oxygen to the air-cathode avoids the need for energy intensive aeration of the wastewater that is currently required for typical activated sludge or aerobic membrane bioreactor processes. In addition, MFCs have lower sludge production than conventional aerobic treatment processes, which could reduce treatment costs and the challenges associated with sludge treatment and disposal.6
MFCs fed with domestic wastewaters have shown promising performance in terms of achieving electricity generation with simultaneous organics removal,7−9 and there continue to be improvements in MFC designs that have produced configurations more suitable for scaling up to larger systems.10−14 Capital costs of the materials used in MFCs are also being reduced, for example, by using cathode catalysts such as inexpensive activated carbon.15,16 One operational aspect of using MFCs for wastewater treatment that has not been sufficiently addressed is the need to meet stringent effluent quality requirements. Effluent chemical oxygen demand (COD) concentrations with domestic wastewater in MFCs have ranged from 23 to 164 mg/L in fed-batch tests, and 60 to 220 mg/L in continuous flow tests, depending on influent COD concentrations, reactor configurations, and cycle time or hydraulic retention time (HRT).8,11,14 One of the reasons for these high effluent CODs is likely inefficient removal of particulate organics,17 as biofilm reactors such as MFCs and trickling filters are more effective for soluble than particulate COD removal. Thus, post-treatment or integrated processes are needed to further improve the quality of the treated wastewater to meet discharge limits.
One approach to improve the overall extent of wastewater treatment has been to integrate the MFC with a membrane-based process in a single reactor. This approach has been referred to either as a membrane bioelectrochemical reactor (MBER)18 or an electrochemical membrane bioreactor (EMBR).19 Although higher treatment efficiencies have been obtained for both acetate solutions and domestic wastewater in tests with this approach, energy consumption has only been balanced with electrical energy production when acetate was used as the substrate.18,19 The main challenges with using both MFCs and membrane processes for domestic wastewater treatment are obtaining high power production from the MFCs, while minimizing membrane fouling.18 Using a shorter hydraulic retention time (HRT) in an MFC treating domestic wastewater will usually improve power production,14 but a shorter HRT could mean a higher organic loading rate on the membrane process, which could result in increased membrane fouling.18 Membrane fouling control remains the biggest challenge in the use of membranes in both aerobic20 and anaerobic systems.21 In previous membrane-based MFC studies, membranes inside the MFCs fouled in 15 days, and therefore these membranes would require frequent cleaning.18 The high maintenance costs due to cleaning processes could limit applications of integrated MFC and membrane bioreactor processes.18
A new approach to obtain high quality effluent with low energy requirements is proposed here based on using a second stage anaerobic fluidized bed membrane bioreactor (AFMBR) following wastewater treatment in the MFC. The AFMBR has recently been shown to be an effective approach for achieving high quality effluent when used as a post-treatment method for an anaerobic fluidized bioreactor (AFBR).1,22 In the AFMBR, membrane fouling is controlled by using granular activated carbon (GAC) as the fluidized particles, as these particles can scour the membrane and minimize fouling.1,22 The properties of particles used in the fluidized bed are important, as spherical plastic particles have been shown to not be as effective as GAC.23 The use of an MFC as the primary treatment process, as opposed to an AFBR, may be useful for several reasons. Electrical energy is directly produced in the MFC, whereas in the AFBR electricity would have to be produced in a separate process from biogas that might need to be cleaned and purified to remove hydrogen sulfide and water to improve utilization efficiencies.24 Any hydrogen sulfide generated in situ in an MFC would be expected to be rapidly oxidized in the MFC as it is a good electron donor to the anode.25 There should be very little methane in the MFC effluent compared to that produced by the AFBR, as organic matter is mainly converted into current or lost to aerobic degradation due to oxygen transfer across the cathode. It is important to remove dissolved methane, which can be supersaturated in these systems, to minimize its release into the atmosphere as it is a potent greenhouse gas.26,27
In this study we examined domestic wastewater treatment using a two-stage MFC-AFMBR system, containing four MFCs and one AFMBR, at ambient temperature. There were two separate flow lines into the AFMBR, with two MFCs connected hydraulically in series (with separate electrical circuits) in each flow line (Figure 1). The use of two MFCs in series avoided large changes in COD concentrations in each MFC, as such large COD changes have previously been shown to adversely affect current generation.14,28 Each pair of MFCs had a different electrode configuration in order to compare two design approaches: using a separator electrode assembly (SEA), where the electrodes are sandwiched together and a separator was placed between them to prevent short circuiting and reduce oxygen crossover from the cathode; and using a spaced electrode assembly (SPA), where the electrodes are kept close to each other, but with sufficient space to avoid direct contact (no separator was used) (Figure S1, Supporting Information (SI)). It has recently been shown that the SPA design can reduce treatment time compared to the SEA, although less energy may be recovered in the SPA configuration due to the loss of organic matter to aerobic processes rather than current generation.29 Treatment efficiency was evaluated in terms of COD and total suspended solid (TSS) removals, and energy efficiency was quantified for both processes in terms of production and demands, under continuous flow conditions.
Figure 1.

Schematic diagram (a) and photo (b) of the two-stage MFC-AFMBR system. (U = the first upstream MFC, and D = the second downstream MFC prior to the AFMBR).
