Highlights
-
•
Insulin/insulin-like signaling reduction alters selenium-induced neurodegeneration.
-
•
Selenium induces nuclear translocation of DAF-16/FOXO3a.
-
•
DAF-16 overexpression decreases GABAergic and cholinergic motor neuron degeneration.
-
•
Loss of DAF-18/PTEN increases sensitivity to selenium-induced movement deficits.
-
•
Glutathione requires DAF-18/PINK-1 to improve selenium-induced movement deficits.
Keywords: Selenium, Neurodegeneration, Insulin/insulin-like signaling, PTEN, PINK1, Amyotrophic lateral sclerosis
Abstract
Exposures to high levels of environmental selenium have been associated with motor neuron disease in both animals and humans and high levels of selenite have been identified in the cerebrospinal fluid of patients with amyotrophic lateral sclerosis (ALS). We have shown previously that exposures to high levels of sodium selenite in the environment of Caenorhabditis elegans adult animals can induce neurodegeneration and cell loss resulting in motor deficits and death and that this is at least partially caused by a reduction in cholinergic signaling across the neuromuscular junction. Here we provide evidence that reduction in insulin/insulin-like (IIS) signaling alters response to high dose levels of environmental selenium which in turn can regulate the IIS pathway. Most specifically we show that nuclear localization and thus activation of the DAF-16/forkhead box transcription factor occurs in response to selenium exposure although this was not observed in motor neurons of the ventral cord. Yet, tissue specific expression and generalized overexpression of DAF-16 can partially rescue the neurodegenerative and behavioral deficits observed with high dose selenium exposures in not only the cholinergic, but also the GABAergic motor neurons. In addition, two modifiers of IIS signaling, PTEN (phosphatase and tensin homolog, deleted on chromosome 10) and PINK1 (PTEN-induced putative kinase 1) are required for the cellular antioxidant reduced glutathione to mitigate the selenium-induced movement deficits. Studies have suggested that environmental exposures can lead to ALS or other neurological diseases and this model of selenium-induced neurodegeneration developed in a genetically tractable organism provides a tool for examining the combined roles of genetics and environment in the neuro-pathologic disease process.
1. Introduction
Selenium (Se) is an essential micronutrient with a narrow recommended dietary range. Insufficient dietary Se can be lethal contributing to the cardiomyopathy of Keshan Disease and the osteochondropathy of Kashin-Beck disease (Lee and Jeong, 2012). And, while supranutritional levels of Se can also be lethal (Spiller and Pfiefer, 2007), high levels of selenite [Se(IV)] and other Se compounds such as Ebselen have been used effectively as cancer therapeutics (Sanmartín et al., 2012; Fairweather-Tait et al., 2011). During the course of a number of cancer treatment trials, it was observed that the higher doses of Se capable of reducing the incidence of various cancers were positively associated with increases in fasting glucose levels and type 2 diabetes (Rocourt and Cheng, 2013; Steinbrenner, 2013). Based on these and other studies, potential mechanisms have been proposed for how Se levels alter diabetic status and include a role for selenoproteins (Rayman and Stranges, 2013; Labunskyy et al., 2011). Selenoproteins are proteins that incorporate Se in the form of selenocysteine and include the antioxidant proteins: glutathione peroxidase (GPx), thioredoxin reductase (TrxR), and selenoprotein P (SeP). Increased levels of SeP have been observed in diabetic and pre-diabetic humans (Yang et al., 2011), and in animal models overexpression of GPx induced insulin resistance (McClung et al., 2004). Targeted knockouts of these selenoproteins in animals models are associated with neurodegeneration (Caito et al., 2011; Wirth et al., 2010), and lower levels are found in individuals with Alzheimer's and other neurological diseases (Vinceti et al., 2013; Zhang et al., 2010).
Supranutritional levels of Se can also exert effects on the insulin/insulin-like growth factor (IGF)-1 signaling (IIS) pathway via the induction of hydrogen peroxide (H2O2) and reactive oxygen species (ROS) formation (Steinbrenner, 2013). High levels of exogenously applied H2O2 prevent phosphorylation/activation of AKT1/PKB (Iwakami et al., 2011), a serine/threonine kinase that is a downstream mediator of IIS signaling (Alessi et al., 1996). Similarly, levels of Se which induce ROS formation prevent AKT phosphorylation (Luo et al., 2012, 2013; Pinto et al., 2011; Wu et al., 2006) or induce its dephosphorylation (Wu et al., 2006). By preventing AKT activation, the downstream forkhead box O transcription factors (FOXO) are not phosphorylated which leads to their activation and subsequent nuclear translocation. Increased incidences of metabolic disorders, including diabetes are associated with transcriptional activation of FOXO proteins (Barthel et al., 2005). In addition, FOXO proteins are known to regulate expression of pro-apoptotic and oxidative stress response genes (Eijkelenboom and Burgering, 2013) which inhibit cancers and aging (Huang and Tindall, 2007) and their activation has also been shown to be neuroprotective (Mojsilovic-Petrovic et al., 2009) and lead to the transcription of SeP (Walter et al., 2008). A mutation in the FOXA2 gene was also significantly associated with the sporadic form of the motor neuron disease, amyotrophic lateral sclerosis (ALS) (Dunckley et al., 2007).
Environmental exposures to high levels of Se, have led to the development of a neurological disorder in both humans and animals that bears striking similarity to ALS (Vinceti et al., 2010; Panter et al., 1996). More recently, Vinceti et al. (2013) reported significantly higher levels of Se(IV) in the cerebrospinal fluid of neurologically impaired patients that were later confirmed to have ALS. Additionally, they reported that SeP levels were reduced in these patients. This coupled with the identification of the FOXA2 association with sporadic ALS, as mentioned above implies that FOXO transcriptional regulation may be altered in ALS. In fact, the Caenorhabditis elegans FOXO3a ortholog, DAF-16 was shown to be required for lifespan extension and improved movement by reducing the toxic effects of TDP-43 (transactive response DNA binding protein 43) and SOD1 (superoxide dismutase 1) (Boccitto et al., 2012; Vaccaro et al., 2012; Zhang et al., 2011) which have both been linked to sporadic and familial forms of ALS (Sreedharan et al., 2008; Kabashi et al., 2008; Rosen et al., 1993; Jones et al., 1993). The importance of FOXO in other neurodegenerative diseases has been demonstrated in both C. elegans and elsewhere (Wong et al., 2013; Parker et al., 2012; Maiese et al., 2007; Morley et al., 2002).
The free-living soil nematode C. elegans has one selenoprotein, a TrxR ortholog TRXR-1 (Gladyshev et al., 1999) which is not required for Se-induced lethality (Boehler et al., 2013). Since selenoproteins do not play a direct role in Se toxicity within C. elegans, it is an ideal model to examine how Se alters other cellular processes within a eukaryotic organism. The nervous system of C. elegans hermaphrodites consists of 302 neurons and 56 support cells, and contains all of the classic neurotransmitters observed in higher organisms including serotonin, dopamine, acetylcholine, ?-aminobutyric acid (GABA), and glutamate (Altun and Hall, 2011). Thus, C. elegans is an excellent model to study neurodegenerative disease (Dimitriadi and Hart, 2010). We have shown previously that in adult animals high dose exposures to Se in the form of sodium selenite [Se(IV)] induce reactive oxygen species (ROS) formation and motor neuron degeneration in C. elegans which results in alterations in the normal movement behaviors leading to paralysis and death (Estevez et al., 2012; Morgan et al., 2010). These phenotypes could be partially ameliorated by exogenous exposure to the cellular antioxidant reduced glutathione (GSH) (Estevez et al., 2012; Morgan et al., 2010). Animals with mutations in genes altering both cholinergic and GABAergic signaling were more sensitive to Se(IV) [i.e. the mutations were shown to further reduce normal movement in Se(IV) exposed animals], and reduced cholinergic signaling across the neuromuscular junction (NMJ) was determined to be partly responsible for these phenotypic changes which mimics aspects of ALS pathology (Estevez et al., 2012). Here we show that reductions in IIS signaling which lead to activation of DAF-16/FOXO3a are neuroprotective against these Se(IV)-induced effects. In addition we show evidence that Se(IV) causes degeneration and loss of GABAergic motor neurons, and that the loss of both the cholinergic and GABAergic neurons with high dose Se(IV) exposure was partially dependent on the levels of DAF-16 expression within the animals. Here we also report that animals with mutations in genes encoding for PTEN (phosphatase and tensin homolog, deleted on chromosome 10) and PINK1 (PTEN-induced putative kinase 1) are resistant to the positive improvements on the Se(IV)-induced movement behaviors previously observed with exogenous application of GSH. This GSH resistance phenotype was only previously seen in animals with a mutation in the dithiol glutaredoxin gene glrx-21 (Estevez et al., 2012; Morgan et al., 2010). The model of Se(IV) toxicity presented here can be used to further elucidate the link between increased Se status and alterations in IIS signaling to disease processes affecting insulin resistance and type 2 diabetes, and cancer, as well as neurodegenerative disorders such as ALS, Parkinson's and Alzheimer's diseases.
2. Materials and methods
2.1. Strains, maintenance, and growth conditions
The following strains were used: N2: wild-type(WT) variety, Bristol, CB1370: daf-2(e1370)III, CB1375: daf-18(e1375)IV, CB5600: ccIs4251 [pSAK2 (Pmyo-3::GFP-LacZ(NLS)) + pSAK4 (Pmyo-3::mitochondrial GFP) + dpy-20(+)]I; him-8(e1489) IV, CF1038: daf-16(mu86)I, CF1442: daf-16(mu86)I;daf-2(e1370)III; muEx169 [pNL206(Punc-119::gfp::daf-16a) + pRF4[rol-6(su1006)]], CF1514: daf-16(mu86)I;daf-2(e1370)III; muEx211 [pNL213(Pges-1::gfp::daf-16a) + pRF4 [rol-6(su1006)]], CF1515: daf-16(mu86)I;daf-2(e1370)III; muEx212 [pNL212(Pmyo-3::gfp::daf-16a) + pRF4[rol-6(su1006)]], DR26: daf-16(m26)I, DR1564: daf-2(m41)III, GR1310: akt-1(mg144)V, OM148: daf-2(e1370)III; yzIs71[Ptph-1::gfp + pRF4[rol-6(su1006)]]V, OM249: daf-16(mu86)I; daf-2(e1370)III; yzIs71[Ptph-1::gfp + pRF4[rol-6(su1006)]]V, OM261: vsIs33[dop-3::rfp]V; wuIs56[Psod-3::sod-3::gfp + pRF4[rol-6(su1006)]], OM285: daf-16(mu86)I; yzIs71[Ptph-1::gfp + pRF4[rol-6(su1006)]], OM324: zIs356[pGP30(Pdaf-16::daf-16a2::gfp) + pRF4[rol-6(su1006)]]IV; vsIs33[Pdop-3::rfp]V, OM325 daf-16(mu86)I; vsIs33[Pdop-3::rfp]V; wdIs20[snb-1::GFP], RB712: daf-18(ok480)IV, RB2547: pink-1(ok3538)II, TJ356: zIs356[pGP30(Pdaf-16::daf-16a2::gfp) + pRF4[rol-6(su1006)]]IV, TJ1052: age-1(hx546)II. The daf-16(mu86)I;daf-2(e1370)III animals examined in Fig. 3A were obtained by selecting non-roller adult animals from those strains expressing the daf-16-tissue specific extra-chromosomal arrays. Animals that did not roll were no longer expressing the extra-chromosomal array and were used as a control for determining the effects of the double mutation on the Se(IV)-induced motility deficits.
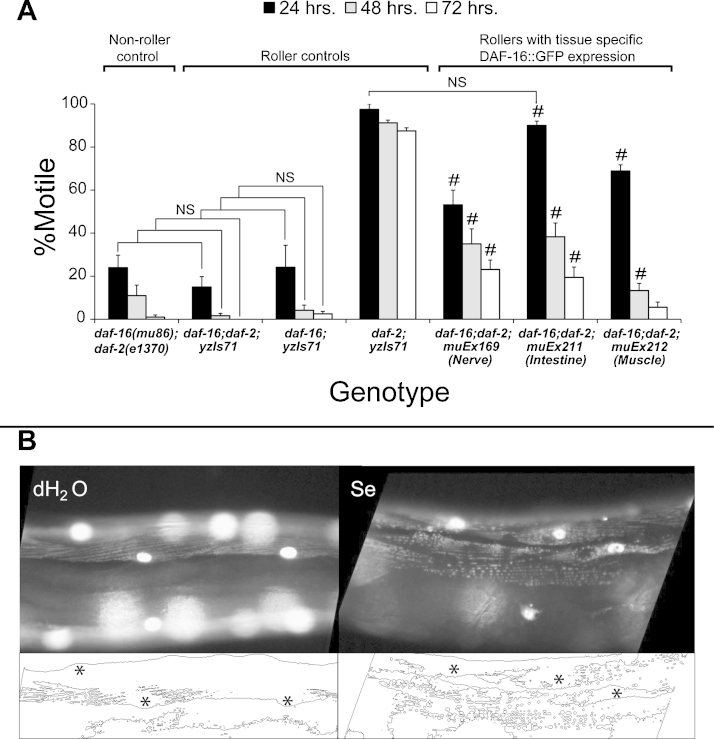
Fig. 3.
Selenium exposure and the effects of tissue-specific expression of DAF-16 on motility. Overexpression of DAF-16 in nerves, intestines, or muscles of adult animals resulted in an increased resistance to the Se(IV)-induced motility defects. (A) Animals expressing the DAF-16 tissue-specific constructs were more resistant (#) to the Se(IV)-induced effects on motility when compared to animals with the same genetic background (daf-16;daf-2) and that expressed the same reporter plasmid, pRF4. This plasmid contained within the yzIs71 transgene induces animals to roll in place (roller phenotype) rather than move forward or backward in a normal sinusoidal wave pattern, a phenotype that results from the expression of a dominant mutation (su1006) in the rol-6 gene (Mello et al., 1991). No significant difference (NS) was observed between the daf-16;daf-2 roller and non-roller strains at all temperatures, between the daf-16 and the daf-16;daf-2 roller strains at all temperatures, nor the daf-2 roller strain and the daf-16;daf-2 intestine expressing strain at 24 h only (as determined by one-way ANOVA). Significance is defined as p = 0.01 as determined by one-way ANOVA followed by post hoc analysis using the Bonferroni–Holm method. All non-Se(IV)-treated strains had a greater than 90% survival at all times tested (data not shown). Each bar graph represents the average of six populations of 20 animals per strain (n = 120); error bars ± SEM. daf-16 = daf-16(mu86); daf-2 = daf-2(e1370); daf-16;daf-2;yzIs71 = OM249; daf-16;yzIs71 = OM285; daf-2;yzIs71 = OM148. (B) Upper panel: Animals of the strain CB5600 transiently express mitochondrial GFP in their muscles and are shown here after 48 h of either mock- (dH2O) or Se(IV)-(Se)exposure. The GFP pattern is tubular indicating healthy mitochondria in the water control (dH2O). Se(IV)-exposure results in fragmentation of the GFP expression indicating damage to the muscle mitochondria (Se). Lower panel: Black and white outline of GFP expression patterns above, nuclei are represented by asterisks for orientation. Se = Se(IV).
