Abstract
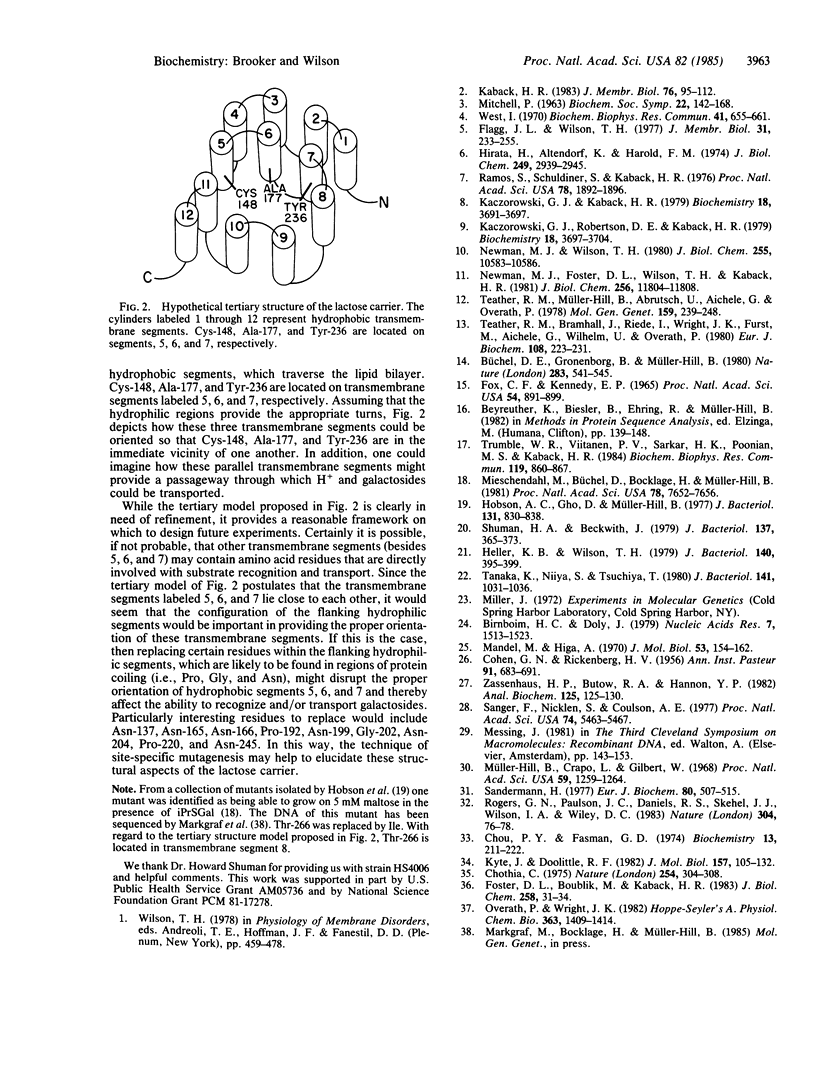
The wild-type lactose carrier of Escherichia coli has a poor ability to transport the disaccharide maltose. However, it is possible to select lactose carrier mutants that have an enhanced ability to transport maltose by growing E. coli cells on maltose minimal plates in the presence of isopropyl thiogalactoside (an inducer of the lac operon). We have utilized this approach to isolate 18 independent lactose permease mutants that transport maltose. The relevant DNA sequences have been determined, and all of the mutations were found to be single base pair changes either at triplet 177 or at triplet 236. The nucleotide changes replace alanine-177 with valine or threonine, or tyrosine-236 with phenylalanine, asparagine, serine, or histidine. Transport experiments indicate that all of the mutants have faster maltose transport compared with the wild-type strain. Position 177 mutants retain the ability to transport galactosides, such as lactose and melibiose, at rates similar to the rate of the wild-type strain. In contrast, the position 236 mutants are markedly defective in the ability to transport galactosides. With regard to secondary structure, alanine-177 and tyrosine-236 are located on adjacent hydrophobic segments of the lactose carrier that are predicted to span the membrane. Thus, the results of this study indicate that the substrate recognition site of the lactose carrier is located within the plane of the lipid bilayer. In addition, a tertiary structure model is proposed that suggests how certain transmembrane segments might be localized relative to one another.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büchel D. E., Gronenborn B., Müller-Hill B. Sequence of the lactose permease gene. Nature. 1980 Feb 7;283(5747):541–545. doi: 10.1038/283541a0. [DOI] [PubMed] [Google Scholar]
- Chothia C. Structural invariants in protein folding. Nature. 1975 Mar 27;254(5498):304–308. doi: 10.1038/254304a0. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Conformational parameters for amino acids in helical, beta-sheet, and random coil regions calculated from proteins. Biochemistry. 1974 Jan 15;13(2):211–222. doi: 10.1021/bi00699a001. [DOI] [PubMed] [Google Scholar]
- Flagg J. L., Wilson T. H. A protonmotive force as the source of energy for galactoside transport in energy depleted Escherichia coli. J Membr Biol. 1977 Mar 8;31(3):233–255. doi: 10.1007/BF01869407. [DOI] [PubMed] [Google Scholar]
- Foster D. L., Boublik M., Kaback H. R. Structure of the lac carrier protein of Escherichia coli. J Biol Chem. 1983 Jan 10;258(1):31–34. [PubMed] [Google Scholar]
- Fox C. F., Kennedy E. P. Specific labeling and partial purification of the M protein, a component of the beta-galactoside transport system of Escherichia coli. Proc Natl Acad Sci U S A. 1965 Sep;54(3):891–899. doi: 10.1073/pnas.54.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller K. B., Wilson T. H. Sucrose transport by the Escherichia coli lactose carrier. J Bacteriol. 1979 Nov;140(2):395–399. doi: 10.1128/jb.140.2.395-399.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H., Altendorf K., Harold F. M. Energy coupling in membrane vesicles of Escherichia coli. I. Accumulation of metabolites in response to an electrical potential. J Biol Chem. 1974 May 10;249(9):2939–2945. [PubMed] [Google Scholar]
- Hobson A. C., Gho D., Müller-Hill B. Isolation, genetic analysis, and characterization of Escherichia coli mutants with defects in the lacY gene. J Bacteriol. 1977 Sep;131(3):830–838. doi: 10.1128/jb.131.3.830-838.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback H. R. The lac carrier protein in Escherichia coli. J Membr Biol. 1983;76(2):95–112. doi: 10.1007/BF02000610. [DOI] [PubMed] [Google Scholar]
- Kaczorowski G. J., Kaback H. R. Mechanism of lactose translocation in membrane vesicles from Escherichia coli. 1. Effect of pH on efflux, exchange, and counterflow. Biochemistry. 1979 Aug 21;18(17):3691–3697. doi: 10.1021/bi00584a009. [DOI] [PubMed] [Google Scholar]