Materials and Methods
Reactors and Operation
The two-stage MFC-AFMBR system consisted of four MFC reactors and one AFMBR reactor. The four MFC reactors were arranged in two groups, each group having two MFC reactors with the same electrode configuration that were hydraulically connected in series (Figure 1). Single-chamber, air-cathode MFCs (130 mL) were constructed as previously described.28 Each reactor contained three brush anodes connected together externally with copper wire, and a single air-cathode. The anodes were graphite fiber brushes with a titanium wire core (25 mm diameter by 35 mm length) (Mill-Rose, Mentor, OH) that were heat treated at 450 °C for 30 min before use. The cathode (35 cm2 projected surface area) was made of wet-proofed carbon cloth (30 wt.%, #CC640WP30, Fuel Cell Earth, Stoneham, MA), with a platinum catalyst (0.5 mg/cm2) on the water side and four PTFE diffusion layers on the air side.30
For the SEA MFCs, the brush anodes were trimmed in half along the direction parallel to the core to prevent contact by the bristles with the cathode through the separator, as both electrodes were pressed against the separator (SI Figure S1). Two layers of textile separator (46% cellulose and 54% polyester, 0.3 mm thickness, Amplitude Prozorb, Contec Inc., Spartanburg, SC) were used in the SEA reactors to prevent short-circuiting and to minimize oxygen crossover. The SPA MFCs did not contain separators, so the edges of the brush anodes were set 0.8 cm from the surface of the cathodes.
The AFMBR (65 mL) consisted of a 300 mm long by 16 mm diameter clear polyvinyl chloride (PVC) tube (U.S. Plastic Corp.) containing 10 g (wet weight) of GAC (DARCO MRX, 10 × 30 mesh, Norit Activated Carbon) as the fluidized bed medium and support for bacterial growth. The GAC was washed with deionized water for three times prior to use to remove any residuals. The AFMBR contained a submerged membrane module with eight 200 mm long polyvinylidenefluoride (PVDF) hollow fiber membranes (2.0 mm outside diameter, 0.8 mm inside diameter, 0.1 μm pore size, Kolon Inc., South Korea), having a total membrane surface area of 0.004 m2. A Hungate tube (10 mL, Bellco Glass Inc., Vineland, NJ) with the bottom cut off was glued onto the top of the PVC tube and sealed with a thick butyl rubber stopper (20 mm diameter, Chemglass Inc., Vineland, NJ), for biogas collection and measurement.
The MFCs were inoculated and fed with domestic wastewater collected from the primary clarifier of the Pennsylvania State University Wastewater Treatment Plant, and operated in continuous flow mode at an HRT of 4 h. The primary clarifier effluent was collected weekly and stored in a refrigerator (4 °C) to minimize COD changes over time. During tests, a container of wastewater was placed in an ice bucket to keep it cool in order to minimize degradation prior to being fed into the MFCs. The wastewater warmed in the tube when it was transferred into the bottom of the reactors using a peristaltic pump (model no. 7523-90, Masterflex, Vernon Hills, IL) at an overall flow rate of 1560 mL/d, with 780 mL/d for each flow line. The effluent from the top of the upstream MFC reactor flowed into the bottom of the downstream MFC due to the hydraulic pressure. The effluent from the two MFCs series was delivered to the AFMBR using another peristaltic pump (as above) at a flow rate of 1560 mL/d, producing an HRT of 1 h. The top of the membrane module was connected to the same peristaltic pump, to maintain a constant permeate flux of 16 L/m2/h (LMH) from the AFMBR. The pump was operated with a 10 min on and 1 min off cycle time, as it was previously shown that periodic relaxation of the membrane reduced trans-membrane pressure (TMP).22 TMP was monitored continuously using a vacuum pressure gauge (Type1490, Ashcroft, Stratford, CT). Fluidization of GAC was maintained with a peristaltic pump (model no. 7523-80 Masterflex, Vernon Hills, IL) at the desired flow rate of 170 mL/min, resulting in bed expansion of 70–80% to a height of 210–240 mm. The two-stage MFC-AFMBR system was operated at room temperature (∼25 °C).