The following caveat must be noted in regards to the sod-3::GFP transgene used here. Expression of sod-3 accounts for only 1% of the total sod mRNA levels in C. elegans adults, thus the strong expression exhibited by the sod-3::gfp transgene examined here is most likely artifactual, but as reported is very likely to express the native pattern (Doonan et al., 2008). Several laboratories have reported similar bright expression patterns with the generation of other Psod-3 transgenes (Wolf et al., 2008; Libina et al., 2003).
All strains were maintained and grown at 20 °C on modified NGM plates without additional calcium as described (Estevez et al., 2004) and all experiments were performed at 20 °C unless otherwise stated.
2.2. Movement assay
Adult animals were developmentally synchronized and placed on plates containing 5 mM sodium selenite (Spectrum Chemicals, Gardena, CA) alone or with 3 mM glutathione (Sigma–Aldrich, St. Louis, MO) as described (Estevez et al., 2012; Morgan et al., 2010). Mock-exposed animals (controls) were placed on plates containing an equivalent amount of carrier (dH2O) to that used on the experimental plates. After plating, animals were scored at 24-h intervals for the movement behaviors as previously described (Morgan et al., 2010). For experiments performed at 25 °C, developmentally synchronized animals at the fourth larval stage (L4) were placed on plates overnight in order to develop to the adult stage at 25 °C. They were transferred the next day to fresh plates with or without the addition of Se(IV) as described (Morgan et al., 2010). The percentage motile (%motile) indicates the number of animals moving normally after a tap to the head and tail as previously described (Morgan et al., 2010).
2.3. Heat-shock
Developmentally synchronized adult animals expressing the Pdaf-16::gfp construct, zIs356 (Henderson and Johnson, 2001) were placed on plates with 5 mM Se(IV) or an equivalent volume of dH2O (mock-exposed control) for 2 h at 20 °C then shifted to 34 °C for 30 min (Lin et al., 2001) before being fluorescently imaged; or they were directly visualized following the 2 h incubation.
2.4. Fluorescence imaging
Following either mock or Se(IV) exposure, ten live animals at a time were anesthetized by placing them into a drop of M9/tricaine solution (0.01% tricaine/0.001% tetramisole) on a glass microscope slide. A coverslip was placed over the animals prior to visualization. Three to five slides (30–50 animals) were visualized for each experiment. Because the animals were visualized alive, only those animals that remained alive and intact were documented.
Animals were visualized on an Olympus BX51 microscope equipped with epifluorescence (Olympus America Inc., Center Valley, PA) and utilizing a FITC (GFP) or Texas Red (RFP) filter. Images were documented with a QImaging Retiga 1300 camera and imaging system (QImaging, Surrey, British Columbia, Canada). Processing of images for publication was accomplished by the Adobe CS5 Software package (Adobe Systems Inc., San Jose, CA) utilizing both Photoshop and Illustrator to create the final images.
For the studies examining the effects of the proteasomal inhibitors levamisole and the MG-132 (Z-Leu-Leu-Leu-CHO), CB5600 animals were simultaneously exposed to Se(IV) and either of the drugs for 48 h. The concentrations of the drugs were as described in Szewczyk et al. (2000).
2.5. Statistical analysis
Statistical analysis was performed by one-way ANOVA with post hoc analysis by the Bonferroni–Holm method using Microsoft Excel 2010 (Microsoft Inc., Seattle, WA) with the add-in Daniel's XL Toolbox version 5.6 (http://xltoolbox.sourceforge.net). Student's t-test was two-tailed with unequal variance.
3. Results
3.1. Mutations in the insulin/insulin-like growth factor pathway alter sensitivity/resistance to environmental selenium
Populations of wild-type (WT) adult C. elegans animals exposed to sodium selenite [Se(IV)] in their growth media exhibit a decline in the percentage of normal moving animals that is both dose and time dependent (Morgan et al., 2010). An exposure of 5 mM Se(IV) was shown to lead to a progressive decline in movement from mild impairment (backing deficient) to paralysis, and death, and to induce oxidative stress and cholinergic motor neuron degeneration (Estevez et al., 2012; Morgan et al., 2010), all of which mimics aspects of amyotrophic lateral sclerosis (ALS). ALS is a neurodegenerative disease characterized by motor neuron loss in the brain and spinal cord and which typically results in death within five years from diagnosis. The causes of ALS are multifaceted and include both genetic factors, and environmental influences (Trojsi et al., 2013). High dose Se exposures have been implicated in ALS-like disease in both animals and humans (Vinceti et al., 2010). Because alterations in insulin/insulin-like growth factor signaling (IIS) have also been associated with ALS, we wanted to determine if alterations in IIS signaling could affect the response of adult animals in our high dose Se(IV) assay. To first determine if alterations in the levels of IIS signaling components can affect the movement behavior of C. elegans adults, animals containing mutations in genes encoding components of the IIS signaling pathway were exposed over time to 5 mM Se(IV) and their movement accessed. When grown at optimal growth temperature (20 °C – Fig. 1A), animals with a reduction-of-function (rf) mutation, m41 in the daf-2 gene which encodes for the C. elegans insulin/insulin-like growth factor receptor (IGFR) (Kimura et al., 1997), were more resistant to the Se(IV)-induced reduction in normal motility at 72 h in comparison to WT (#, m41, Fig. 1A), although at 24 h they were initially more sensitive (*, m41, Fig. 1A). Both hx546 a rf mutation in the age-1 gene [which encodes for the phosphoinositide 3-kinase (PI(3)K) (Morris et al., 1996) downstream of DAF-2 (Ogg et al., 1997)] and the rf mutation sa709 in the worm PDK1 encoding gene pdk-1, conferred increased resistance to the Se(IV)-induced motility impairment at 72 h (#, hx546, Fig. 1A; data not shown for pdk-1, p = 2.2 × 10-3 by one-way ANOVA comparing sa709 to WT at 72 h). In contrast, an activating mutation in the gene encoding for the C. elegans ortholog of the serine/threonine kinase AKT/PKB, akt-1(mg144) (Paradis and Ruvkun, 1998) and an rf mutation in the forkhead box transcription factor (FOXO3a) gene, daf-16(m26) (Ogg et al., 1997; Lin et al., 1997) significantly increased the sensitivity of these animals to Se(IV) by reducing the number of animals moving normally in comparison to the WT strain (*, mg144 and m26, Fig. 1A). The increased sensitivity to Se(IV) observed with these two strains was manifest across all the time points tested. Thus, reductions in IIS signaling are protective against the decline in normal movement behavior previously observed to occur when WT adult animals were exposed to high levels of environmental Se(IV).
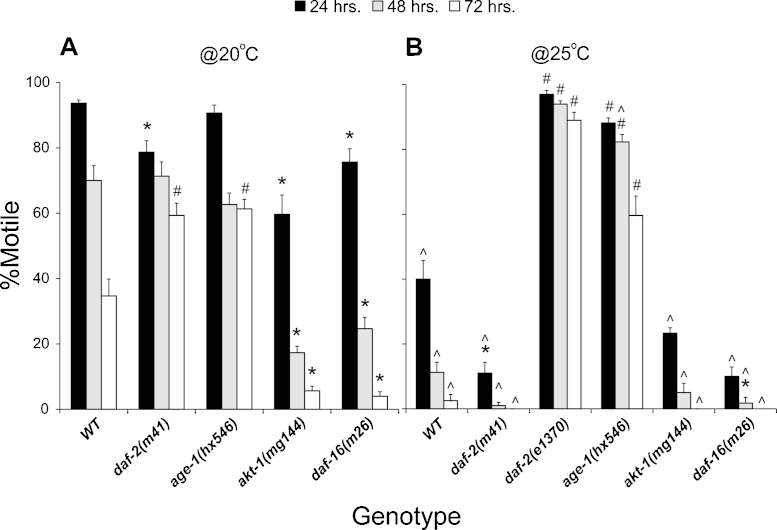
Fig. 1.
Mutations in the IGF pathway affect selenium sensitivity. Animals containing mutations in the daf-2 IGF pathway show an altered sensitivity to Se(IV)-induced motility effects that is enhanced by increasing temperature. (A) When grown at 20 °C, adult animals were found to be more resistant (#) or to exhibit significantly increased sensitivity (*) to Se(IV) in comparison to the WT strain by one-way ANOVA performed across each time point (p = 0.05; significance was determined by post hoc analysis using the Bonferroni–Holm method). (B) The phenotypes (sensitivity or resistance) observed at 20 °C were significantly enhanced by Se(IV)-exposure at 25 °C at some or all of the time points tested for all strains [^ significantly different at 25 ° when each strain was compared to the same strain and time exposure at 20 °C; p < 0.05 by Student's t-test (two tailed, unequal variance)]. Comparisons of the mutant strains to WT when all were grown at 25 °C, showed significant increases in resistance (#) or sensitivity (*) in the animals at some or all of the time points tested. Analysis was by one-way ANOVA performed across each time point (p = 1.4 × 10-11; significance was determined by post hoc analysis using the Bonferroni–Holm method). When not exposed to Se(IV), all strains had =95% motility at each time point and temperature tested (data not shown), with the only exception being akt-1 which exhibited 80% motility across all time points at 25 °C only (data not shown). Each bar graph represents the average of three populations (n = 150 at 20 °C; n = 60 at 25 °C) with error bars indicating SEM. WT = N2 strain; Se = Se(IV). All mutations are reduction-of-function mutations with the exception of mg144 which is an activating mutation in the akt-1 gene.
3.2. The effects of high dose selenium exposure on movement is enhanced with increased temperature
Stressors including oxidative stress and heat can reduce IIS signaling which leads to activation of DAF-16/FOXO3a (Landis and Murphy, 2010; Honda and Honda, 2002). We have shown that high dose Se(IV) exposures also result in increased stress as indicated by the detection of reactive oxygen species (ROS) formation in exposed animals and that increased temperature reduced movement in WT animals (Morgan et al., 2010). To determine whether the alterations in normal movement observed within populations of the IIS mutant animals exposed to high dose Se(IV) could be enhanced by increased temperature (i.e. increased stress), animals grown at 20 °C until the adult stage were exposed to Se(IV) at 25 °C. Many of the original components of the IIS signaling pathway were isolated as temperature sensitive (ts) regulators of a developmental process that can lead to the formation of an alternate third stage larva known as a dauer larva (Golden and Riddle, 1984; Riddle et al., 1981; Swanson and Riddle, 1981; Riddle, 1977), and 25 °C is a temperature at which the ts dauer larva formation phenotype is fully penetrant in many of the IIS mutant strains (Swanson and Riddle, 1981). At this higher temperature, the WT animals, those with the rf mutations daf-2(m41) or daf-16(m26), or the akt-1(mg144) activating mutation had significantly fewer normal moving animals at all of the time points when compared to animals of the same genotype exposed at 20 °C for the given 24 h time period (^, p < 0.05: Fig. 1B). Thus, there was an overall enhancement of the sensitivity to the Se(IV)-induced effects on movement when these animals were exposed at the higher temperature. Similarly, the populations of age-1(hx546) animals were observed to have a significantly greater number of normal moving animals within their populations, but only when they were exposed for 48 h at 25 °C (^, p < 0.05: Fig. 1B). This was an enhancement of the resistance phenotype observed at 48 h for the age-1(hx546) animals exposed at 20 °C. Finally, when compared to the WT strain exposed to Se(IV) at 25 °C, all of the IIS mutant strains exposed under the same conditions exhibited the same Se(IV) responsive phenotype that they exhibited at 20 °C [i.e. more sensitive or resistant to Se(IV) in comparison to WT] with the exception of akt-1(mg144) which was no different than WT at this temperature (mg144, Fig. 1B). Thus, we show that the additional stressor of increasing temperature during Se(IV)-exposure results in enhancement of the Se(IV)-induced movement phenotypes that were observed with exposures at 20 °C, and that high dose Se(IV)-exposure induces a stress response process that appears to be mediated by IIS signaling similar to its control of other stress response pathways (Shore and Ruvkun, 2013).
3.3. daf-2 alleles have opposite selenium responsive phenotypes under high temperature stress
There are over 40 daf-2 alleles that have been isolated and these have been described to express a range of phenotypes in response to environmental and oxidative stressors (Ackerman and Gems, 2012; Honda and Honda, 2002; Scott et al., 2002; Gems et al., 1998). Most recently, Vaccaro et al. (2012) showed that the e1370 allele enhanced neurodegeneration in a worm TDP43/TDP-1 ALS model while another allele e1368 suppressed the neurodegeneration. The m41 allele has been previously described as thermolabile or a true temperature-sensitive allele (Gems et al., 1998) and has demonstrated differences in phenotype from the hypomorphic alleles of daf-2 in regards to life span, intrinsic thermal tolerance, and enhancements of its Daf-c phenotype by mutations in the tyrosine phosphatase-like sdf-9 (Gems et al., 1998; Jensen et al., 2007). Here we observed that m41 allele of daf-2 was more sensitive to the Se(IV)-induced effects on normal movement when exposed at 25 °C, a phenotype it shared with the daf-16(m26) animals (Fig. 1B). Yet, at 20 °C although it was also initially more sensitive, after 72 h of exposure time the daf-2(m41) animals were more resistant to Se(IV)’s effect on normal movement; a phenotype opposite to that of the daf-16 mutant animals exposed under the same conditions (Fig. 1A). Because of this discrepancy in phenotypes exhibited by the m41 allele after Se(IV)-exposure, we tested an additional allele e1370 for its effect on movement behavior during the combined Se(IV) and heat-induced stress conditions. The e1370 allele was previously shown to be more resistant to the combined effects of oxidative stress and hyperoxia, a phenotype that was opposite to and suppressed by a mutation in the daf-16 gene (Honda and Honda, 2002). Here we similarly observed that the daf-2(e1370) animals were significantly and consistently more resistant to the Se(IV)-induced reductions in normal movement when compared to WT animals grown under the same conditions at 25 °C (#, e1370, Fig. 1B). Because the combined stressors of high heat and high dose Se(IV) exposure enhanced the phenotypes of the other IIS signaling component mutants, it is very likely that the phenotype expressed by the daf-2(e1370) allele under these conditions is more indicative of the phenotype that other daf-2 alleles (other than m41) would be expected to express.
