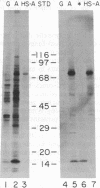
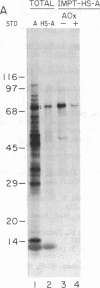
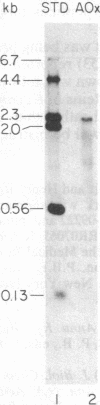
Abstract
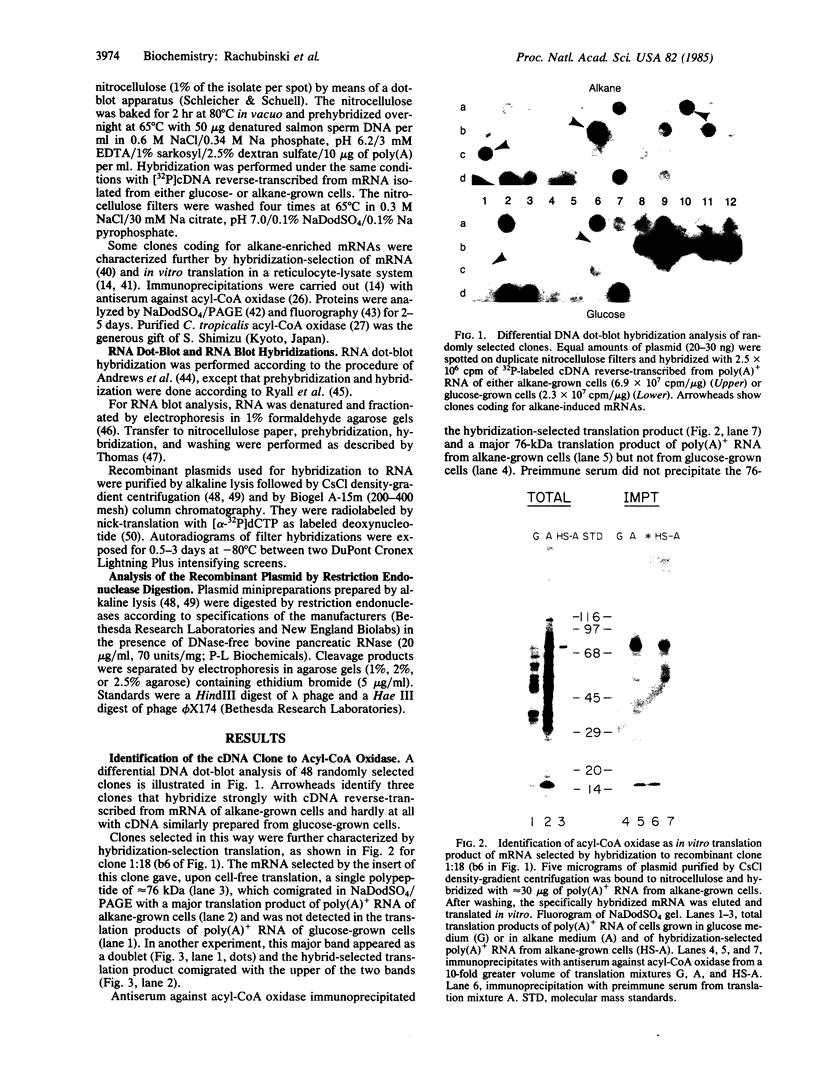
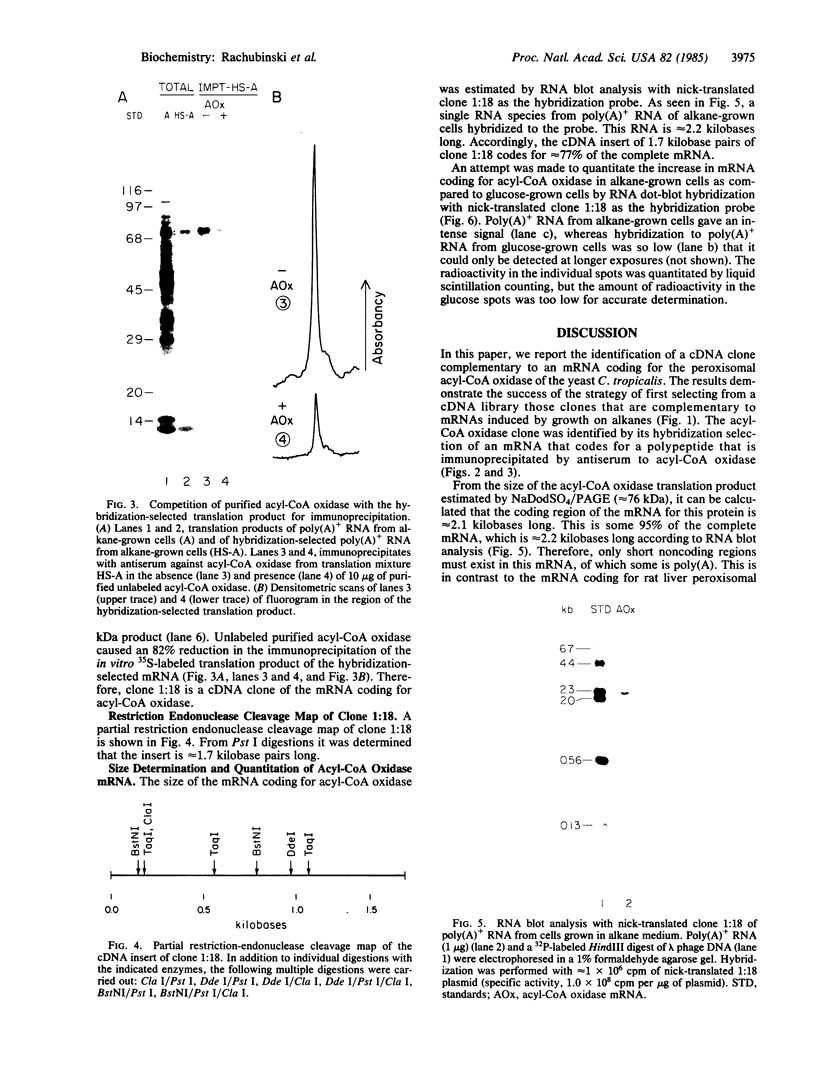
Candida tropicalis pK233 cells were grown with n-alkanes as carbon source to induce the synthesis of peroxisomal proteins and the proliferation of peroxisomes. Poly-(A)+ RNA was isolated and used to construct a cDNA library by insertion of double-stranded reverse transcripts into the Pst I site of pBR322 followed by cloning in Escherichia coli. Clones complementary to mRNAs induced by growth on alkanes were selected by differential DNA dot-blot analysis using [32P]cDNA reverse-transcribed from poly(A)+ RNA of glucose-grown cells (which contain few peroxisomes) or of alkane-grown cells. Among these clones, one containing a 1.7-kilobase insert coding for acyl-CoA oxidase (the first enzyme in the peroxisomal Beta-oxidation pathway) was identified by hybridization-selection translation and immunoprecipitation. By RNA blot analysis, the acyl-CoA oxidase mRNA was estimated to be approximately equal to 2.2 kilobases long, of which 2.1 kilobases are required to code for the approximately equal to 76-kDa protein. Since the mRNA is polyadenylylated, there appears to be little additional nontranslated region. Cell-free mRNA translation and RNA dot-blot hybridization analyses demonstrated that, whereas glucose-grown C. tropicalis contained little or no acyl-CoA oxidase mRNA, alkane-grown cells contained so much of this mRNA as to make acyl-CoA oxidase one of the major in vitro translation products.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews G. K., Dziadek M., Tamaoki T. Expression and methylation of the mouse alpha-fetoprotein gene in embryonic, adult, and neoplastic tissues. J Biol Chem. 1982 May 10;257(9):5148–5153. [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coudron P. E., Frerman F. E., Schowalter D. B. Chemical and catalytic properties of the peroxisomal acyl-coenzyme A oxidase from Candida tropicalis. Arch Biochem Biophys. 1983 Oct 1;226(1):324–336. doi: 10.1016/0003-9861(83)90299-0. [DOI] [PubMed] [Google Scholar]
- Derman E., Krauter K., Walling L., Weinberger C., Ray M., Darnell J. E., Jr Transcriptional control in the production of liver-specific mRNAs. Cell. 1981 Mar;23(3):731–739. doi: 10.1016/0092-8674(81)90436-0. [DOI] [PubMed] [Google Scholar]
- Dommes P., Dommes V., Kunau W. H. beta-Oxidation in Candida tropicalis. Partial purification and biological function of an inducible 2,4-dienoyl coenzyme A reductase. J Biol Chem. 1983 Sep 25;258(18):10846–10852. [PubMed] [Google Scholar]