Measurements and Chemical Analyses
The voltage across an external resistor for the MFC circuit was measured every 20 min using a multimeter (model 2700; Keithley Instruments, Inc.). Current was calculated using Ohm’s law (I = U/R), with power calculated as P = IU, where U is the measured voltage (V), and R the external resistance (Ω).31 A reference electrode [Ag/AgCl; +200 mV vs standard hydrogen electrode (SHE); BASi] was inserted into the upper middle of the MFC reactor to determine the anode and cathode potentials. Polarization and power curves were obtained by changing the external resistances from open circuit to 1600, 800, 400, 200, and 100 Ω, with one day at each resistance (six HRTs). The averaged voltage at each external resistance was used to obtain the polarization curve. Columbic efficiency (CE) was calculated as CE = Ct/Cth × 100%, where Ct was the total coulombs calculated by integrating the current over time (Ct = Σ I Δt, where Δt is the time interval of one HRT), and Cth was the theoretical amount of coulombs available based on the COD removed in the MFC over the same amount of time, calculated as Cth = [Fb (CODin – CODout) Q Δt]/M, where F is Faraday’s constant, b = 4 is the number of electrons exchanged per mole of oxygen, CODin and CODout are the influent and effluent COD, Q is the flow rate, Δt is the time interval (HRT), and M = 32 is the molecular weight of oxygen.32
Total suspended solids (TSS) were measured using standard methods (APHA, 1998). Total COD (tCOD) and soluble COD (sCOD) were measured using standard methods (method 5220, HACH Company, Loveland, CO). All samples for sCOD measurement were filtered through 0.45 μm pore diameter syringe filters (polyvinylidenedifluoride, PVDF, 25 mm size, Restek Corporation). Conductivity and pH were measured immediately after sampling using a probe (SevenMulti, Mettler-Toledo International Inc.). The sampling points on the flow line for the chemical analyses were indicated in the SI (Figure S2). Biogas (200 μL samples) of the AFMBR headspace was sampled using gastight syringes (250 μL; Hamilton Samplelock Syringe) and analyzed using two gas chromatographs (SRI Instruments) for H2, N2, CH4 and CO2, as described previously.33 Gas was collected and measured directly using a 10 mL glass syringe (Air-Tite Products Co., Inc., VA) inserted into the top of the AFMBR. Dissolved methane was also measured as described previously34 by transferring a liquid sample from the AFMBR reactor into a serum bottle (6.5 mL, Wheaton, Millville, NJ) without any air contact or headspace, and sealed with a thick butyl rubber stopper (20 mm diameter, Chemglass Inc.). The serum bottle full of the liquid sample was then autoclaved to prevent biological activity. Some liquid sample (1.5 mL) was then replaced by N2 gas from this serum bottle with a syringe. After establishing gas–liquid equilibrium by shaking the serum bottle for six hours at room temperature, the amount of dissolved methane was back-calculated from the measured methane amount in the headspace (detailed information in the SI). All samples were collected and analyzed in triplicate.
Results and Discussion
Performance of MFCs
The start-up time needed for the SEA MFCs was shorter than that required for the SPAs. The SEA MFCs produced a stable voltage of 0.59 ± 0.03 V (1000 Ω) after 3 days, while the SPAs produced 0.51 ± 0.04 V after 3 days, and required 10 days to achieve a stable voltage of 0.58 ± 0.01 V. Stable voltage production was indicated by a deviation between the daily averaged voltage values that was <0.006 V (∼1% of the daily averaged voltage) over three consecutive days. There was no appreciable difference in start-up time between the upstream or downstream MFC within the individual flow paths (data not shown).
The power produced by the SEAs and SPAs changed over time. Based on the polarization data obtained after 1 month, the SEA-U MFC produced a maximum power of 0.31 mW (89 mW/m2, normalized to the cathode projected surface area of 35 cm2), which was comparable to that of SPA-U (0.33 mW) (Figure 2). Although the same current was produced with these two configurations, the SEA-U had better cathode performance but showed poorer anode performance than the SPA-U (Figure 3b and c). The downstream MFCs produced slightly lower maximum power than the upstream ones, with 0.28 mW for SEA-D and 0.27 mW for SPA-D (Figure 2). The downstream MFCs generally had more positive anode potentials than the first MFCs (Figure 3b), likely due to the lower substrate concentrations in the downstream MFCs (SI Table S1), as it was shown that the anode potentials became more positive at lower substrate concentrations in a previous study.35
Figure 2.

Power production of the SEA and SPA MFCs at different time after start-up, after (a) 1 month and (b) 5 months. (U = the first upstream MFC, and D = the second downstream MFC prior to the AFMBR).
Figure 3.
Voltage, anode potential and cathode potential of the SEA and SPA MFCs at different time after start-up: (a) voltage, (b) anode potential and (c) cathode potential at 1 month, and (d) voltage, (e) anode potential and (f) cathode potential at 5 month. The letters “A” in (b) indicated the anodes, and “C” in (c) the cathodes. All electrode potentials were reported versus the Ag/AgCl reference electrode [+200 mV vs a standard hydrogen electrode (SHE); BASi].
After 5 months, the maximum power densities of the SEA MFCs were relatively unchanged (0.33 mW for SEA-U and 0.32 mW for SEA-D), and the wastewater composition fed into the reactor was relatively unchanged based on the influent tCOD concentrations (210 ± 11 mg/L at 5 months, compared to 224 ± 17 mg/L at 1 month). However, the maximum power produced by SPA MFCs substantially decreased to 0.16 mW (SPA-U) and 0.18 mW (SPA-D). The reason for these decreases was a large reduction in cathode potentials (Figure 3f), which was likely due to biofouling.15,36 While the cathodes used for the SEA configuration contained a separator that covered the cathode, the SPA cathodes were directly exposed to the wastewater, and thus they were more prone to fouling (Figure 3f).