3.4. Selenium induces nuclear translocation of DAF-16/FOXO3a
DAF-16 has been observed to translocate into the nucleus in response to starvation, heat shock, and oxidative stressors (Henderson and Johnson, 2001; Lin et al., 2001). Here we have shown that reduction in IIS signaling coupled with high dose Se(IV) exposure reduces the percentage of normal moving animals and that this can be enhanced when exposure occurs at an increased temperature. Since reduction in IIS signaling can activate DAF-16 and reduction of daf-16 function leads to increased Se(IV)-sensitivity (Fig. 1), we hypothesized that high dose Se(IV) exposure would lead to nuclear translocation of DAF-16. In order to investigate this, we examined a strain TJ356 that overexpresses the DAF-16a2 isoform linked to the green fluorescent protein (GFP) and which is driven by the daf-16 promoter (Henderson and Johnson, 2001). Under normal growth conditions (well-fed and grown at 20 °C) expression of this DAF-16::GFP reporter is diffuse and primarily localized to the cytoplasm (Fig. 2A), as was reported previously (Henderson and Johnson, 2001). Translocation of the DAF-16::GFP to the nucleus occurs with mild heat shock (34 °C for 30 min) (Henderson and Johnson, 2001; Lin et al., 2001). It was observed here that mild heat shock was capable of inducing nuclear translocation in adults as evidenced by the localized punctate expression of the GFP although some cytoplasmic localization was still observed (Fig. 2B). Our previous studies had shown that exposures to high dose Se(IV) could induce phenotypic changes in the normal movement and egg-laying behaviors of adult animals in as early as 6 h (Estevez et al., 2012; Morgan et al., 2010). Here we observed translocation of DAF-16::GFP to the nucleus within only 2 h after exposure to Se(IV) although cytoplasmic localization was still apparent (Fig. 2C). Henderson and Johnson (2001) similarly showed that the localization of DAF-16::GFP after exposure to the pro-oxidant juglone was not as strong as when animals were exposed to heat shock. Since we had shown that in WT animals coupling Se(IV) exposure with increased temperature could enhance the Se(IV)-induced reduction in normal movement (Fig. 1B; Morgan et al., 2010), we expected that exposing animals to Se(IV) for 2 h followed by mild heat shock would enhance the nuclear localization of DAF-16. As expected, when the 2 h Se(IV) exposure was followed by heat shock, the majority of the DAF-16 expression was observed to be located in the nucleus (Fig. 2D). This nuclear localization was stronger than when animals were exposed to mild heat shock (Fig. 2B) or Se(IV) alone (Fig. 2C). Thus, as predicted exposure to high dose Se(IV) levels lead to activation of DAF-16 similar to that previously observed to occur with reductions in IIS signaling. This activation could be enhanced by the additional stress of high temperature exposure as was observed for the movement behaviors.
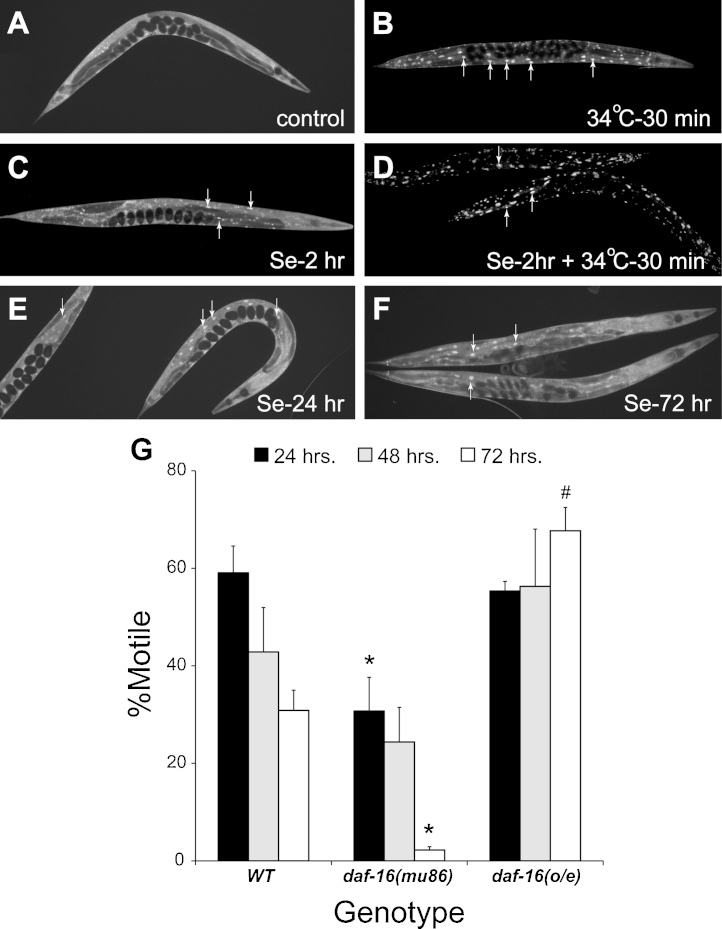
Fig. 2.
DAF-16 nuclear localization and dosage effects on motility with selenium exposure. Se(IV) induces DAF-16::GFP to translocate to the nucleus which is required for protection from the Se(IV)-induced effects on motility. (A–F) A strain (TJ356) containing a transgene (zIs356) that overexpresses wild-type DAF-16::GFP under control of the daf-16 promoter (Henderson and Johnson, 2001) was examined to determine its subcellular localization under various conditions. (A) Well-fed animals maintained at 20 °C show cytoplasmic expression of DAF-16::GFP. (B) Heat-shock-induced stress results in nuclear localization of DAF-16 (indicated by arrows) as previously described (Henderson and Johnson, 2001; Lin et al., 2001). (C) Se(IV) exposure induced DAF-16::GFP translocation after 2 h. (D) Exposure to Se(IV) for 2 h followed by heat shock increased nuclear translocation. (E and F) Longer exposures to Se(IV) (without heat shock) did not vastly increase localized GFP expression. All animals were maintained in a well-fed state at 20 °C and were exposed in the presence of food. Magnification = 100×. (G) In comparison to the WT strain, the presumptive null-mutation, daf-16(mu86) conferred significantly more sensitivity to the Se(IV)-induced effects on motility at 24 and 72 h [*, sensitivity to Se(IV), p = 8.0 × 10-3] while animals overexpressing DAF-16 [daf-16(o/e) = zIs356] were significantly more resistant at 72 h [# resistance to Se(IV); p = 4.3 × 10-6] by one-way ANOVA performed across each time point with significance determined by post hoc analysis using the Bonferroni–Holm method. Each graph bar represents at least three populations of 20 animals per strain (n = 60); error bars indicate SEM. Se = Se(IV).
3.5. DAF-16 is required to alleviate the selenium induced effects on normal movement
WT adult animals chronically exposed to high dose Se(IV) levels experienced behavioral deficits that increased with the time of exposure (Estevez et al., 2012; Morgan et al., 2010). Similarly, it was shown here that the percentage of normal moving animals continued to decrease over time when Se(IV)-exposure was coupled with IIS signaling reduction (Fig. 1A). Thus, it was expected that increasing the time of exposure to high dose Se(IV) would also increase the number of cells with activated DAF-16 that was localized to the nucleus. This was not the case. Instead increasing the exposure time to 24 (Fig. 2E) or 72 h (Fig. 2F) did not alter the expression pattern from that observed after the 2 h Se(IV) only exposure (Fig. 2C). One possible explanation for this unexpected result is that with continued exposure to Se(IV), a greater percentage of animals die or are significantly impaired (Morgan et al., 2010), thus only animals that are experiencing less Se(IV)-induced stress are able to survive the photo-documentation process. To circumvent this issue we examined the effect of high dose Se(IV)-exposure over time on animals experiencing different levels of DAF-16 expression and examined their motility (Fig. 2G). daf-16(mu86) is a deletion allele that is a presumptive null mutation (Lin et al., 1997). Animals with this mutation were significantly more sensitive to the effects of Se(IV) on movement than the animals with WT levels of DAF-16 at both 24 h and 72 h after Se(IV) exposure (*, Fig. 2G; Table 1). This was similar to the levels observed with the daf-16 rf allele, m26 (Fig. 1A). Thus, loss of DAF-16 leads to a predicted sensitivity to high dose Se(IV). In contrast, overexpression of DAF-16 [daf-16(o/e)] improves motility over time since significantly more animals are moving normally within this population than in populations of animals with WT levels of DAF-16 at the 72 h exposure time point (#, Fig. 2G). Thus, increasing the expression level of DAF-16 is capable of minimizing the Se(IV)-induced declines in normal movement observed over time with Se(IV)-exposure.
Table 1.
Motility of selenium exposed animals with tissue-specific daf-16 expression.
| Strain name | Genetic background | Transgene | Phenotype | Time (h) | Mean motility ± SEM (%) | p valuea against non-transgeneb | p valuea against daf-2 | p valuea against daf-16 |
|---|---|---|---|---|---|---|---|---|
| N2 | WT | None | Non-roller | 24 | 65.5 ± 5.5 | – | – | – |
| 48 | 42.8.0 ± 6.5 | – | – | – | ||||
| 72 | 32.0.0 ± 3.5 | – | – | – | ||||
| CB1370 | daf-2 (e1370) | None | 24 | 97.0 ± 1.2 | – | – | – | |
| 48 | 94.0 ± 1.0 | – | – | – | ||||
| 72 | 89.0 ± 2.4 | – | – | – | ||||
| CF1038 | daf-16 (mu86) | None | 24 | 39.6 ± 5.2 | – | – | – | |
| 48 | 23.8 ± 3.2 | – | – | – | ||||
| 72 | 6.1 ± 1.9 | – | – | – | ||||
| Nonec | daf-16;daf-2 | None | 24 | 24.0 ± 5.8 | – | 1.7 × 10-6 | NS | |
| 48 | 11.0 ± 4.8 | – | 1.6 × 10-7 | NS | ||||
| 72 | 1.0 ± 1.0 | – | 7.3 × 10-10 | NS | ||||
| TJ356 | WT | zIs356 (native) | Roller | 24 | 55.3 ± 2.0 | NS | – | – |
| 48 | 56.3 ± 11.8 | NS | – | – | ||||
| 72 | 67.7 ± 4.8 | 1.5 × 10-6 | – | – | ||||
| CF1442 | daf-16;daf-2 | muEx169 (nerve) | 24 | 53.1 ± 6.8 | 0.01 | 4.2 × 10-4 | NS | |
| 48 | 35.0 ± 7.0 | NS# | 4.3 × 10-5 | NS | ||||
| 72 | 23.1 ± 4.6 | 3.6 × 10-3 | 4.3 × 10-7 | 3.6 × 10-4 | ||||
| CF1514 | muEx211 (intestine) | 24 | 90.0 ± 2.0 | 1.7 × 10-8 | NS | 1.3 × 10-7 | ||
| 48 | 38.3 ± 6.4 | 0.01 | 3.7 × 10-5 | NS | ||||
| 72 | 19.4 ± 4.5 | 0.01 | 1.5 × 10-10 | 3.0 × 10-3 | ||||
| CF1515 | muEx212 (muscle) | 24 | 68.9 ± 2.9 | 4.5 × 10-6 | 1.4 × 10-5 | 1.4 × 10-4 | ||
| 48 | 13.3 ± 3.3 | NS# | 6.6 × 10-10 | NS | ||||
| 72 | 5.6 ± 2.3 | NS | 2.3 × 10-11 | NS |
NS = not significant (p > 0.01) as determined by one-way ANOVA with post hoc testing by the Bonferroni–Holm method. p-Values were for strain comparisons at the same time (h).
Non-transgene = the strain with the same genetic background as the strain that it is being compared to, but this strain differs because it does not express a transgene; e.g. TJ356 animals were compared to N2 since both strain have a WT genetic background, but TJ356 contains a transgene while N2 does not.
The daf-16;daf-2 animals used here were obtained by selecting non-roller adult animals from those strains expressing the daf-16-tissue specific extra-chromosomal arrays (CF1442, CF1514, CF1515). Animals that did not roll were no longer expressing the extra-chromosomal array and were used as a control for determining the effects of the double mutation on the Se(IV)-induced motility deficits.
p = 0.01when compared to the roller control strain (daf-16;daf-2;yzIs71; Fig. 3A, “#”).
3.6. Tissue-specific expression of DAF-16 partially rescues the daf-16-deficient selenium sensitivity
DAF-16 is natively expressed in all tissue types except some pharyngeal cells (Henderson and Johnson, 2001). The prior experiment demonstrated that overexpression of DAF-16 in native tissue is capable of partially alleviating the Se(IV)-induced effects on motility (Fig. 2G), but could not address which specific tissue, if any DAF-16 was exerting this effect. Previous studies showed that expression of DAF-16 in intestines could partially restore the reduced lifespan of daf-16(-) animals (Libina et al., 2003).
To determine whether expression of DAF-16 in specific tissues was capable of improving the movement of animals exposed to high dose Se(IV), we took advantage of the availability of strains CF1442, CF1514, and CF1515 (Table 1 and Section 2.1) which express GFP::DAF-16 under control of promoters for the nervous system (muEx169), intestines (muEx211), and muscles (muEx212), respectively (Libina et al., 2003). All of these strains were constructed to have the same genetic background and include the mutations daf-16(mu86) and daf-2(e1370). The presence of the mu86 mutation presumably eliminates any native DAF-16 expression since it is believed to be a null mutation in the daf-16 gene (discussed in Section 3.5). Thus, DAF-16 expression in each of these strains would be limited to the specific tissues controlled by the promoter regulating its expression. In addition, each of the transgenes also contain a reporter plasmid pRF4 expressing the mutation su1006, an allele of a collagen gene rol-6 which results in a dominant rolling (Rol) phenotype in the animals that express this mutation. Rolling movement is different than normal sinusoidal movement therefore we had to confirm that the Rol phenotype had no effect on the Se(IV)-induced movement deficits. To accomplish this we generated a strain (OM249; Section 2.1) that expresses a different transgene (yzIs71), but contains the same reporter plasmid pRF4, and is in the same genetic background daf-16(mu86);daf-2(e1370) as the strains expressing the tissue specific transgenes (Roller control: daf-16;daf-2;yzIs71; Fig. 3A). When compared to daf-16(mu86);daf-2(e1370) animals containing no transgene (Non-roller control; Fig. 3A), the OM249 animals (Roller control: daf-16;daf-2;yzIs71; Fig. 3A) were not significantly different (NS; Fig. 3A) over all the time points tested. In addition, there was also no difference between OM249 and OM285 (Section 2.1) a strain that was identical to OM249, but lacking the daf-2 mutation (Roller control: daf-16;yzIs71; Fig. 3A). These data suggest that the Rol phenotype did not affect the Se(IV)-induced movement deficits.