- Fujiki Y., Hubbard A. L., Fowler S., Lazarow P. B. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J Cell Biol. 1982 Apr;93(1):97–102. doi: 10.1083/jcb.93.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki Y., Rachubinski R. A., Lazarow P. B. Synthesis of a major integral membrane polypeptide of rat liver peroxisomes on free polysomes. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7127–7131. doi: 10.1073/pnas.81.22.7127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki Y., Rachubinski R. A., Mortensen R. M., Lazarow P. B. Synthesis of 3-ketoacyl-CoA thiolase of rat liver peroxisomes on free polyribosomes as a larger precursor. Induction of thiolase mRNA activity by clofibrate. Biochem J. 1985 Mar 15;226(3):697–704. doi: 10.1042/bj2260697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta S., Hashimoto T., Miura S., Mori M., Tatibana M. Cell-free synthesis of the enzymes of peroxisomal beta-oxidation. Biochem Biophys Res Commun. 1982 Mar 30;105(2):639–646. doi: 10.1016/0006-291x(82)91482-6. [DOI] [PubMed] [Google Scholar]
- Goldman B. M., Blobel G. Biogenesis of peroxisomes: intracellular site of synthesis of catalase and uricase. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5066–5070. doi: 10.1073/pnas.75.10.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman J. M., Scott C. W., Donahue P. N., Atherton J. P. Alcohol oxidase assembles post-translationally into the peroxisome of Candida boidinii. J Biol Chem. 1984 Jul 10;259(13):8485–8493. [PubMed] [Google Scholar]
- Hashimoto T. Individual peroxisomal beta-oxidation enzymes. Ann N Y Acad Sci. 1982;386:5–12. doi: 10.1111/j.1749-6632.1982.tb21403.x. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Jiang Z., Thorpe C. Acyl-CoA oxidase from Candida tropicalis. Biochemistry. 1983 Aug 2;22(16):3752–3758. doi: 10.1021/bi00285a006. [DOI] [PubMed] [Google Scholar]
- Kamiryo T., Abe M., Okazaki K., Kato S., Shimamoto N. Absence of DNA in peroxisomes of Candida tropicalis. J Bacteriol. 1982 Oct;152(1):269–274. doi: 10.1128/jb.152.1.269-274.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiryo T., Okazaki K. High-level expression and molecular cloning of genes encoding Candida tropicalis peroxisomal proteins. Mol Cell Biol. 1984 Oct;4(10):2136–2141. doi: 10.1128/mcb.4.10.2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto S., Nozaki C., Tanaka A., Fukui S. Fatty acid beta-oxidation system in microbodies of n-alkane-grown Candida tropicalis. Eur J Biochem. 1978 Feb;83(2):609–613. doi: 10.1111/j.1432-1033.1978.tb12130.x. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lazarow P. B. Rat liver peroxisomes catalyze the beta oxidation of fatty acids. J Biol Chem. 1978 Mar 10;253(5):1522–1528. [PubMed] [Google Scholar]