The maximum power density of 89 ± 6 mW/m2 produced by the SEA MFC in these continuous flow tests was lower than the maximum power densities obtained in two other studies with domestic wastewater when the MFC was operated in fed-batch mode (120 mW/m214 or 328 ± 11 mW/m229). The lower power density here was likely due to a lower influent COD (217 ± 18 mg/L, compared to 275 ± 71 mg/L14 and 303 ± 69 mg/L29), and operation under continuous flow conditions, where the average substrate concentration was lower than that in the fed-batch reactors at the beginning of the cycle.28
The different electrode configurations (SEA or SPA) did not appreciably affect the extent of COD removal. tCOD removals were 28 ± 7% for SEA-U, and 34 ± 3% for SPA-U, which are comparable removals within the calculated standard deviations. The downstream MFCs had slightly lower tCOD removals than the upstream MFCs, with 17 ± 5% for SEA-D and 19 ± 5% for SPA-D, likely due to the lower substrate concentrations. Fed-batch tests with domestic wastewater have shown that COD removal in MFCs is first order with respect to concentration (unpublished data). Thus, the reduction in COD concentration would have reduced removal rates in the downstream reactors. sCOD removals showed the same trends as tCOD, with greater removals in the upstream reactors (27 ± 10% for SEA-U, 32 ± 5% for SPA-U) than the downstream ones (19 ± 5% for SEA-D, and 26 ± 7% for SPA-D). Note that these COD removals were based on the combination of all data in tests at the different external resistances used in the polarization tests, as the effluent COD concentrations in these tests did not change substantially with the different external resistances (COD concentrations in the SI, Table S1). These COD removals were lower than those obtained in previous studies operated in fed-batch mode using the same domestic wastewater source (62–93%), due to the short HRT (4 h) in this study compared to much longer fed-batch cycle times (12–36 h).14,29 In additional tests, increasing the HRT to 24 h increased COD removals to 67 ± 2%, which was about the same as that obtained in fed-batch mode with a cycle length of 24 h (65 ± 1%; 500 Ω resistance, data not shown). However, a long HRT is not desirable for efficient wastewater treatment, and thus the shorter HRT was used here.
The CEs increased in proportion to the current (lower resistance), even though the COD removals remained relatively constant with different resistances. At the maximum power density (0.31 ± 0.02 mW, 0.86 ± 0.02 mA), the overall CE of the SEA MFCs was 18% (13% for SEA-U and 28% for SEA-D). Over time, changes in the CEs paralleled those on maximum power densities. The SEA and SPA MFCs had comparable overall CEs at 1 month, (range of 6–29%), but after 5 months the SEA MFCs remained relatively unchanged while those of the SPA MFCs decreased (range of 4–20%) with the decreased currents. CE values obtained here under continuous flow conditions were comparable to those previously reported for fed-batch conditions (2–31%).29 Overall, these results suggest that the SEA configuration was superior to the SPA design on the basis of fixed HRTs as it maintained higher power densities and CEs over time with the same level of wastewater treatment.
Wastewater Treatment with the Second Stage AFMBR
The two-stage MFC-AFMBR system achieved excellent treatment levels in terms of COD and TSS removals. The AFMBR was first inoculated with anaerobic sludge and fed the effluent from MFCs for two weeks. After that, the membrane module was installed, and preliminary tests were conducted to optimize the design and operation of the AFMBR over a period of approximately two months, with occasional system shutdown to address problems related to consistent flow and treatment. Following this system optimization, a new membrane module was installed, and the MFC-AFMBR system was operated continuously for 50 days in concert with the MFCs operation over the last two of the five months.
tCOD further decreased from the influent concentration to the AFMBR of 107 ± 10 mg/L to 16 ± 3 mg/L in the effluent, providing an overall tCOD removal for the two stages of 92.5% (49.1% for the MFCs, and 43.4% for the AFMBR) (Figure 4 and SI Table S2). The effluent sCOD and tCOD concentrations from the AFMBR were identical, and therefore there was a lower overall sCOD removal of 86.2% (influent sCOD to MFCs of 114 ± 8 mg/L, compared to tCOD of 210 ± 11 mg/L) (Figure 4 and SI Table S2). A larger percent of sCOD was removed by the MFCs (50.3%) than by the AFMBR (35.9%), while particulate COD removal was 47.9% for the MFCs compared to 100% for the AFMBR. Changes in the forms of COD might occur through hydrolysis from particulate to soluble COD, and through biomass growth from soluble to particulate. The particulate COD removal in MFCs might be partially due to settling in the reactor chambers, while that in the AFMBR was primarily due to membrane filtration. The effluent contained <1 mg/L of TSS due to filtration of the wastewater through the membrane, resulting in >99.6% TSS removal (Figure 4 and SI Table S2). These COD and TSS removals are comparable to those obtained using a staged anaerobic fluidized membrane bioreactor (SAF-MBR) treating domestic wastewater.22 There was little overall change in pH, as the influent pH to the MFCs of 7.6 ± 0.1 decreased to 7.1 ± 0.1 in the MFCs effluent, but it increased to 7.5 ± 0.2 following treatment in the AFMBR (SI Table S2). These pH changes might result from losses of CO2 and volatile fatty acids, for example from methanogenesis processes occurring in the MFC-AFMBR system. Also there were no large changes in conductivity, with 1473 ± 33 mS/cm for the MFCs influent, 1457 ± 15 mS/cm for the MFCs effluent, and 1420 ± 19 mS/cm for the AFMBR effluent (SI Table S2).
Figure 4.
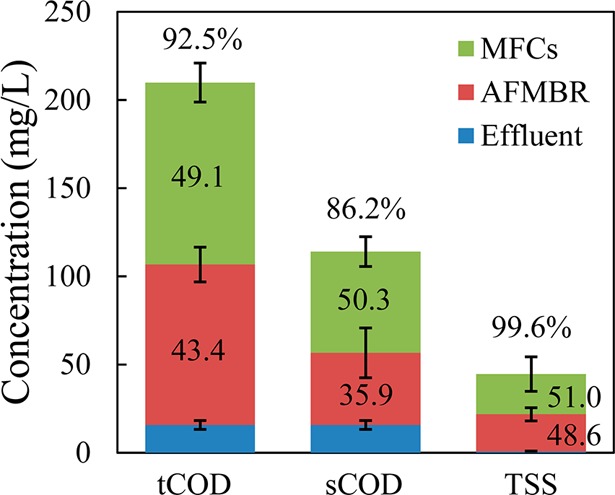
Influent and effluent concentrations, and removals of tCOD, sCOD and TSS for the combined MFC-AFMBR system. The values inside the figures were the percent of the influent concentration that was removed by the MFCs, AFMBR, and the whole system.