Next animals from each of the three tissue specific DAF-16 expression strains (CF1442, CF1514, CF1515; Rollers with tissue specific DAF-16::GFP expression: Fig. 3A; Table 1) were exposed to Se(IV) and compared at all the time points to both OM249 (Roller control: daf-16;daf-2;yzIs71; Fig. 3A) and the daf-16;daf-2 animals expressing no transgene (Non-roller control; Fig. 3A). All of the strains expressing the tissue specific transgenes showed significant improvement when compared to OM249 (#, Fig. 3A) as well as to the daf-16;daf-2 non-roller control animals (Table 1), but with a few exceptions (NS, p value against non-transgene, Table 1). The most notable improvement was observed at the 24 h exposure time point in animals expressing DAF-16 in the intestines (CF1514: Table 1). When the CF1514 animals were compared to either the daf-2 non-roller (CB1370, Table 1) or daf-2 roller animals (Roller control: daf-2;yzIs71; Fig. 3A), the CF1514 animals were not significantly different (NS; Table 1 and Fig. 3A). Both of these daf-2 strains exhibit the Se(IV)-resistance phenotype previously observed for the daf-2(e1370) allele (Fig. 1B) and do not have a mutation in the daf-16 gene, thus they express WT levels of DAF-16. This demonstrates that the Se(IV)-induced movement deficits observed in animals lacking DAF-16 could be fully suppressed at 24 h by intestinal expression of DAF-16 in a daf-16;daf-2 mutant background. After 24 h, intestinal suppression was still significantly improved, but the percentage of normal moving animals within the population trended downward (Fig. 3A). This trend downward was observed over time for all three of the DAF-16 tissue specific strains when exposed to Se(IV) and with the exception of the 72 h muscle specific expression, they all still maintained a significant improvement over the daf-16;daf-2 non-roller and roller control strains, (Fig. 3A and Table 1). Thus, expression of DAF-16 in the nerves, intestines, or muscles is capable of at least partially preventing the Se(IV)-induced effects on movement with the greatest improvement observed in the intestines at 24 h.
3.7. Selenium cause mitochondrial damage to muscles
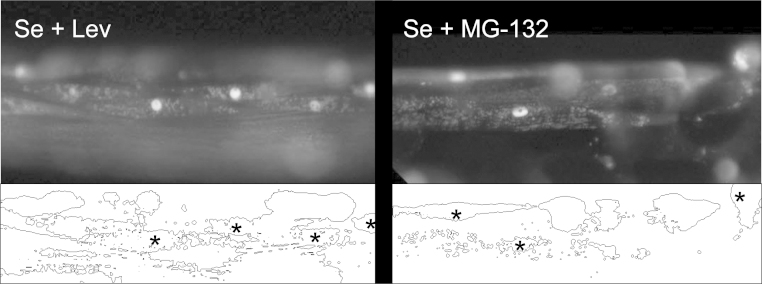
Muscle damage after exposure to high levels of Se is well documented in livestock and humans (Davis et al., 2012; Spiller and Pfiefer, 2007; Tiwary et al., 2006), but in C. elegans the body wall and egg-laying muscles were shown to be functionally and structurally intact after 48 h of exposure to high dose Se(IV) (Estevez et al., 2012). Yet, here we have demonstrated that muscle specific expression of DAF-16 can lead to partial rescue of normal movement (Fig. 3A) suggesting that Se(IV) does affect muscle in C. elegans. Recent studies have shown that activation of FOXO3a leads to ROS accumulation in the mitochondrial membrane which is followed by additional accumulation of FOXO3a in the nucleus and later more intense mitochondrial ROS accumulation culminating in apoptosis (Hagenbuchner and Ausserlechner, 2013). If a similar cascade of events is occurring in the muscle after Se(IV) exposure than it is possible that the reduction in normal movement observed after the 48 h time point in animals with DAF-16 muscle specific expression may be due to the accumulation of mitochondrial damage. To examine this possibility, we used a strain CB5600 which expresses GFP in the mitochondria and nuclei of muscle and exposed the animals for 48 h to Se(IV) (Fig. 3B). Since after the 48 h time point Se(IV)-exposed animals experienced increased lethality and fragility, it was not possible to examine these older animals. At 48 h, the mitochondria of the water treated age-matched controls exhibited normal tubular shaped expression (H2O, Fig. 3B) while the GFP expression of the mitochondria in Se(IV) exposed animals was observed to be patchy and disorganized an indication that fragmentation of the mitochondrial network had occurred (Se, Fig. 3B). Although levamisole and MG-132 (Z-Leu-Leu-Leu-CHO) have been shown to block proteasome mediated degradation in muscle (Muñoz-Lobato et al., 2013; Estevez et al., 2012), they were not able to here block the Se(IV)-induced mitochondrial fragmentation (Supp. Fig. 1). These data demonstrate that Se(IV) exposure is capable of inducing mitochondrial damage to muscle and that this damage is not prevented by proteasomal blockade.
3.8. DAF-16 prevents motor neuron degeneration
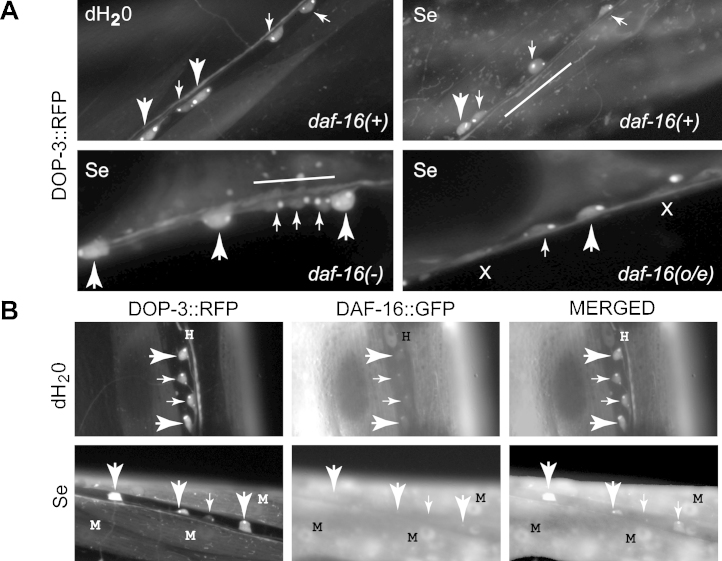
Because expression of DAF-16 in neurons partially rescued the Se(IV)-sensitivity phenotype of daf-16(mu86), we examined whether this partial rescue was due to activation of DAF-16 in the motor neurons specifically. In animals expressing vsIs33, cholinergic motor neurons weakly express RFP making them easily identifiable from the GABAergic motor neurons which strongly express RFP under control of the promoter for the dop-3 gene which encodes for the C. elegans ortholog of the mammalian D2 dopamine receptor (Chase et al., 2004). In animals expressing dop-3::rfp in a WT daf-16 background [daf-16(+); Fig. 4A], Se(IV)-exposure induced the cholinergic motor neurons (small arrows, Fig. 4) to round and lose their fusiform shape, and blebbing of the ventral cord (white lines, Fig. 4A), as had been previously described (Estevez et al., 2012). Reduction in GABAergic signaling through the UNC-49 receptor was shown to decrease the percentage of forward moving animals after Se(IV)-exposure (Estevez et al., 2012) and here we show that the GABAergic motor neurons (large arrows, Fig. 4) showed signs of neurodegeneration similar to that observed in the cholinergic motor neurons (small arrows, Fig. 4). Loss of DAF-16 [daf-16(-); Fig. 4A] seemed to enhance these Se(IV) induced effects. The over-expression of DAF-16 [daf-16(o/e); Fig. 4A] under control of the daf-16 promoter was partially protective against these changes since axonal blebbing was reduced, as was cell-rounding although evidence of cell loss was still apparent (x, Fig. 4A). Cytoplasmic and nuclear localization of DAF-16::GFP has been reported in the neurons of daf-16(+) animals grown under standard conditions (Henderson and Johnson, 2001) and specifically in the ventral cord neurons of dauer larvae (Lin et al., 2001). In the animals overexpressing DAF-16, the protein did not appear to be localized to the nucleus in the motor neuron cells (compare dH2O and Se, Fig. 4B), but was expressed in the nuclei of muscle cells (M, Fig. 4B) after Se(IV) exposure. Together, these data demonstrate that although Se(IV) does not cause detectable nuclear translocation of the DAF-16::GFP within motor neurons, some of the neurotoxic effects induced by Se(IV) on motor neurons can be ameliorated by increased global expression of DAF-16.
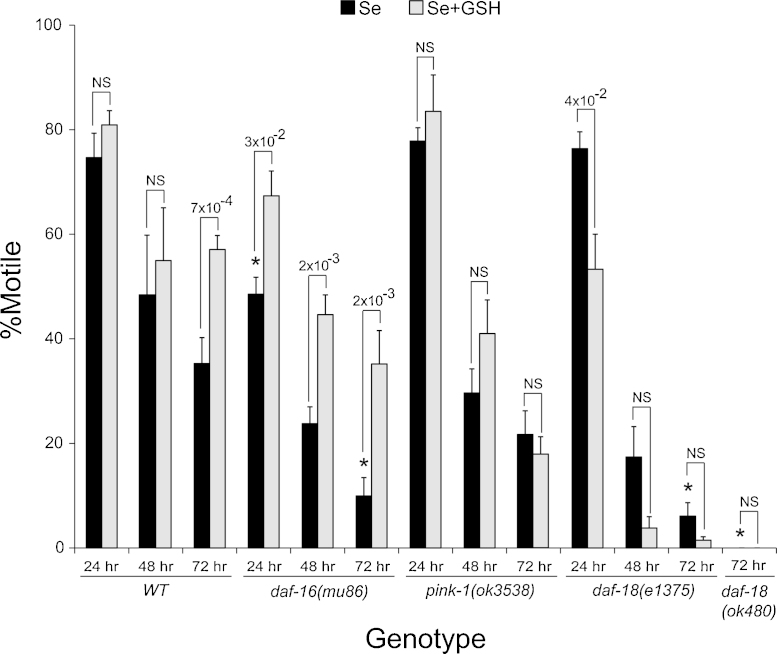
Fig. 4.
Selenium exposure and the dosage effects of DAF-16 on neurodegeneration. The level of DAF-16 expression affects the observed levels of neurodegeneration in the ventral cord. (A) The DOP-3::RFP construct (vsIs33) expresses in both cholinergic (small arrows) and GABAergic (large arrows) motor neurons, but more weakly expresses in the former than the latter (Chase et al., 2004). In animals with WT levels of DAF-16 [daf16(+) = strain OM261], the normal fusiform shape of the motor neuron cell bodies observed in the control animals [daf-16(+), dH2O] is altered in animals exposed to Se(IV) [daf-16(+), Se]. Axonal blebbing of the ventral cord (line) is also observed with Se(IV) exposure, but not in the control animals; as was previously reported (Estevez et al., 2012). With loss of DAF-16 [daf-16(-) = strain OM325], neuronal damage is increased. Over-expression of DAF-16 [daf-16(o/e) = strain OM324] reduced the blebbing in the cord as well as cell body rounding. Cell loss was still observed in the DAF-16 over-expressing animals. This had been shown previously to occur in WT animals exposed to Se(IV) (Estevez et al., 2012). (B) Se(IV) does not induce DAF-16::GFP (zIs356) localization to the nucleus in either the cholinergic (small arrows) or GABAergic motor neurons (large arrows) of adult animals (strain = OM324), but was observed in muscle cells (M). Nuclear localization of DAF-16 in the hypodermal cells (H) of animals not exposed to Se(IV) (dH2O) is most likely due to stress induced on the animals which were alive, but paralyzed while being imaged, as previously observed (Lin et al., 2001). Magnification = 400×. Se = Se(IV). The genotype of each strain is listed in Section 2. Animals shown here are representative of 30–50 animals examined under.