- Kaczorowski G. J., Robertson D. E., Kaback H. R. Mechanism of lactose translocation in membrane vesicles from Escherichia coli. 2. Effect of imposed delata psi, delta pH, and Delta mu H+. Biochemistry. 1979 Aug 21;18(17):3697–3704. doi: 10.1021/bi00584a010. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Mieschendahl M., Büchel D., Bocklage H., Müller-Hill B. Mutations in the lacY gene of Escherichia coli define functional organization of lactose permease. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7652–7656. doi: 10.1073/pnas.78.12.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Hill B., Crapo L., Gilbert W. Mutants that make more lac repressor. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1259–1264. doi: 10.1073/pnas.59.4.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman M. J., Foster D. L., Wilson T. H., Kaback H. R. Purification and reconstitution of functional lactose carrier from Escherichia coli. J Biol Chem. 1981 Nov 25;256(22):11804–11808. [PubMed] [Google Scholar]
- Newman M. J., Wilson T. H. Solubilization and reconstitution of the lactose transport system from Escherichia coli. J Biol Chem. 1980 Nov 25;255(22):10583–10586. [PubMed] [Google Scholar]
- Overath P., Wright J. K. Lactose permease and the molecular biology of transport. Hoppe Seylers Z Physiol Chem. 1982 Dec;363(12):1409–1414. doi: 10.1515/bchm2.1982.363.2.1409. [DOI] [PubMed] [Google Scholar]
- Ramos S., Schuldiner S., Kaback H. R. The electrochemical gradient of protons and its relationship to active transport in Escherichia coli membrane vesicles. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1892–1896. doi: 10.1073/pnas.73.6.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers G. N., Paulson J. C., Daniels R. S., Skehel J. J., Wilson I. A., Wiley D. C. Single amino acid substitutions in influenza haemagglutinin change receptor binding specificity. Nature. 1983 Jul 7;304(5921):76–78. doi: 10.1038/304076a0. [DOI] [PubMed] [Google Scholar]
- Sandermann H., Jr beta-D-Galactoside transport in Escherichia coli: substrate recognition. Eur J Biochem. 1977 Nov 1;80(2):507–515. doi: 10.1111/j.1432-1033.1977.tb11906.x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuman H. A., Beckwith J. Escherichia coli K-12 mutants that allow transport of maltose via the beta-galactoside transport system. J Bacteriol. 1979 Jan;137(1):365–373. doi: 10.1128/jb.137.1.365-373.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Niiya S., Tsuchiya T. Melibiose transport of Escherichia coli. J Bacteriol. 1980 Mar;141(3):1031–1036. doi: 10.1128/jb.141.3.1031-1036.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teather R. M., Bramhall J., Riede I., Wright J. K., Fürst M., Aichele G., Wilhelm U., Overath P. Lactose carrier protein of Escherichia coli. Structure and expression of plasmids carrying the Y gene of the lac operon. Eur J Biochem. 1980;108(1):223–231. doi: 10.1111/j.1432-1033.1980.tb04715.x. [DOI] [PubMed] [Google Scholar]
- Teather R. M., Müller-Hill B., Abrutsch U., Aichele G., Overath P. Amplification of the lactose carrier protein in Escherichia coli using a plasmid vector. Mol Gen Genet. 1978 Feb 27;159(3):239–248. doi: 10.1007/BF00268260. [DOI] [PubMed] [Google Scholar]
- Trumble W. R., Viitanen P. V., Sarkar H. K., Poonian M. S., Kaback H. R. Site-directed mutagenesis of cys148 in the lac carrier protein of Escherichia coli. Biochem Biophys Res Commun. 1984 Mar 30;119(3):860–867. doi: 10.1016/0006-291x(84)90853-2. [DOI] [PubMed] [Google Scholar]
- West I. C. Lactose transport coupled to proton movements in Escherichia coli. Biochem Biophys Res Commun. 1970 Nov 9;41(3):655–661. doi: 10.1016/0006-291x(70)90063-x. [DOI] [PubMed] [Google Scholar]
- Zassenhaus H. P., Butow R. A., Hannon Y. P. Rapid electroelution of nucleic acids from agarose and acrylamide gels. Anal Biochem. 1982 Sep 1;125(1):125–130. doi: 10.1016/0003-2697(82)90392-x. [DOI] [PubMed] [Google Scholar]