The AFMBR was operated continuously for 50 days at a high membrane flux of 16 LMH, even without cleaning by backwashing or using chemicals. Most of the increase in the TMP, from 0.015 to 0.035 bar, occurred during the first 8 days of operation (Figure 5). Thereafter, it slowly increased to 0.050 bar during the rest of the test (Figure 5). Liquid (9 mL) was withdrawn from the AFMBR every 3.5 days (0.16% of the total influent flow) to removal finer material and excess suspended solids from days 8 to 50, as suggested in a previous AFMBR study.1 The membrane flux of 16 LMH here is higher than that previously reported for the AFMBR following an AFBR (11 LMH), with a PVDF hollow-fiber membrane with the same pore size as the one here (0.1 μm).22 In that study, the TMP reached 0.25 bar in 3 days when the membrane flux was increased to 14 LMH,22 which is much higher than the maximum TMP observed here. The stable operation of the flux through the AFMBR without appreciable membrane fouling was likely due to a combination of factors here that included the scouring effect of the GAC particles on the membrane surface, intermittent filtration, and periodic removal of suspended solids. The use of MFCs as the primary treatment process likely contributed to the high flux and stable performance of the AFMBR, due to the removals of COD and TSS in the MFCs. The improved flux with the first-stage MFC treatment, compared to that previously obtained with a first-stage AFBR treatment, suggests that MFCs might be a better first stage treatment than AFBR, but this cannot be concluded without direct side-by-side tests of the two different systems. The operation of the AFMBR without wastewater pretreatment was not examined here as that would represent a different treatment process, and one that would not allow for electrical power generation and recovery from COD removal.
Figure 5.
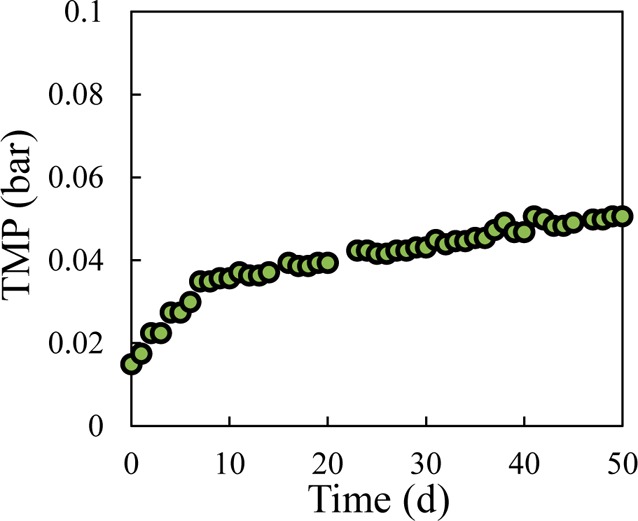
TMP for the AFMBR over 50 days of operation.
Energy Balance
Energy usage for the two-stage MFC-AFMBR system was calculated as previously described.1,22 All the volumetric energy densities were reported on the basis of normalizing to 1 m3 of wastewater treated. The energy requirements were calculated as 0.0107 kWh/m3 for fluidizing the GAC particles, and 0.0014 kWh/m3 for pumping permeate through the membranes, resulting in a total electrical energy requirement for the AFMBR of 0.0186 kWh/m3 (Table 1). The electrical energy requirement for pumping liquid through the MFCs was negligible compared to that needed for the AFMBR (Table 1). The higher energy requirement for the AFMBR was primarily due to pumping needed for liquid recirculation to maintain the GAC fluidization. The energy needed for this is proportional to the total reactor flow rate and the hydraulic head loss of the system.37 In an AFMBR reactor with a given configuration, the minimum recirculation flow rate and the hydraulic head loss are fixed, and thus the energy requirement for recirculation is inversely proportional to the permeate flow rate or the HRT.18 Therefore, the high permeate flux and low HRT achieved for the AFMBR in this study were favorable for achieving a low energy requirement of 0.0186 kWh/m3. This energy requirement is lower than previous reports using the AFMBR (0.027–0.040 kWh/m3),1,18,22 but there are many differences in these studies that preclude a direct comparison of these values.
Table 1. Electrical Energy Requirements and Production for the Two-Stage MFC-AFMBR System.
| characteristic | MFCs | AFMBR | system total |
|---|---|---|---|
| Electrical Energy required | |||
| Energy for Hydraulic Loss | |||
| reactor head loss, cm H2O | 0.5 | 2.5 | |
| reactor influent plus recirculation flow rate, mL/min | 1.1 | 171.1 | |
| hydraulic energy requirement, kWa | 0.001(10–6) | 0.699(10–6) | |
| required pumping energy, kWh/m3b | 0.00001 | 0.0107 | 0.0107 |
| Energy for Permeate Extraction | |||
| average TMP, cm H2O | 50.8 | ||
| permeate flow rate, mL/min | 1.1 | ||
| permeate energy requirement, kW | 0.090(10–6) | ||
| required pumping energy, kWh/m3 | 0.0014 | ||
| total pumping energy required for system, kWh/m3 | 0.00001 | 0.0121 | 0.0121 |
| total electrical energy required for pumps, kWh/m3c | 0.000015 | 0.0186 | 0.0186 |
| Electrical Energy Produced | |||
| MFC maximum power, mWd | 1.28 | ||
| electrical energy production, kWh/m3 | 0.0197 | 0.0197 | |
| electrical energy produced/requirede | 1.06 | ||
Energy requirement =9.8QE, where Q (m3/s) is flow rate and E (m H2O) is head loss.1
Energy per m3 of wastewater treated.