3.9. Mutation in the DAF-16 target gene sod-3 leads to selenium-resistance
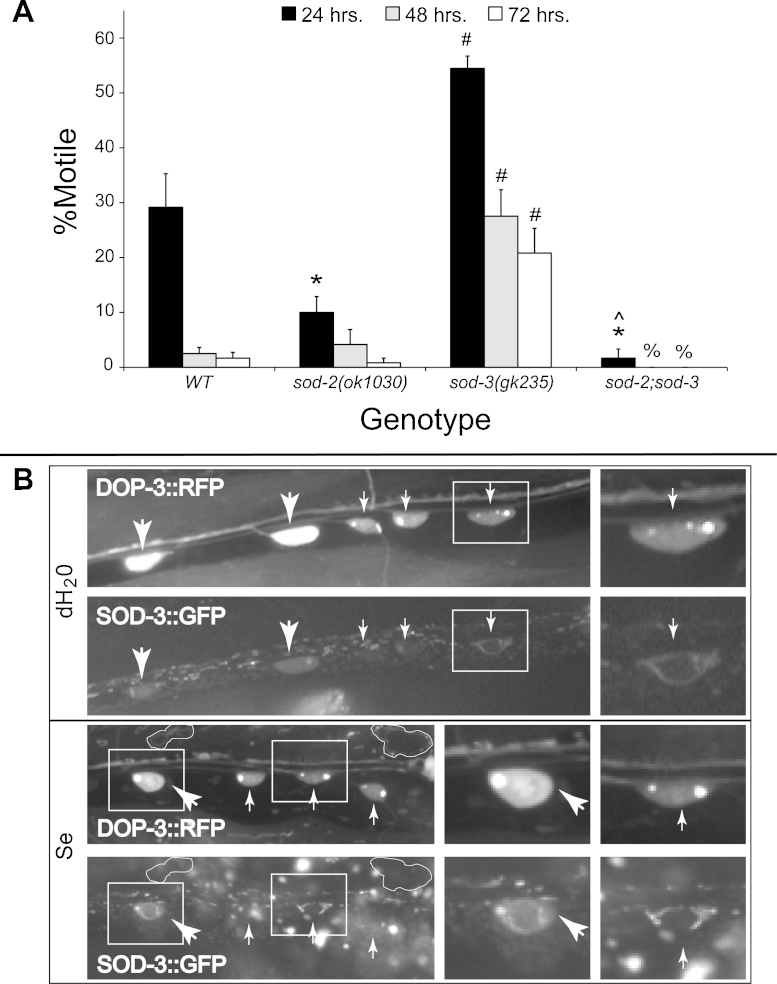
The superoxide dismutases (SOD) are required for oxidative stress responses in many organisms including C. elegans (Honda et al., 2010; Doonan et al., 2008). There are three general types of SOD: copper/zinc (Cu/Zn SOD or SOD1), manganese (Mn-SOD or SOD2), and extracellular (EC-SOD or SOD3) (Miao and St Clair, 2009). Deletion of SOD2 in mice leads to progressive motor weakness and neurodegeneration (Lebovitz et al., 1996). In addition, SOD2 has been implicated in mitochondrial dysfunction and examined for its role in models of neurodegenerative diseases including stroke, ALS, and Parkinson's, Alzheimer's, and Huntington's Diseases (Flynn and Melov, 2013; Maier and Chan, 2002; Andreassen et al., 2000). In C. elegans, there are two Mn-SODs encoded by the sod-2 and sod-3 genes (Hunter et al., 1997) which have been shown to be transcriptionally regulated by DAF-16 (Cabreiro et al., 2011; Oh et al., 2006). Knockout/reduction mutations in the sod-3 gene typically have no effect or are sensitivity to oxidative stressors such as paraquat and juglone (Back et al., 2012), but have been shown to be more resistant to cadmium toxicity (Roh et al., 2009); similar results have been reported for sod-2 mutations in regards to stress (Back et al., 2012). When exposed to high dose Se(IV), animals with a mutation in sod-2 were more sensitive after 24 h of exposure in comparison to WT animals (p = 3.8 × 10-3, Fig. 5A, *) while those with a mutation in sod-3 exhibited resistance across all time points tested (p = 0.02; Fig. 5A, #) with regards to their movement phenotype. The Se(IV)-resistance exhibited by the sod-3 mutant animals was suppressed by the sod-2 mutation in sod-2;sod-3 double mutant animals, and the double mutant strain was more sensitive to Se(IV) than was sod-2 alone (p = 4.2 × 10-4, compared at the 24 h time point; Fig. 5A). Increased sensitivity for sod-2;sod-3 double mutant animals was previously reported for stress induced by paraquat-hyperoxia and juglone treatments (Back et al., 2012). Thus, loss of sod-3 or sod-2 had opposite phenotypes in regards to their response to high dose Se(IV)-exposure while the sod-2;sod-3 double mutant exhibited a phenotype similar to the sod-2 mutation alone.
Fig. 5.
The effects of mutations on downstream target genes of DAF-16 transcription. C. elegans encodes for two superoxide dismutase 2 (SOD2) genes which are regulated by DAF-16 and have opposite effects on the Se(IV)-induced movement deficits. (A) Animals with a mutation in the sod-2 gene are initially more sensitive to the effects of Se(IV) than WT animals (at 24 h: p = 3.8 × 10-3 by one-way ANOVA), but at later time points are no different than WT animals (p = 0.5) while animals with a sod-3 mutation are consistently and significantly more resistant to Se(IV)’s effect on movement than WT (p = 0.03 by one-way ANOVA across all time points tested). The sod-2;sod-3 double mutant animals are phenotypically similar to the sod-2 single mutant animals, the animals are initially more sensitive than WT to Se(IV) at 24 h (p = 4.3 × 10-4 by one-way ANOVA), but are no different at the later time points (p > 0.1). Animals were grown at 25 °C, but the trends and significances were the same for animals grown at 20 °C (data not shown). (B) Animals of the strain OM261 which expresses DOP-3::RFP and over-expresses a SOD-3::GFP translational fusion under control of its native promoter (wuIs56) were exposed to either water (dH2O) or Se(IV) (Se) for 24 h. The SOD-3::GFP was observed to co-localize with DOP-3::RFP to the ventral cord and the motor neurons (large arrows = GABAergic; small = cholinergic), but was unable to protect either from the Se(IV)-induced neurodegenerative effects. This SOD-3::GFP expression is discontinuous in the Se(IV)-exposed motor neurons (Se, SOD-3::GFP, right panels) in comparison to the water exposed ones (H2O, SOD-3::GFP, right panel) suggesting that mitochondrial fragmentation has occurred as was observed in the Se(IV)-exposed muscle (Fig. 3B). In the Se(IV) exposed animals, muscles are observed to exhibit increased expression of DOP-3::RFP that did not colocalize with the SOD-3::GFP expression (Se, white outline) and was not observed in the water controls. Smaller panels on the right focus in on the motor neurons that are boxed in the larger image immediately to their left. Magnification = 400×. Se = Se(IV).
Since animals with loss of SOD-3 expression are resistant to the movement deficits normally elicited by high dose Se(IV) exposure, we hypothesized that SOD-3 overexpression would not protect motor neurons from Se-induced neurodegeneration. To determine this we examined the ventral cord of animals overexpressing SOD-3 translationally fused to GFP (Fig. 5B). These animals coexpressed the dop-3::rfp transgene examined previously (Fig. 4) allowing for ease in identifying the GABAergic (large arrows, Fig. 5B) and cholinergic (small arrows, Fig. 5B) motor neurons. SOD-3::GFP expression was observed in both types of motor neurons and along the ventral cord in the mock-exposed animals (dH2O, Fig. 5B). After 24 h of exposure to Se(IV), the SOD-3::GFP expression pattern appeared fragmented within the motor neurons (Se, Fig. 5B, larger images and smaller panels to the right). Since SOD-3 was shown to co-localize with mitochondrial staining (Wolf et al., 2008), this fragmentation pattern most likely reflects mitochondrial fragmentation similar to that observed in the muscle (Fig. 3B). In addition, signs of degeneration including rounding of the motor neurons and blebbing of the ventral cord were observed with Se(IV) exposure (Se, Fig. 5B). Thus, as predicted by the loss-of-function sod-3 phenotype, SOD-3 overexpression did not prevent the neurodegeneration previously observed to occur in the GABAergic (Fig. 4) and cholinergic (Fig. 4; Estevez et al., 2012) motor neurons after high dose Se(IV)-exposure.
3.10. Selenium exposure increases expression of the DOP-3/D2 dopamine receptor in muscle
Muscles expressing the DOP-3::RFP and exposed to Se(IV), showed increased expression levels of DOP-3::RFP not observed in the muscles of water exposed animals (Fig. 5B; compare DOP-3::RFP, dH2O to Se). This expression did not appear to co-localize with the SOD-3::GFP expression [Fig. 5B, outlined regions in Se(IV)-exposed animals]. Expression of DOP-3::RFP in muscles has been previously reported as weak (Chase et al., 2004). No previous studies to our knowledge have described DOP-3 expression patterns in muscles under stress. Here we demonstrate that stress induced by high dose Se(IV) exposure increased expression of DOP-3 in the muscle.
3.11. Deletion of daf-18/PTEN leads to selenium sensitivity
Mutations in PTEN (phosphatase and tensin homolog deleted on chromosome 10) and PINK1 (PTEN-induced putative kinase 1) have been linked to neurodegenerative disease processes affecting ALS, Parkinson's and Alzheimer's Diseases (Domanskyi et al., 2011; Kirby et al., 2011; Wilhelmus et al., 2011; Morimoto et al., 2010; Sonoda et al., 2010; Valente et al., 2004). PTEN has been shown to regulate neuronal outgrowth and induces apoptotic cell death in neurons (Wong et al., 2013; Christensen et al., 2011) by inhibiting PI(3)K-dependent activation of AKT (Stambolic et al., 1998) allowing for the activation of FOXO transcription. Conversely, PINK1 is required for the IIS dependent activation of AKT (Akundi et al., 2012) which leads to the phosphorylation inactivation of FOXO. Because of their opposite roles in regulating FOXO transcription, we hypothesized that mutations in the C. elegans orthologs of PTEN and PINK1, daf-18 and pink-1, respectively, would have opposite phenotypes in regards to their responsiveness to high dose Se(IV) exposure: daf-18/PTEN mutants would be expected to express a sensitivity phenotype similar to that observed with loss or reduction of daf-16 while PINK1 mutants should be more resistant to Se(IV). As expected, animals with the daf-18 mutations e1375(rf) and ok480(lf) were both more sensitive although this sensitivity was not observed until 72 h after exposure to high dose Se(IV) (*, Fig. 6). This sensitivity phenotype was similar to previous studies in which it was demonstrated that genetic or RNA inhibition of daf-18 resulted in increased sensitivity to the oxidative stressor paraquat (Masse et al., 2008; Honda and Honda, 2002). Animals with a mutation in the pink-1(ok3538) were no more sensitive or resistant to Se(IV) than was WT (Fig. 6). Thus, although PINK-1 does not appear to be required for Se(IV) to induce its neurotoxic effects on movement in C. elegans, loss of daf-18 does increase sensitivity to Se(IV) suggesting that it plays a protective role against Se(IV)-induced toxicity.
Fig. 6.
The cellular antioxidant glutathione and its effect on modulators and a downstream regulator of IGF signaling when exposed to selenium. Animals containing mutations in the genes encoding the C. elegans orthologs of PTEN (phosphatase and tensin homolog, daf-18) and PINK1 (PTEN-induced putative kinase, pink-1), modulators of the IGFR signaling are not rescued from the Se(IV)-induced motility defects by exposure to the cellular antioxidant glutathione (GSH), but the Se(IV)-sensitivity observed with the daf-16(mu86) mutation was rescued. All the strains were sensitive to Se(IV), but increased sensitivity to Se(IV) (*, p = 0.05) was not observed until 24 h of exposure in the animals with a mutation in daf-16, and at 72 h in the daf-16 and daf-18(e1375 and ok480) mutant animals when compared to wild-type (WT). Analysis was by one-way ANOVA performed across each time point [significance (indicated above bar graphs) was determined by post hoc analysis using the Bonferroni–Holm method; NS = not significant]. Glutathione was able to significantly rescue the Se(IV)-induced deficits in WT animals after 72 h of continuous exposure (significance is indicated above each pair of bars and was determined by Student's t-test). When not exposed to Se(IV), all strains had =95% motility at each time point and temperature tested (data not shown). The addition of GSH [without Se(IV)] did not alter motility in comparison to the control (water only exposed) animals (data not shown). Each bar graph represents the average of three populations (n = 60) with error bars indicating SEM. Se = Se(IV).
3.12. Glutathione requires DAF-18 and PINK-1 to ameliorate the selenium-induced movement deficits
The cellular antioxidant reduced glutathione (GSH) plays an essential role in protecting against the damaging effects ROS. In C. elegans, exogenous GSH has been shown to reduce oxidative stress and to partially rescue the movement and egg-laying deficits caused by Se(IV)-exposure in WT animals (Estevez et al., 2012; Morgan et al., 2010). Previously, we showed the dithiol glutaredoxin, GLRX-21 was required for GSH to exert these protective effects (Estevez et al., 2012; Morgan et al., 2010). When daf-16(mu86) animals were concomitantly exposed to Se(IV) and GSH, they responded no differently than WT, i.e. GSH was able to partially rescue the movement deficits observed with Se(IV) exposure alone at all the time points tested (Fig. 6) as was previously shown for WT animals (Morgan et al., 2010). This improvement was observed across all the time points tested for the daf-16 lf animals. Although the improvement observed in WT animals was only significant at the 72 h time point here, we had previously observed differences at both 24 and 48 h depending on dosage (Morgan et al., 2010). Thus, this data suggests that DAF-16 is not required for GSH to provide protection from high dose Se(IV) exposure.
Hydrogen peroxide induced oxidation of PTEN has been shown to be reduced by glutathione in yeast and human cells (Kim et al., 2011) while oxidative stress significantly increased levels of GSH in cells from patients with a missense mutation in PINK1 in comparison to controls (Grünewald et al., 2009). When animals with the pink-1 or daf-18 mutations were treated with GSH, they did not respond favorably; none of these animals showed improved percentages of animals moving normally after treatment (Fig. 6). In fact when both sets of animals were exposed to Se(IV), at 24 h the GSH-treated daf-18(e1375) animals showed a significant reduction in the percentage of animals moving normally in comparison to animals not treated with GSH (Fig. 6). At later time points, both daf-18 mutant animals showed a trend downward in response to GSH treatment although it was not significant (Fig. 6). Thus, both PTEN and PINK1 appear to be required for GSH to ameliorate the Se(IV)-induced motility deficits.
4. Discussion
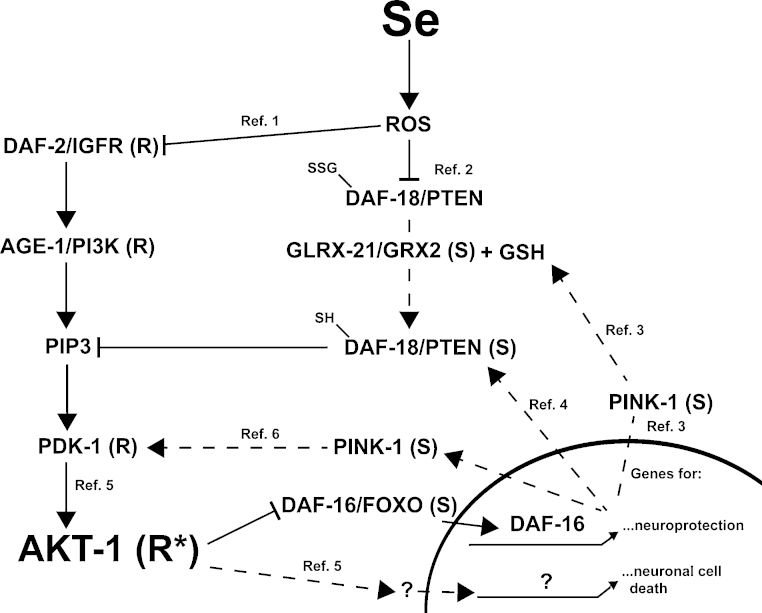
We have shown previously that exposures to high levels of selenium [Se(IV)] in the environment of adult animals can induce neurodegeneration and cell loss resulting in motor deficits and death (Estevez et al., 2012; Morgan et al., 2010) and that this is at least partially caused by a reduction in cholinergic signaling across the neuromuscular junction (Estevez et al., 2012). Here we provide evidence that a reduction in the insulin/insulin-like (IIS) stress response pathway regulates the response to high dose levels of environmental selenium which in turn regulates the IIS pathway. Most specifically we show that nuclear localization and thus activation of the DAF-16/forkhead box transcription factor occurs in response to Se(IV) exposure although this was not observed in motor neurons of the ventral cord. Yet, tissue specific expression and generalized overexpression of DAF-16 can partially rescue the neurodegenerative and behavioral deficits observed with high dose Se(IV) exposures. In addition, we determined that two modifiers of AKT activation, PINK1 and PTEN are required for the cellular antioxidant glutathione to mitigate the Se(IV)-induced movement deficits, previously shown to also require the dithiol glutaredoxin, GLRX-21 (Morgan et al., 2010). The data presented here and previously are summarized in Fig. 7.