- Lazarow P. B., de Duve C. The synthesis and turnover of rat liver peroxisomes. V. Intracellular pathway of catalase synthesis. J Cell Biol. 1973 Nov;59(2 Pt 1):507–524. doi: 10.1083/jcb.59.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccecchini M. L., Rudin Y., Blobel G., Schatz G. Import of proteins into mitochondria: precursor forms of the extramitochondrially made F1-ATPase subunits in yeast. Proc Natl Acad Sci U S A. 1979 Jan;76(1):343–347. doi: 10.1073/pnas.76.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura S., Mori M., Takiguchi M., Tatibana M., Furuta S., Miyazawa S., Hashimoto T. Biosynthesis and intracellular transport of enzymes of peroxisomal beta-oxidation. J Biol Chem. 1984 May 25;259(10):6397–6402. [PubMed] [Google Scholar]
- Osumi M., Miwa N., Teranishi Y., Tanaka A., Fukui S. Ultrastructure of Candida yeasts grown on n-alkanes. Appearance of microbodies and its relationship to high catalase activity. Arch Microbiol. 1974;99(3):181–201. doi: 10.1007/BF00696234. [DOI] [PubMed] [Google Scholar]
- Osumi T., Ozasa H., Hashimoto T. Molecular cloning of cDNA for rat acyl-CoA oxidase. J Biol Chem. 1984 Feb 25;259(4):2031–2034. [PubMed] [Google Scholar]
- Ozasa H., Miyazawa S., Osumi T. Biosynthesis of carnitine octanoyltransferase and carnitine palmitoyltransferase. J Biochem. 1983 Aug;94(2):543–549. doi: 10.1093/oxfordjournals.jbchem.a134385. [DOI] [PubMed] [Google Scholar]
- Parnes J. R., Velan B., Felsenfeld A., Ramanathan L., Ferrini U., Appella E., Seidman J. G. Mouse beta 2-microglobulin cDNA clones: a screening procedure for cDNA clones corresponding to rare mRNAs. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2253–2257. doi: 10.1073/pnas.78.4.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Rachubinski R. A., Fujiki Y., Mortensen R. M., Lazarow P. B. Acyl-Coa oxidase and hydratase-dehydrogenase, two enzymes of the peroxisomal beta-oxidation system, are synthesized on free polysomes of clofibrate-treated rat liver. J Cell Biol. 1984 Dec;99(6):2241–2246. doi: 10.1083/jcb.99.6.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman C. M., Grab D. J., Irukulla R. The intracellular pathway of newly formed rat liver catalase. Arch Biochem Biophys. 1972 Oct;152(2):496–501. doi: 10.1016/0003-9861(72)90244-5. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Roa M., Blobel G. Biosynthesis of peroxisomal enzymes in the methylotrophic yeast Hansenula polymorpha. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6872–6876. doi: 10.1073/pnas.80.22.6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbi M., Lazarow P. B. Peptide mapping of peroxisomal catalase and its precursor. Comparison to the primary wheat germ translation product. J Biol Chem. 1982 Jan 25;257(2):964–970. [PubMed] [Google Scholar]