Assume energy efficiency of 65% in conversion of electrical energy to pump energy.1
Based on the maximum power produced by the SEA MFCs in series. This maximum power output was quite similar to that obtained during steady operation, and therefore it represents power production that could be obtained during continuous treatment tests (SI Figure S3).
The ratio of the electrical energy produced to that required by the MFC-AFMBR system.
Electrical energy could be produced directly from the MFCs. Based on the maximum power that could be produced by the SEA configuration after 5 months of operation (0.33 mW for SEA-U and 0.32 mW for SEA-D, Figure 2), the total power that could be produced by the four MFCs coupled to the AFMBR was 1.28 mW (four times 0.32 mW) (Table 1). If all of this electrical energy was recovered, the net electrical energy available for the system operation would be 0.0197 kWh/m3. This would be enough to supply the 0.0186 kWh/m3 required to operate the system if these values could be maintained for larger-scale systems. However, this energy balance would likely change as the size of the system increases. Also, in practice there might be other energy losses that would affect overall energy recovery, that have not been included here. Direct electricity production by MFCs is advantageous, compared to methanogenic reactors that require combustion of the methane to produce power, as the conversion efficiency of methane to electricity is typically only 33%.1 However, there will also be energy losses in converting the low voltage DC power into higher voltage DC or AC power.38,39
The additional energy that could be recovered from the methane production in the AFMBR was not included in this energy balance as it would have been difficult to recover. Total methane production in the AFMBR was 1.67 mL/L liquid treated at ambient temperature and pressure, with most of this present as dissolved methane (1.5 mL/L) (detailed calculations in the SI). This concentration was estimated to be 125% oversaturation relative to the concentration of methane in the AFMBR headspace. The energy value of this amount of methane is 0.016 kWh/m3 (methane combustion, assuming 800 kJ/mol), equivalent to electrical energy of 0.005 kWh/m3 (33% energy recovery) which could theoretically add 27% more energy production into the system. However, as most of this methane is dissolved, and at a low concentration, it would have been difficult to recover. The methane yield from the AFMBR was 0.75 mmol/g COD removed, indicating only 5% of the COD removal in the AFMBR could be attributed to methane generation. The amount of methane that theoretically could be produced in the AFMBR was estimated as 17 mL/L, based on the COD removal and assuming a conversion of 0.017 mol CH4/g COD (detailed information and calculations in the SI). It is not clear what other processes occurred relative to COD removal as the methane production was only about 1/10 of that possible by methanogenesis alone. This subject will require further study. More methane might be recovered in the future with improvements in the configuration and operation of the AFMBR.
Outlook
The two-stage MFC-AFMBR system was shown to effectively treat domestic wastewater (primary clarifier effluent) at ambient temperatures in terms of COD and TSS removals, producing a high effluent quality with a near neutral (or net positive) energy requirement, without the need for membrane cleaning even after 50 days of operation. tCOD was reduced from 210 ± 11 mg/L to 16 ± 3 mg/L, resulting in 92.5% overall COD removal with >99% removal of TSS to a final effluent concentration of <1 mg/L. The energy requirement of the AFMBR is much less than that needed for aerobic MBRs with internal membranes that require air sparging to control membrane fouling.1 The high permeate flux (16 LMH) and low HRT (1 h) here resulted in an overall low energy requirement for the AFMBR of only 0.0186 kWh/m3. Thus, the energy produced by only the MFCs (0.0197 kWh/m3) was theoretically sufficient here to meet the energy demands for the system operation, although the energy balance for a larger system would likely change. An additional benefit of the MFC-AFMBR system should be a low sludge production rate. Although sludge production was not measured here (for an estimate, see the SI), previous studies have shown that the sludge production by anaerobic MFC and AFMBR processes are less than those of conventional aerobic processes such as activated sludge.6,22
While feasibility of the combined process was shown here, additional work will be needed to optimize the performance of the individual and combined MFC and AFMBR reactors. More optimal treatment could likely be obtained by adjusting the HRTs of the two systems, and examining benefits of treatment rates compared to electrical power production. The current findings were sufficient to show that the two-stage MFC-AFMBR system is useful for treatment of low strength wastewater even at ambient temperatures. The robustness of the system at other temperatures, particularly lower ones, would need to be investigated. However, MFCs have been shown to function over a wide range of temperatures.2,40 The assessment of nitrogen, phosphorus, and pathogen removals will be examined in future studies to see to what extent the MFC-AFMBR system can be used for these wastewater components. Other issues to address are capture and use(or destruction) of the methane to avoid its release into the air, and efficient use of the electricity produced by MFCs. Following these optimization studies, it should be possible to better evaluate the economics of the system compared to traditional treatment systems.