Fig. 7.
Summary model of the effects of sodium selenite in C. elegans. Adult animals with mutations in components of the DAF-2/IGFR signaling cascade responded to high dose Se(IV) within their environment similar to that observed when exposed to other stressors (Honda and Honda, 2002), i.e. the loss- or reduction-of-function daf-16 alleles had an opposite phenotype from that of many of the upstream components that inhibit its nuclear translocation. Yet, although the pattern was predictable, the specific sensitivity or resistance phenotypes were not. A model depicting the effects of Se(IV) on the IGFR signaling cascade including modulatory branches is shown and summarizes the data presented here as well as previously by us (Morgan et al., 2010), and elsewhere (Luo et al., 2013; Akundi et al., 2012; Ponugoti et al., 2012; Kim et al., 2011; Loh et al., 2009; Mei et al., 2009; Budovskaya et al., 2008). The Se(IV) (selenite) responsive phenotypes, listed in parentheses as R (resistant) or S (sensitive), are based on the phenotypes of the reduction-of-function mutants. *R, resistance phenotype of reduction-of-function mutant is assumed since the mg144 gain-of-function mutation is sensitive (Fig. 1); Dotted lines represent pathways that are assumed based on data observed in other organisms, but not confirmed in C. elegans. IGFR = insulin/insulin growth factor-like receptor; PI3K = phosphatidylinositide 3-kinase; FOXO = forkhead box transcription factor; PTEN = phosphatase and tensin homolog; GSH = reduced glutathione; GSSG = oxidized glutathione; Ref. 1 = Ponugoti et al. (2012); Ref. 2 = Kim et al. (2011), Loh et al. (2009); Ref. 3 = Mei et al. (2009); Ref. 4 = Luo et al. (2013); Ref. 5 = Budovskaya et al. (2008); Ref. 6 = Akundi et al. (2012).
Overexpression of DAF-16 under native promoter control leads to the partial rescue of the Se(IV)-induced effects on movement and neurodegeneration (Table 1 and Fig. 2G; Fig. 4A) while loss of daf-16 had the opposite effect on both phenotypes (Table 1; Fig. 2G). This suggests a neuroprotective role for DAF-16 during Se(IV)-induced toxicosis in C. elegans (Fig. 7). Gene expression through activation of DAF-16 prevents ROS-induced neuronal degeneration (Calixto et al., 2012), as well as protects from neurodegeneration in worm models of Huntington's (Parker et al., 2012), and motor neuron diseases (Mojsilovic-Petrovic et al., 2009). Yet, surprisingly neuron-specific expression of DAF-16 did not fully rescue the movement deficits (Fig. 3A). Limiting expression of DAF-16 to the intestines on the other hand fully rescued the movement deficits, but only for the first 24 h after exposure (Fig. 3A). If DAF-16 can function non-cell autonomously as previously suggested (Iser et al., 2007; Libina et al., 2003), then it is possible that intestinal expression of DAF-16 could result in secretion of a diffusible factor that activates DAF-16 within the motor neurons preventing the movement deficits normally observed with Se(IV) exposure (Morgan et al., 2010). Yet, the inability to detect DAF-16 nuclear localization in motor neurons (Fig. 4B) after Se(IV) exposure although localization in other cell types was observed (Fig. 2C–F; Fig. 4B) does not seem to support this notion, but suggests another possibility. Activation of DAF-16 in hypodermal tissue was observed by Qi et al. (2012) to affect germ-line proliferation without activating DAF-16 in the gonad, thus DAF-16 activation in the intestine may similarly non-autonomously protect the motor neurons without activating DAF-16 in these neurons. Our observation that the level of DAF-16 expression could affect the amount of damage to the motor neurons and the ventral cord with Se(IV)-exposure (Fig. 4A) without inducing detectable translocation of DAF-16 into the neurons (Fig. 4B) seems to support this hypothesis.
The sod-3 gene is reported to be a direct target of the DAF-16 transcriptional activation (Oh et al., 2006) and is required by DAF-16 for oxidative stress response (Honda et al., 2010). Yet, if activation of the sod-3 gene occurred in response to DAF-16 transcription, than sod-3 mutant animals would be expected to have the same phenotype as the daf-16(-) animals in response to Se(IV)-exposure. We did not observe this to be the case since the sod-3 mutant animals were resistant to Se(IV) while the daf-16 mutant animals were more sensitive (compare Fig. 5A to Figs. 1A and 2G). In addition, overexpression of SOD-3 did not appear to prevent the Se(IV)-induced neurodegeneration in the motor neurons (Fig. 5B) although overexpression of DAF-16 did partially rescue this effect (Fig. 4A). Given this result it seems unlikely that sod-3 expression is activated by DAF-16 in response to high dose Se(IV) exposures. In addition, transcriptional repression of sod-3 expression by DAF-16 is unlikely since SOD-3 expression was observed in both the GABAergic and cholinergic motor neurons (Fig. 5B) and DAF-16 was not (Fig. 4B). Thus, the data presented here would suggest that SOD-3 expression in the motor neurons is regulated by a transcription factor other than DAF-16. Indeed SOD-3 expression in other cells has been reported to be regulated by the GATA transcription factor ELT-3 (Budovskaya et al., 2008). Further studies aimed at examining motor neuron expression of SOD-3::GFP in the background of animals with mutations in other transcription factors would address this issue, but is outside the scope of this paper.
PINK1 and PTEN have both been shown to be induced by FOXO3a transcription (Luo et al., 2013; Mei et al., 2009) and although it is possible that such an interaction occurs in worms, it has not been demonstrated. Here we report that loss or reduction of either daf-18/PTEN or pink-1 results in a decreased percentage of normal moving animals when either strain is exposed to Se(IV). Yet although the daf-18/PTEN animals were significantly more sensitive to Se(IV) than was WT [i.e. daf-18 mutant animals were Se(IV)-sensitive], the pink-1 animals were no worse than WT (Fig. 6). The similarity of the phenotypes displayed by the daf-18/PTEN and the daf-16 mutant animals in response to Se(IV) exposure suggests the possibility that daf-18 is transcriptionally regulated by daf-16 (Fig. 7) although further studies would need to be performed to confirm this. PINK1 was initially identified as a PTEN inducible kinase (Valente et al., 2004) which was more recently shown to be regulated by PTEN through its inhibition of AKT signaling and subsequent activation of FOXO3a transcription of the PINK1 gene (Mei et al., 2009). In addition to being induced by PTEN, PINK1 has also been shown to induce AKT phosphorylation and the subsequent inactivation of FOXO3a (Fig. 7) (Akundi et al., 2012). Thus, loss of PINK1 in C. elegans may not induce an increased Se(IV)-sensitivity phenotype simply because phosphorylation of AKT by PINK1 can be achieved by other kinases including PDK-1 and mTOR (Sánchez-Mora et al., 2012; Murata et al., 2011; Maj et al., 2010; Paradis et al., 1999).
The loss of pink-1 or daf-18/PTEN was here shown to produce a GSH insensitivity phenotype (Fig. 6) which had only previously been observed by loss of GLRX-21, the glutaredoxin 2 (GRX)2-like protein (Estevez et al., 2012; Morgan et al., 2010). ROS has been demonstrated to oxidize and thus inactivate PTEN and this process requires both GRX and GSH to be reduced (Kim et al., 2011). Thus, a similar mechanism may occur in C. elegans accounting for the GSH insensitivity phenotype of the daf-18 mutants (Fig. 7). Loss of PINK1 was observed to lead to a dramatic decrease in GSH levels (Mei et al., 2009), as well as an increase in the activity of the enzyme glutathione S-transferase (GST) (Hoepken et al., 2007). GST has been shown to increase in response to high levels of sodium selenite (Sun et al., 2013; Zhang et al., 2008). Thus, the inability of exogenous GSH to improve movement in the pink-1 mutant animals may reflect an increase in catabolism of GSH through the increased activities of GST. Further studies examining the GST levels in both WT and pink-1 mutants would be able to address this issue in more depth.
Selenite can induce mitochondrial fragmentation in glioma cells (Kim et al., 2007) and was shown here to induce mitochondrial fragmentation in muscles and neurons (Figs. 3B and 5B, respectively). Overexpression of Mn-SOD was shown to prevent this selenite-induced mitochondrial damage in the glioma cells, but in C. elegans overexpression of the SOD-3/Mn-SOD protein did not (Fig. 5B). The increased expression of the D2-like dopamine receptor DOP-3 observed in muscle after Se(IV) exposure (Fig. 5B) might represent an attempt to alter the mitochondrial damage induced by Se(IV) even though the DOP-3::RFP was not observed to co-localize with the SOD-3 expression in the mitochondria (compare outlines Fig. 5B). Examining the effects of D2 receptor activation in a Parkinson's model, Chau et al. (2009) showed receptor activation improved mitochondrial membrane potential and up-regulated intracellular levels of GSH. Thus the upregulation of DOP-3 observed in the muscle may be an attempt to stabilize the mitochondria and prevent mitochondrial damage. This question could not be directly addressed within the context of these studies since the DOP-3 construct used was a promoter fusion only and not a rescuing clone (Chase et al., 2004).
5. Conclusion
Here we present more extensive evidence that high dose exposures to sodium selenite can induce neurodegeneration and demonstrate damage of both the cholinergic and GABAergic motor neurons in the ventral cord. Reductions in both cholinergic and GABAergic signaling were previously shown to alter normal movement. Similarly, movement deficits were observed to occur with reductions in insulin/insulin-like signaling that are known to activate DAF-16/forkhead box-3a and increased expression of DAF-16 was shown to both improve movement and reduce neurodegeneration. This suggests that DAF-16 alters expression of genes that are neuroprotective against the toxic insult of high dose selenium exposure. Two gene products that are known to be regulated by forkhead box transcription, PINK1 and PTEN were shown here to be required for the cellular anti-oxidant reduced glutathione to reduce the neurodegenerative effects on movement while overexpression of a third gene, sod-3 known to be regulated in C. elegans by DAF-16 did not alter the neurodegenerative process. Taken together these data suggest a link between toxicant exposures and complex regulation of signaling processes that can alter the course of neurological decline. That environmental exposures can lead to ALS or other neurological diseases is suspected through previous observations, and this model of selenium-induced neurodegeneration developed in a genetically tractable organism provides a tool for examining the combined roles of genetics and environment in neuro-pathologic disease progression.
Conflict of interest statement
None declared.
Acknowledgements
This research was supported by grants from the Jim Himelic Foundation (ME), Southern Arizona Foundation (ME), Keatings Institute (ME), National Institutes of Environmental Health Sciences [R21-ES012305 to AE and ME], and a Career Development Award [ME] provided through the Office of Research and Development, Department of Veterans Affairs. Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources (NCRR) and the International C. elegans Gene Knockout Consortium.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Contributor Information
Annette O. Estevez, Email: neurometrica@gmail.com, annette_estevez@yahoo.com.
Kathleen L. Morgan, Email: kathleen@immunetrics.com.
Nathaniel J. Szewczyk, Email: mbzns3@exmail.nottingham.ac.uk.
David Gems, Email: david.gems@ucl.ac.uk.
Miguel Estevez, Email: m_estevez@yahoo.com.
Appendix A. Supplementary data
The following are the supplementary data to this article:
Supplemental Fig. I.
Inhibition of proteasome does not prevent Se(IV)-induced damage to mitochondria. Upper panel: Animals of the strain CB5600 transiently express mitochondrial GFP in their muscles that became fragmented after Se(IV) exposure for 48 h (Fig. 1). This damage could not be prevented by inhibition of proteasomal degradation with either levamisole (Se + Lev) or MG-132 (Se + MG-132) when adult animals were exposed simultaneously to Se(IV) and either compound for 48 h. Lower panel: Black and white outline of GFP expression patterns above, nuclei are represented by asterisks for orientation. Lev = 1 mM levamisol; MG-132 = 200 µM Z-Leu-Leu-Leu-CHO (MG-132). Se = Se(IV).