- Robbi M., Lazarow P. B. Synthesis of catalase in two cell-free protein-synthesizing systems and in rat liver. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4344–4348. doi: 10.1073/pnas.75.9.4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roychoudhury R., Jay E., Wu R. Terminal labeling and addition of homopolymer tracts to duplex DNA fragments by terminal deoxynucleotidyl transferase. Nucleic Acids Res. 1976 Apr;3(4):863–877. doi: 10.1093/nar/3.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryall J., Rachubinski R. A., Nguyen M., Rozen R., Broglie K. E., Shore G. C. Regulation and expression of carbamyl phosphate synthetase I mRNA in developing rat liver and Morris hepatoma 5123D. J Biol Chem. 1984 Jul 25;259(14):9172–9176. [PubMed] [Google Scholar]
- Shimizu S., Yasui K., Tani Y., Yamada H. Acyl-CoA oxidase from Candida tropicalis. Biochem Biophys Res Commun. 1979 Nov 14;91(1):108–113. doi: 10.1016/0006-291x(79)90589-8. [DOI] [PubMed] [Google Scholar]
- Tanaka A., Osumi M., Fukui S. Peroxisomes of alkane-grown yeast: fundamental and practical aspects. Ann N Y Acad Sci. 1982;386:183–199. doi: 10.1111/j.1749-6632.1982.tb21416.x. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobe T., Higashi T. Studies on rat liver catalase. XI. Site of synthesis and segregation by stripped ER membranes. J Biochem. 1980 Nov;88(5):1341–1347. doi: 10.1093/oxfordjournals.jbchem.a133102. [DOI] [PubMed] [Google Scholar]
- Tolbert N. E. Metabolic pathways in peroxisomes and glyoxysomes. Annu Rev Biochem. 1981;50:133–157. doi: 10.1146/annurev.bi.50.070181.001025. [DOI] [PubMed] [Google Scholar]
- Villa-Komaroff L., Efstratiadis A., Broome S., Lomedico P., Tizard R., Naber S. P., Chick W. L., Gilbert W. A bacterial clone synthesizing proinsulin. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3727–3731. doi: 10.1073/pnas.75.8.3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens M. P., Buell G. N., Schimke R. T. Synthesis of double-stranded DNA complementary to lysozyme, ovomucoid, and ovalbumin mRNAs. Optimization for full length second strand synthesis by Escherichia coli DNA polymerase I. J Biol Chem. 1978 Apr 10;253(7):2483–2495. [PubMed] [Google Scholar]
- Yamada T., Tanaka A., Horikawa S., Numa S., Fukui S. Cell-free translation and regulation of Candida tropicalis catalase messenger RNA. Eur J Biochem. 1982 Dec 15;129(2):251–255. doi: 10.1111/j.1432-1033.1982.tb07046.x. [DOI] [PubMed] [Google Scholar]