Acknowledgments
We thank David Jones for help with the analytical measurements, Dr. Xiaoyuan Zhang for preparing the photo of the two-stage MFCs-AFMBR system, and KOLON Inc.(South Korea) for providing hollow-fiber membranes for this research. This research is supported by Award KUS-I1-003-13 from the King Abdullah University of Science and Technology(KAUST).
Supporting Information Available
Additional information is provided in the Supporting Information that includes: schematic diagrams of the SEA and SPA MFCs, a schematic diagram of the sampling points, voltage and power of the MFCs with steady operation, COD removals of the SEA and SPA MFCs after 1 month, wastewater treatment efficiencies for the MFC-AFMBR system, estimation of biomass solids production, estimation of methane production in the AFMBR, batch test results used to estimate methane production, a COD balance for the AFMBR, and methane production of the AFMBR. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Present Address
† Department of Energy Engineering, Gyeongnam National University of Science and Technology, Dongjin-ro 33, Jinju, 584 Gyeongnam, 660-758, Republic of Korea.
The authors declare no competing financial interest.
Supplementary Material
References
- Kim J.; Kim K.; Ye H.; Lee E.; Shin C.; McCarty P. L.; Bae J. Anaerobic fluidized bed membrane bioreactor for wastewater treatment. Environ. Sci. Technol. 2011, 452576–581. [DOI] [PubMed] [Google Scholar]
- Logan B. E.; Rabaey K. Conversion of wastes into bioelectricity and chemicals by using microbial electrochemical technologies. Science 2012, 3376095686–690. [DOI] [PubMed] [Google Scholar]
- He Z. Microbial fuel cells: Now let us talk about energy. Environ. Sci. Technol. 2013, 471332–333. [DOI] [PubMed] [Google Scholar]
- Logan B. E.; Regan J. M. Electricity-producing bacterial communities in microbial fuel cells. Trends Microbiol. 2006, 1412512–518. [DOI] [PubMed] [Google Scholar]
- Logan B. E.; Call D.; Cheng S.; Hamelers H. V. M; Sleutels T.; Jeremiasse A. W.; Rozendal R. A. Microbial electrolysis cells for high yield hydrogen gas production from organic matter. Environ. Sci. Technol. 2008, 42238630–8640. [DOI] [PubMed] [Google Scholar]
- Rabaey K.; Verstraete W. Microbial fuel cells: Novel biotechnology for energy generation. Trends Biotechnol. 2005, 236291–298. [DOI] [PubMed] [Google Scholar]
- Liu H.; Ramnarayanan R.; Logan B. E. Production of electricity during wastewater treatment using a single chamber microbial fuel cell. Environ. Sci. Technol. 2004, 3872281–2285. [DOI] [PubMed] [Google Scholar]
- Min B.; Logan B. E. Continuous electricity generation from domestic wastewater and organic substrates in a flat plate microbial fuel cell. Environ. Sci. Technol. 2004, 38215809–5814. [DOI] [PubMed] [Google Scholar]
- You S. J.; Zhao Q. L.; Jiang J. Q.; Zhang J. N. Treatment of domestic wastewater with simultaneous electricity generation in microbial fuel cell under continuous operation. Chem. Biochem. Eng. Q. 2006, 204407–412. [Google Scholar]
- Du Z. W.; Li H. R.; Gu T. Y. A state of the art review on microbial fuel cells: A promising technology for wastewater treatment and bioenergy. Biotechnol. Adv. 2007, 255464–482. [DOI] [PubMed] [Google Scholar]
- Hays S.; Zhang F.; Logan B. E. Performance of two different types of anodes in membrane electrode assembly microbial fuel cells for power generation from domestic wastewater. J. Power Sources 2011, 196208293–8300. [Google Scholar]
- Zhuang L.; Zheng Y.; Zhou S. G.; Yuan Y.; Yuan H. R.; Chen Y. Scalable microbial fuel cell(MFC) stack for continuous real wastewater treatment. Bioresour. Technol. 2012, 106, 82–88. [DOI] [PubMed] [Google Scholar]
- Zhang F.; Ge Z.; Grimaud J.; Hurst J.; He Z. Long-term performance of liter-scale microbial fuel cells treating primary effluent installed in a municipal wastewater treatment facility. Environ. Sci. Technol. 2013, 4794941–4948. [DOI] [PubMed] [Google Scholar]
- Ahn Y.; Logan B. E. Domestic wastewater treatment using multi-electrode continuous flow MFCs with a separator electrode assembly design. Appl. Microbiol. Biotechnol. 2013, 971409–416. [DOI] [PubMed] [Google Scholar]
- Zhang F.; Pant D.; Logan B. E. Long-term performance of activated carbon air cathodes with different diffusion layer porosities in microbial fuel cells. Biosens. Bioelectron. 2011, 30149–55. [DOI] [PubMed] [Google Scholar]
- Zhang F.; Cheng S. A.; Pant D.; Van Bogaert G.; Logan B. E. Power generation using an activated carbon and metal mesh cathode in a microbial fuel cell. Electrochem. Commun. 2009, 11112177–2179. [Google Scholar]
- Huang L. P.; Logan B. E. Electricity generation and treatment of paper recycling wastewater using a microbial fuel cell. Appl. Microbiol. Biotechnol. 2008, 802349–355. [DOI] [PubMed] [Google Scholar]