References
- Ackerman D., Gems D. Insulin/IGF-1 and hypoxia signaling act in concert to regulate iron homeostasis in Caenorhabditis elegans. PLoS Genet. 2012;8(3):e1002498. doi: 10.1371/journal.pgen.1002498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akundi R.S., Zhi L., Büeler H. PINK1 enhances insulin-like growth factor-1-dependent Akt signaling and protection against apoptosis. Neurobiol Dis. 2012;45(1):469–478. doi: 10.1016/j.nbd.2011.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessi D.R., Andjelkovic M., Caudwell B., Cron P., Morrice N., Cohen P. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15(23):6541–6551. [PMC free article] [PubMed] [Google Scholar]
- Altun Z.F., Hall D.H. WormAtlas. 2011. Nervous system, general description. [Google Scholar]
- Andreassen O.A., Ferrante R.J., Klivenyi P., Klein A.M., Shinobu L.A., Epstein C.J. Partial deficiency of manganese superoxide dismutase exacerbates a transgenic mouse model of amyotrophic lateral sclerosis. Ann Neurol. 2000;47(4):447–455. [PubMed] [Google Scholar]
- Back P., Braeckman B.P., Matthijssens F. ROS in aging Caenorhabditis elegans: damage or signaling? Oxid Med Cell Longev. 2012;2012:608478. doi: 10.1155/2012/608478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthel A., Schmoll D., Unterman T.G. FoxO proteins in insulin action and metabolism. Trends Endocrinol Metab. 2005;16(4):183–189. doi: 10.1016/j.tem.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Boccitto M., Lamitina T., Kalb R.G. Daf-2 signaling modifies mutant SOD1 toxicity in C. elegans. PLoS One. 2012;7(3):e33494. doi: 10.1371/journal.pone.0033494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehler C.J., Raines A.M., Sunde R.A. Deletion of thioredoxin reductase and effects of selenite and selenate toxicity in Caenorhabditis elegans. PLoS One. 2013;8(8):e71525. doi: 10.1371/journal.pone.0071525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budovskaya Y.V., Wu K., Southworth L.K., Jiang M., Tedesco P., Johnson T.E. An elt-3/elt-5/elt-6 GATA transcription circuit guides aging in C. elegans. Cell. 2008;134(2):291–303. doi: 10.1016/j.cell.2008.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabreiro F., Ackerman D., Doonan R., Araiz C., Back P., Papp D. Increased life span from overexpression of superoxide dismutase in Caenorhabditis elegans is not caused by decreased oxidative damage. Free Radic Biol Med. 2011;51(8):1575–1582. doi: 10.1016/j.freeradbiomed.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caito S.W., Milatovic D., Hill K.E., Aschner M., Burk R.F., Valentine W.M. Progression of neurodegeneration and morphologic changes in the brains of juvenile mice with selenoprotein P deleted. Brain Res. 2011;1398:1–12. doi: 10.1016/j.brainres.2011.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calixto A., Jara J.S., Court F.A. Diapause formation and downregulation of insulin-like signaling via DAF-16/FOXO delays axonal degeneration and neuronal loss. PLoS Genet. 2012;8(12):e1003141. doi: 10.1371/journal.pgen.1003141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase D.L., Pepper J.S., Koelle M.R. Mechanism of extrasynaptic dopamine signaling in Caenorhabditis elegans. Nat Neurosci. 2004;7(10):1096–1103. doi: 10.1038/nn1316. [DOI] [PubMed] [Google Scholar]
- Chau K.Y., Korlipara L.V., Cooper J.M., Schapira A.H. Protection against paraquat and A53T alpha-synuclein toxicity by cabergoline is partially mediated by dopamine receptors. J Neurol Sci. 2009;278(1–2):44–53. doi: 10.1016/j.jns.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Christensen R., de la Torre-Ubieta L., Bonni A., Colón-Ramos D.A. A conserved PTEN/FOXO pathway regulates neuronal morphology during C. elegans development. Development. 2011;138(23):5257–5267. doi: 10.1242/dev.069062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis T.Z., Stegelmeier B.L., Panter K.E., Cook D., Gardner D.R., Hall J.O. Toxicokinetics and pathology of plant-associated acute selenium toxicosis in steers. J Vet Diagn Invest. 2012;24(2):319–327. doi: 10.1177/1040638711435407. [DOI] [PubMed] [Google Scholar]
- Dimitriadi M., Hart A.C. Neurodegenerative disorders: insights from the nematode Caenorhabditis elegans. Neurobiol Dis. 2010;40(1):4–11. doi: 10.1016/j.nbd.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domanskyi A., Geissler C., Vinnikov I.A., Alter H., Schober A., Vogt M.A. PTEN ablation in adult dopaminergic neurons is neuroprotective in Parkinson's disease models. FASEB J. 2011;25(9):2898–2910. doi: 10.1096/fj.11-181958. [DOI] [PubMed] [Google Scholar]
- Doonan R., McElwee J.J., Matthijssens F., Walker G.A., Houthoofd K., Back P. Against the oxidative damage theory of aging: superoxide dismutases protect against oxidative stress but have little or no effect on life span in Caenorhabditis elegans. Genes Dev. 2008;22(23):3236–3241. doi: 10.1101/gad.504808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunckley T., Huentelman M.J., Craig D.W., Pearson J.V., Szelinger S., Joshipura K. Whole-genome analysis of sporadic amyotrophic lateral sclerosis. N Engl J Med. 2007;357(8):775–788. doi: 10.1056/NEJMoa070174. [DOI] [PubMed] [Google Scholar]
- Eijkelenboom A., Burgering B.M. FOXOs: signalling integrators for homeostasis maintenance. Nat Rev Mol Cell Biol. 2013;14(2):83–97. doi: 10.1038/nrm3507. [DOI] [PubMed] [Google Scholar]
- Estevez M., Estevez A.O., Cowie R.H., Gardner K.L. The voltage-gated calcium channel UNC-2 is involved in stress-mediated regulation of tryptophan hydroxylase. J Neurochem. 2004;88(1):102–113. doi: 10.1046/j.1471-4159.2003.02140.x. [DOI] [PubMed] [Google Scholar]
- Estevez A.O., Mueller C.L., Morgan K.L., Szewczyk N.J., Teece L., Miranda-Vizuete A. Selenium induces cholinergic motor neuron degeneration in Caenorhabditis elegans. Neurotoxicology. 2012;33(5):1021–1032. doi: 10.1016/j.neuro.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairweather-Tait S.J., Bao Y., Broadley M.R., Collings R., Ford D., Hesketh J.E. Selenium in human health and disease. Antioxid Redox Signal. 2011;14(7):1337–1383. doi: 10.1089/ars.2010.3275. [DOI] [PubMed] [Google Scholar]
- Flynn J.M., Melov S. SOD2 in mitochondrial dysfunction and neurodegeneration. Free Radic Biol Med. 2013;62:4–12. doi: 10.1016/j.freeradbiomed.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gems D., Sutton A.J., Sundermeyer M.L., Albert P.S., King K.V., Edgley M.L. Two pleiotropic classes of daf-2 mutation affect larval arrest, adult behavior, reproduction and longevity in Caenorhabditis elegans. Genetics. 1998;150(1):129–155. doi: 10.1093/genetics/150.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladyshev V.N., Krause M., Xu X.M., Korotkov K.V., Kryukov G.V., Sun Q.A. Selenocysteine-containing thioredoxin reductase in C. elegans. Biochem Biophys Res Commun. 1999;259(2):244–249. doi: 10.1006/bbrc.1999.0765. [DOI] [PubMed] [Google Scholar]
- Golden J.W., Riddle D.L. A pheromone-induced developmental switch in Caenorhabditis elegans: temperature-sensitive mutants reveal a wild-type temperature-dependent process. Proc Natl Acad Sci USA. 1984;81(3):819–823. doi: 10.1073/pnas.81.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grünewald A., Gegg M.E., Taanman J.W., King R.H., Kock N., Klein C. Differential effects of PINK1 nonsense and missense mutations on mitochondrial function and morphology. Exp Neurol. 2009;219(1):266–273. doi: 10.1016/j.expneurol.2009.05.027. [DOI] [PubMed] [Google Scholar]
- Hagenbuchner J., Ausserlechner M.J. Mitochondria and FOXO3: breath or die. Front Physiol. 2013;4:147. doi: 10.3389/fphys.2013.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson S.T., Johnson T.E. daf-16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans. Curr Biol. 2001;11(24):1975–1980. doi: 10.1016/s0960-9822(01)00594-2. [DOI] [PubMed] [Google Scholar]
- Hoepken H.H., Gispert S., Morales B., Wingerter O., Del Turco D., Mülsch A. Mitochondrial dysfunction, peroxidation damage and changes in glutathione metabolism in PARK6. Neurobiol Dis. 2007;25(2):401–411. doi: 10.1016/j.nbd.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Honda Y., Honda S. Oxidative stress and life span determination in the nematode Caenorhabditis elegans. Ann N Y Acad Sci. 2002;959:466–474. doi: 10.1111/j.1749-6632.2002.tb02117.x. [DOI] [PubMed] [Google Scholar]
- Honda Y., Tanaka M., Honda S. Redox regulation, gene expression and longevity. Geriatr Gerontol Int. 2010;10(Suppl. 1):S59–S69. doi: 10.1111/j.1447-0594.2010.00591.x. [DOI] [PubMed] [Google Scholar]
- Huang H., Tindall D.J. Dynamic FoxO transcription factors. J Cell Sci. 2007;120(Pt 15):2479–2487. doi: 10.1242/jcs.001222. [DOI] [PubMed] [Google Scholar]
- Hunter T., Bannister W.H., Hunter G.J. Cloning, expression, and characterization of two manganese superoxide dismutases from Caenorhabditis elegans. J Biol Chem. 1997;272(45):28652–28659. doi: 10.1074/jbc.272.45.28652. [DOI] [PubMed] [Google Scholar]
- Iser W.B., Gami M.S., Wolkow C.A. Insulin signaling in Caenorhabditis elegans regulates both endocrine-like and cell-autonomous outputs. Dev Biol. 2007;303(2):434–447. doi: 10.1016/j.ydbio.2006.04.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwakami S., Misu H., Takeda T., Sugimori M., Matsugo S., Kaneko S. Concentration-dependent dual effects of hydrogen peroxide on insulin signal transduction in H4IIEC hepatocytes. PLoS One. 2011;6(11):e27401. doi: 10.1371/journal.pone.0027401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen V.L., Albert P.S., Riddle D.L. Caenorhabditis elegans SDF-9 enhances insulin/insulin-like signaling through interaction with DAF-2. Genetics. 2007;177(1):661–666. doi: 10.1534/genetics.107.076703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C.T., Brock D.J., Chancellor A.M., Warlow C.P., Swingler R.J. Cu/Zn superoxide dismutase (SOD1) mutations and sporadic amyotrophic lateral sclerosis. Lancet. 1993;342(8878):1050–1051. doi: 10.1016/0140-6736(93)92905-9. [DOI] [PubMed] [Google Scholar]
- Kabashi E., Valdmanis P.N., Dion P., Spiegelman D., McConkey B.J., Vande Velde C. TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat Genet. 2008;40(5):572–574. doi: 10.1038/ng.132. [DOI] [PubMed] [Google Scholar]
- Kim E.H., Sohn S., Kwon H.J., Kim S.U., Kim M.J., Lee S.J. Sodium selenite induces superoxide-mediated mitochondrial damage and subsequent autophagic cell death in malignant glioma cells. Cancer Res. 2007;67(13):6314–6324. doi: 10.1158/0008-5472.CAN-06-4217. [DOI] [PubMed] [Google Scholar]
- Kim Y., Chay K.O., Kim I., Song Y.B., Kim T.Y., Han S.J. Redox regulation of the tumor suppressor PTEN by glutaredoxin 5 and Ycp4. Biochem Biophys Res Commun. 2011;407(1):175–180. doi: 10.1016/j.bbrc.2011.02.133. [DOI] [PubMed] [Google Scholar]
- Kimura K.D., Tissenbaum H.A., Liu Y., Ruvkun G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science. 1997;277(5328):942–946. doi: 10.1126/science.277.5328.942. [DOI] [PubMed] [Google Scholar]
- Kirby J., Ning K., Ferraiuolo L., Heath P.R., Ismail A., Kuo S.W. Phosphatase and tensin homologue/protein kinase B pathway linked to motor neuron survival in human superoxide dismutase 1-related amyotrophic lateral sclerosis. Brain. 2011;134(Pt 2):506–517. doi: 10.1093/brain/awq345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labunskyy V.M., Lee B.C., Handy D.E., Loscalzo J., Hatfield D.L., Gladyshev V.N. Both maximal expression of selenoproteins and selenoprotein deficiency can promote development of type 2 diabetes-like phenotype in mice. Antioxid Redox Signal. 2011;14(12):2327–2336. doi: 10.1089/ars.2010.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis J.N., Murphy C.T. Integration of diverse inputs in the regulation of Caenorhabditis elegans DAF-16/FOXO. Dev Dyn. 2010;239(5):1405–1412. doi: 10.1002/dvdy.22244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebovitz R.M., Zhang H., Vogel H., Cartwright J., Jr., Dionne L., Lu N. Neurodegeneration, myocardial injury, and perinatal death in mitochondrial superoxide dismutase-deficient mice. Proc Natl Acad Sci USA. 1996;93(18):9782–9787. doi: 10.1073/pnas.93.18.9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.H., Jeong D. Bimodal actions of selenium essential for antioxidant and toxic pro-oxidant activities: the selenium paradox (Review) Mol Med Rep. 2012;5(2):299–304. doi: 10.3892/mmr.2011.651. [DOI] [PubMed] [Google Scholar]
- Libina N., Berman J.R., Kenyon C. Tissue-specific activities of C. elegans DAF-16 in the regulation of lifespan. Cell. 2003;115(4):489–502. doi: 10.1016/s0092-8674(03)00889-4. [DOI] [PubMed] [Google Scholar]
- Lin K., Hsin H., Libina N., Kenyon C. Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nat Genet. 2001;28(2):139–145. doi: 10.1038/88850. [DOI] [PubMed] [Google Scholar]
- Lin K., Dorman J.B., Rodan A., Kenyon C. daf-16: an HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science. 1997;278(5341):1319–1322. doi: 10.1126/science.278.5341.1319. [DOI] [PubMed] [Google Scholar]
- Loh K., Deng H., Fukushima A., Cai X., Boivin B., Galic S. Reactive oxygen species enhance insulin sensitivity. Cell Metab. 2009;10(4):260–272. doi: 10.1016/j.cmet.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H., Yang Y., Duan J., Wu P., Jiang Q., Xu C. PTEN-regulated AKT/FoxO3a/Bim signaling contributes to reactive oxygen species-mediated apoptosis in selenite-treated colorectal cancer cells. Cell Death Dis. 2013;4:e481. doi: 10.1038/cddis.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H., Yang Y., Huang F., Li F., Jiang Q., Shi K. Selenite induces apoptosis in colorectal cancer cells via AKT-mediated inhibition of ß-catenin survival axis. Cancer Lett. 2012;315(1):78–85. doi: 10.1016/j.canlet.2011.10.014. [DOI] [PubMed] [Google Scholar]
- Maier C.M., Chan P.H. Role of superoxide dismutases in oxidative damage and neurodegenerative disorders. Neuroscientist. 2002;8(4):323–334. doi: 10.1177/107385840200800408. [DOI] [PubMed] [Google Scholar]
- Maiese K., Chong Z.Z., Shang Y.C. “Sly as a FOXO”: new paths with Forkhead signaling in the brain. Curr Neurovasc Res. 2007;4(4):295–302. doi: 10.2174/156720207782446306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maj M.C., Tkachyova I., Patel P., Addis J.B., Mackay N., Levandovskiy V. Oxidative stress alters the regulatory control of p66Shc and Akt in PINK1 deficient cells. Biochem Biophys Res Commun. 2010;399(3):331–335. doi: 10.1016/j.bbrc.2010.07.033. [DOI] [PubMed] [Google Scholar]
- Masse I., Molin L., Mouchiroud L., Vanhems P., Palladino F., Billaud M. A novel role for the SMG-1 kinase in lifespan and oxidative stress resistance in Caenorhabditis elegans. PLoS One. 2008;3(10):e3354. doi: 10.1371/journal.pone.0003354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung J.P., Roneker C.A., Mu W., Lisk D.J., Langlais P., Liu F. Development of insulin resistance and obesity in mice overexpressing cellular glutathione peroxidase. Proc Natl Acad Sci USA. 2004;101(24):8852–8857. doi: 10.1073/pnas.0308096101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei Y., Zhang Y., Yamamoto K., Xie W., Mak T.W., You H. FOXO3a-dependent regulation of Pink1 (Park6) mediates survival signaling in response to cytokine deprivation. Proc Natl Acad Sci USA. 2009;106(13):5153–5158. doi: 10.1073/pnas.0901104106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello C.C., Kramer J.M., Stinchcomb D., Ambros V. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 1991;10(12):3959–3970. doi: 10.1002/j.1460-2075.1991.tb04966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao L., St Clair D.K. Regulation of superoxide dismutase genes: implications in disease. Free Radic Biol Med. 2009;47(4):344–356. doi: 10.1016/j.freeradbiomed.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojsilovic-Petrovic J., Nedelsky N., Boccitto M., Mano I., Georgiades S.N., Zhou W. FOXO3a is broadly neuroprotective in vitro and in vivo against insults implicated in motor neuron diseases. J Neurosci. 2009;29(25):8236–8247. doi: 10.1523/JNEUROSCI.1805-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan K.L., Estevez A.O., Mueller C.L., Cacho-Valadez B., Miranda-Vizuete A., Szewczyk N.J. The glutaredoxin GLRX-21 functions to prevent selenium-induced oxidative stress in Caenorhabditis elegans. Toxicol Sci. 2010;118(2):530–543. doi: 10.1093/toxsci/kfq273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto N., Nagai M., Miyazaki K., Ohta Y., Kurata T., Takehisa Y. Induction of parkinsonism-related proteins in the spinal motor neurons of transgenic mouse carrying a mutant SOD1 gene. J Neurosci Res. 2010;88(8):1804–1811. doi: 10.1002/jnr.22341. [DOI] [PubMed] [Google Scholar]
- Morley J.F., Brignull H.R., Weyers J.J., Morimoto R.I. The threshold for polyglutamine-expansion protein aggregation and cellular toxicity is dynamic and influenced by aging in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2002;99(16):10417–10422. doi: 10.1073/pnas.152161099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J.Z., Tissenbaum H.A., Ruvkun G. A phosphatidylinositol-3-OH kinase family member regulating longevity and diapause in Caenorhabditis elegans. Nature. 1996;382(6591):536–539. doi: 10.1038/382536a0. [DOI] [PubMed] [Google Scholar]
- Muñoz-Lobato F., Rodríguez-Palero M.J., Naranjo-Galindo F.J., Shephard F., Gaffney C.J., Szewczyk N.J. Protective role of DNJ-27/ERdj5 in Caenorhabditis elegans models of human neurodegenerative diseases. Antioxid Redox Signal. 2013 doi: 10.1089/ars.2012.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata H., Sakaguchi M., Jin Y., Sakaguchi Y., Futami J., Yamada H. A new cytosolic pathway from a Parkinson disease-associated kinase, BRPK/PINK1: activation of AKT via mTORC2. J Biol Chem. 2011;286(9):7182–7189. doi: 10.1074/jbc.M110.179390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogg S., Paradis S., Gottlieb S., Patterson G.I., Lee L., Tissenbaum H.A. The fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. 1997;389(6654):994–999. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- Oh S.W., Mukhopadhyay A., Dixit B.L., Raha T., Green M.R., Tissenbaum H.A. Identification of direct DAF-16 targets controlling longevity, metabolism and diapause by chromatin immunoprecipitation. Nat Genet. 2006;38(2):251–257. doi: 10.1038/ng1723. [DOI] [PubMed] [Google Scholar]
- Panter K.E., Hartley W.J., James L.F., Mayland H.F., Stegelmeier B.L., Kechele P.O. Comparative toxicity of selenium from seleno-dl-methionine, sodium selenate, and Astragalus bisulcatus in pigs. Fundam Appl Toxicol. 1996;32(2):217–223. [PubMed] [Google Scholar]
- Paradis S., Ruvkun G. Caenorhabditis elegans Akt/PKB transduces insulin receptor-like signals from AGE-1 PI3 kinase to the DAF-16 transcription factor. Genes Dev. 1998;12(16):2488–2498. doi: 10.1101/gad.12.16.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis S., Ailion M., Toker A., Thomas J.H., Ruvkun G. A PDK1 homolog is necessary and sufficient to transduce AGE-1 PI3 kinase signals that regulate diapause in Caenorhabditis elegans. Genes Dev. 1999;13(11):1438–1452. doi: 10.1101/gad.13.11.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker J.A., Vazquez-Manrique R.P., Tourette C., Farina F., Offner N., Mukhopadhyay A. Integration of ß-catenin, sirtuin, and FOXO signaling protects from mutant huntingtin toxicity. J Neurosci. 2012;32(36):12630–12640. doi: 10.1523/JNEUROSCI.0277-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto A., Speckmann B., Heisler M., Sies H., Steinbrenner H. Delaying of insulin signal transduction in skeletal muscle cells by selenium compounds. J Inorg Biochem. 2011;105(6):812–820. doi: 10.1016/j.jinorgbio.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Ponugoti B., Dong G., Graves D.T. Role of forkhead transcription factors in diabetes-induced oxidative stress. Exp Diabetes Res. 2012;2012:939751. doi: 10.1155/2012/939751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi W., Huang X., Neumann-Haefelin E., Schulze E., Baumeister R. Cell-nonautonomous signaling of FOXO/DAF-16 to the stem cells of Caenorhabditis elegans. PLoS Genet. 2012;8(8):e1002836. doi: 10.1371/journal.pgen.1002836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayman M.P., Stranges S. Epidemiology of selenium and type 2 diabetes: can we make sense of it? Free Radic Biol Med. 2013 doi: 10.1016/j.freeradbiomed.2013.04.003. pii:S0891-5849(13)00143-3. [DOI] [PubMed] [Google Scholar]
- Riddle D.L. A genetic pathway for dauer larva formation in Caenorhabditis elegans. Stadler Genet Symp. 1977;9:101–120. [Google Scholar]
- Riddle D.L., Swanson M.M., Albert P.S. Interacting genes in nematode dauer larva formation. Nature. 1981;290:668–671. doi: 10.1038/290668a0. [DOI] [PubMed] [Google Scholar]
- Rocourt C.R., Cheng W.H. Selenium supranutrition: are the potential benefits of chemoprevention outweighed by the promotion of diabetes and insulin resistance? Nutrients. 2013;5(4):1349–1365. doi: 10.3390/nu5041349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh J.Y., Park Y.J., Choi J. A cadmium toxicity assay using stress responsive Caenorhabditis elegans mutant strains. Environ Toxicol Pharmacol. 2009;28(3):409–413. doi: 10.1016/j.etap.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Rosen D.R., Siddique T., Patterson D., Figlewicz D.A., Sapp P., Hentati A. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362(6415):59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Sánchez-Mora R.M., Arboleda H., Arboleda G. PINK1 overexpression protects against C2-ceramide-induced CAD cell death through the PI3K/AKT pathway. J Mol Neurosci. 2012;47(3):582–594. doi: 10.1007/s12031-011-9687-z. [DOI] [PubMed] [Google Scholar]
- Sanmartín C., Plano D., Sharma A.K., Palop J.A. Selenium compounds, apoptosis and other types of cell death: an overview for cancer therapy. Int J Mol Sci. 2012;13(8):9649–9672. doi: 10.3390/ijms13089649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott B.A., Avidan M.S., Crowder C.M. Regulation of hypoxic death in C. elegans by the insulin/IGF receptor homolog DAF-2. Science. 2002;296(5577):2388–2391. doi: 10.1126/science.1072302. [DOI] [PubMed] [Google Scholar]
- Shore D.E., Ruvkun G. A cytoprotective perspective on longevity regulation. Trends Cell Biol. 2013;23(9):409–420. doi: 10.1016/j.tcb.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda Y., Mukai H., Matsuo K., Takahashi M., Ono Y., Maeda K. Accumulation of tumor-suppressor PTEN in Alzheimer neurofibrillary tangles. Neurosci Lett. 2010;471(1):20–24. doi: 10.1016/j.neulet.2009.12.078. [DOI] [PubMed] [Google Scholar]
- Spiller H.A., Pfiefer E. Two fatal cases of selenium toxicity. Forensic Sci Int. 2007;171(1):67–72. doi: 10.1016/j.forsciint.2006.06.077. [DOI] [PubMed] [Google Scholar]
- Sreedharan J., Blair I.P., Tripathi V.B., Hu X., Vance C., Rogelj B. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319(5870):1668–1672. doi: 10.1126/science.1154584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stambolic V., Suzuki A., de la Pompa J.L., Brothers G.M., Mirtsos C., Sasaki T. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95(1):29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- Steinbrenner H. Interference of selenium and selenoproteins with the insulin-regulated carbohydrate and lipid metabolism. Free Radic Biol Med. 2013;65:1538–1547. doi: 10.1016/j.freeradbiomed.2013.07.016. [DOI] [PubMed] [Google Scholar]
- Sun K., Wu S., Wang Y., Wan X., Thompson H.J., Zhang J. High-dose sodium selenite toxicity cannot be prevented by the co-administration of pharmacological levels of epigallocatechin-3-gallate which in turn aggravates the toxicity. Food Chem Toxicol. 2013;52:36–41. doi: 10.1016/j.fct.2012.10.038. [DOI] [PubMed] [Google Scholar]
- Swanson M.M., Riddle D.L. Critical periods in the development of the Caenorhabditis elegans dauer larva. Dev Biol. 1981;84(1):27–40. doi: 10.1016/0012-1606(81)90367-5. [DOI] [PubMed] [Google Scholar]
- Szewczyk N.J., Hartman J.J., Barmada S.J., Jacobson L.A. Genetic defects in acetylcholine signalling promote protein degradation in muscle cells of Caenorhabditis elegans. J Cell Sci. 2000;113(Pt 11):2003–2010. doi: 10.1242/jcs.113.11.2003. [DOI] [PubMed] [Google Scholar]
- Tiwary A.K., Stegelmeier B.L., Panter K.E., James L.F., Hall J.O. Comparative toxicosis of sodium selenite and selenomethionine in lambs. J Vet Diagn Invest. 2006;18(1):61–70. doi: 10.1177/104063870601800108. [DOI] [PubMed] [Google Scholar]
- Trojsi F., Monsurrò M.R., Tedeschi G. Exposure to environmental toxicants and pathogenesis of amyotrophic lateral sclerosis: state of the art and research perspectives. Int J Mol Sci. 2013;14(8):15286–15311. doi: 10.3390/ijms140815286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccaro A., Tauffenberger A., Ash P.E., Carlomagno Y., Petrucelli L., Parker J.A. TDP-1/TDP-43 regulates stress signaling and age-dependent proteotoxicity in Caenorhabditis elegans. PLoS Genet. 2012;8(7):e1002806. doi: 10.1371/journal.pgen.1002806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente E.M., Abou-Sleiman P.M., Caputo V., Muqit M.M., Harvey K., Gispert S. Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science. 2004;304(5674):1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- Vinceti M., Solovyev N., Mandrioli J., Crespi C.M., Bonvicini F., Arcolin E. Cerebrospinal fluid of newly diagnosed amyotrophic lateral sclerosis patients exhibits abnormal levels of selenium species including elevated selenite. Neurotoxicology. 2013;38:25–32. doi: 10.1016/j.neuro.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinceti M., Bonvicini F., Bergomi M., Malagoli C. Possible involvement of overexposure to environmental selenium in the etiology of amyotrophic lateral sclerosis: a short review. Ann Ist Super Sanita. 2010;46(3):279–283. doi: 10.4415/ANN_10_03_09. [DOI] [PubMed] [Google Scholar]
- Walter P.L., Steinbrenner H., Barthel A., Klotz L.O. Stimulation of selenoprotein P promoter activity in hepatoma cells by FoxO1a transcription factor. Biochem Biophys Res Commun. 2008;365(2):316–321. doi: 10.1016/j.bbrc.2007.10.171. [DOI] [PubMed] [Google Scholar]
- Wilhelmus M.M., van der Pol S.M., Jansen Q., Witte M.E., van der Valk P., Rozemuller A.J. Association of Parkinson disease-related protein PINK1 with Alzheimer disease and multiple sclerosis brain lesions. Free Radic Biol Med. 2011;50(3):469–476. doi: 10.1016/j.freeradbiomed.2010.11.033. [DOI] [PubMed] [Google Scholar]
- Wirth E.K., Conrad M., Winterer J., Wozny C., Carlson B.A., Roth S. Neuronal selenoprotein expression is required for interneuron development and prevents seizures and neurodegeneration. FASEB J. 2010;24(3):844–852. doi: 10.1096/fj.09-143974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M., Nunes F., Henkel A., Heinick A., Paul R.J. The MAP kinase JNK-1 of Caenorhabditis elegans: location, activation, and influences over temperature-dependent insulin-like signaling, stress responses, and fitness. J Cell Physiol. 2008;214(3):721–729. doi: 10.1002/jcp.21269. [DOI] [PubMed] [Google Scholar]
- Wong H.K., Veremeyko T., Patel N., Lemere C.A., Walsh D.M., Esau C. De-repression of FOXO3a death axis by microRNA-132 and -212 causes neuronal apoptosis in Alzheimer's disease. Hum Mol Genet. 2013;22(15):3077–3092. doi: 10.1093/hmg/ddt164. [DOI] [PubMed] [Google Scholar]
- Wu Y., Zu K., Warren M.A., Wallace P.K., Ip C. Delineating the mechanism by which selenium deactivates Akt in prostate cancer cells. Mol Cancer Ther. 2006;5(2):246–252. doi: 10.1158/1535-7163.MCT-05-0376. [DOI] [PubMed] [Google Scholar]
- Yang S.J., Hwang S.Y., Choi H.Y., Yoo H.J., Seo J.A., Kim S.G. Serum selenoprotein P levels in patients with type 2 diabetes and prediabetes: implications for insulin resistance, inflammation, and atherosclerosis. J Clin Endocrinol Metab. 2011;96(8):E1325–E1329. doi: 10.1210/jc.2011-0620. [DOI] [PubMed] [Google Scholar]
- Zhang J., Wang H., Peng D., Taylor E.W. Further insight into the impact of sodium selenite on selenoenzymes: high-dose selenite enhances hepatic thioredoxin reductase 1 activity as a consequence of liver injury. Toxicol Lett. 2008;176(3):223–229. doi: 10.1016/j.toxlet.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Zhang S., Rocourt C., Cheng W.H. Selenoproteins and the aging brain. Mech Ageing Dev. 2010;131(4):253–260. doi: 10.1016/j.mad.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Zhang T., Mullane P.C., Periz G., Wang J. TDP-43 neurotoxicity and protein aggregation modulated by heat shock factor and insulin/IGF-1 signaling. Hum Mol Genet. 2011;20(10):1952–1965. doi: 10.1093/hmg/ddr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.