- Ge Z.; Ping Q.; He Z. Hollow-fiber membrane bioelectrochemical reactor for domestic wastewater treatment. J. Chem. Technol. Biotechnol. 2013, 8881584–1590. [Google Scholar]
- Wang Y. K.; Sheng G. P.; Shi B. J.; Li W. W.; Yu H. Q. A novel electrochemical membrane bioreactor as a potential net energy producer for sustainable wastewater treatment. Sci. Rep. 2013, 318641–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng F. G.; Chae S. R.; Drews A.; Kraume M.; Shin H. S.; Yang F. L. Recent advances in membrane bioreactors(MBRs): Membrane fouling and membrane material. Water Res. 2009, 4361489–1512. [DOI] [PubMed] [Google Scholar]
- Liao B. Q.; Kraemer J. T.; Bagley D. M. Anaerobic membrane bioreactors: Applications and research directions. Crit. Rev. Environ. Sci. Technol. 2006, 366489–530. [Google Scholar]
- Yoo R.; Kim J.; McCarty P. L.; Bae J. Anaerobic treatment of municipal wastewater with a staged anaerobic fluidized membrane bioreactor(SAF-MBR) system. Bioresour. Technol. 2012, 120, 133–139. [DOI] [PubMed] [Google Scholar]
- Kim J.; Kim K.; Lee R.; McCarty P. L.; Bae J. Physical aspects of GAC fluidization on membrane fouling in anaerobic fluidized membrane bioreactor. IWA World Water Congress Busan, Korea. 2012
- Pipatmanomai S.; Kaewluan S.; Vitidsant T. Economic assessment of biogas-to-electricity generation system with H2S removal by activated carbon in small pig farm. Appl. Energy 2009, 865669–674. [Google Scholar]
- Rabaey K.; Van de Sompel K.; Maignien L.; Boon N.; Aelterman P.; Clauwaert P.; De Schamphelaire L.; Pham H. T.; Vermeulen J.; Verhaege M.; Lens P.; Verstraete W. Microbial fuel cells for sulfide removal. Environ. Sci. Technol. 2006, 40175218–5224. [DOI] [PubMed] [Google Scholar]
- IPCC IPCC fourth assessment report (AR4). Working Group 1, The Physical Science Basis. 2007
- Howarth R. W.; Santoro R.; Ingraffea A. Methane and the greenhouse-gas footprint of natural gas from shale formations. Clim. Chang. 2011, 1064679–690. [Google Scholar]
- Ahn Y.; Logan B. E. A multi-electrode continuous flow microbial fuel cell with separator electrode assembly design. Appl. Microbiol. Biotechnol. 2012, 9352241–2248. [DOI] [PubMed] [Google Scholar]
- Ahn Y.; Hatzell M. C.; Zhang F.; Logan B. E. Different electrode configurations to optimize performance of multi-electrode microbial fuel cells for generating power or treating domestic wastewater. J. Power Sources 2014, 249, 440–445. [Google Scholar]
- Cheng S.; Liu H.; Logan B. E. Increased performance of single-chamber microbial fuel cells using an improved cathode structure. Electrochem. Commun. 2006, 83489–494. [Google Scholar]
- Hong Y. Y.; Call D. F.; Werner C. M.; Logan B. E. Adaptation to high current using low external resistances eliminates power overshoot in microbial fuel cells. Biosens. Bioelectron. 2011, 28171–76. [DOI] [PubMed] [Google Scholar]
- Logan B. E.; Hamelers B.; Rozendal R. A.; Schrorder U.; Keller J.; Freguia S.; Aelterman P.; Verstraete W.; Rabaey K. Microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 2006, 40175181–5192. [DOI] [PubMed] [Google Scholar]
- Call D. F.; Wagner R. C.; Logan B. E. Hydrogen production by Geobacter species and a mixed consortium in a microbial electrolysis cell. Appl. Environ. Microbiol. 2009, 75247579–7587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin C.; Lee E.; McCarty P. L.; Bae J. Effects of influent DO/COD ratio on the performance of an anaerobic fluidized bed reactor fed low-strength synthetic wastewater. Bioresour. Technol. 2011, 102219860–9865. [DOI] [PubMed] [Google Scholar]
- Ren L.; Ahn Y.; Hou H.; Zhang F.; Logan B. E. Electrochemical study of multi-electrode microbial fuel cells under fed-batch and continuous flow conditions. J. Power Sources 2014, 10.1016/j.jpowsour.2013.11.085. [DOI] [Google Scholar]
- Zhang F.; Ahn Y.; Logan B. E. Treating refinery wastewaters in microbial fuel cells using separator electrode assembly or spaced electrode configurations. Bioresour. Technol. 2014, 152, 46–52. [DOI] [PubMed] [Google Scholar]
- Vennard J. K.; Street R. L., Elementary Fluid Mechanics; John Wiley & Sons: New York, 1982. [Google Scholar]
- Alaraj M.; Ren Z. J.; Park J.-D. Microbial fuel cell energy harvesting using synchronous flyback converter. J. Power Sources 2014, 247, 636–642. [Google Scholar]
- Park J. D.; Ren Z. Y. High efficiency energy harvesting from microbial fuel cells using a synchronous boost converter. J. Power Sources 2012, 208, 322–327. [Google Scholar]
- Pham T. H.; Rabaey K.; Aelterman P.; Clauwaert P.; De Schamphelaire L.; Boon N.; Verstraete W. Microbial fuel cells in relation to conventional anaerobic digestion technology. Eng. Life Sci. 2006, 63285–292. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



