Abstract
Progesterone receptors (PR) are expressed throughout the brain. However, their functional significance remains understudied. Here we report a novel role of PR as crucial mediators of neuroprotection using a model of transient middle cerebral artery occlusion and PR knockout mice. Six hours after ischemia, we observed a rapid increase in progesterone and 5α-dihydroprogesterone, the endogenous PR ligands, a process that may be a part of the natural neuroprotective mechanisms. PR deficiency, and even haploinsufficiency, increases the susceptibility of the brain to stroke damage. Within a time window of 24 h, PR-dependent signaling of endogenous brain progesterone limits the extent of tissue damage and the impairment of motor functions. Longer-term improvement requires additional treatment with exogenous progesterone and is also PR dependent. The potent and selective PR agonist Nestorone is also effective. In contrast to progesterone, levels of the neurosteroid allopregnanolone, which modulates γ-aminobutyric acid type A receptors, did not increase after stroke, but its administration protected both wild-type and PR-deficient mice against ischemic damage. These results show that 1) PR are linked to signaling pathways that influence susceptibility to stroke, and 2) PR are direct key targets for both endogenous neuroprotection and for therapeutic strategies after stroke, and they suggest a novel indication for synthetic progestins already validated for contraception. Although allopregnanolone may not be an endogenous neuroprotective agent, its administration protects the brain against ischemic damage by signaling mechanisms not involving PR. Collectively, our data clarify the relative roles of PR and allopregnanolone in neuroprotection after stroke.
Progesterone is receiving much attention as a neuroprotective agent and is making its way into neurological practice (1–3). Indeed, two phase II trials have already assessed the beneficial effects of progesterone after traumatic brain injury (TBI) (4, 5), and their encouraging outcomes have spurred the launching of two large phase III multicenter trials (Protect III, 2011, at http://www.clinicaltrials.gov/NCT00822900 and SyNAPSe, 2011, at http://www.synapse-trial.com). Progesterone is also a promising candidate for neuroprotective strategies after stroke, a major cause of death and neurological disability (6). The only approved treatment for acute stroke is thrombolysis with tissue plasminogen activator, but it can be used in only 10% of patients (7, 8). Progesterone treatment may represent a new, safe, and effective therapy that can be offered to a higher percentage of patients. Indeed, experimental studies have demonstrated its efficiency in reducing lesion volume and improving functional recovery after either transient or permanent occlusion of brain arteries (9–11).
Progesterone modulates the transcription of target genes by interacting with intracellular progesterone receptors (PR), which belong to the nuclear receptor superfamily of transcription factors (12). In addition to uterus, ovaries, and mammary glands, PR are also expressed throughout the brain, and they are abundant not only within hypothalamic nuclei involved in the control of reproductive functions but also in cerebral cortex and subcortical structures (13, 14). However, the significance of their widespread distribution in the brain is unknown, and still, recently, brain PR have been qualified as the neglected ones (15).
That so little attention has been devoted to brain functions of PR may relate to the widely accepted assumption that neuroprotective effects of progesterone may be mainly mediated by its metabolite allopregnanolone. Indeed, the neuroprotective actions of progesterone can be mimicked by its metabolite allopregnanolone (16), which does not interact with PR, but instead potentiates membrane γ-aminobutyric acid type A (GABAA) receptors, the major inhibitory neurotransmitter receptors in the brain (17, 18). However, it has been reported that allopregnanolone may be back-oxidized to 5 α-dihydroprogesterone (5α-DHP), which then interacts with PR, activates gene transcription, and regulates neuronal functions (19, 20). The signaling mechanisms involved in progesterone and allopregnanolone neuroprotection thus require clarification. A second possible reason why brain PR have been so much neglected may be the growing interest in new membrane receptors of progesterone [mPR and progesterone membrane receptor component 1 (PGRMC1)], although their biological significance remains to be explored (21).
We hypothesize that PR may be key mediators of the neuroprotective effects of progesterone and may have a contributory role in mediating the effects of allopregnanolone after stroke. To test this hypothesis, we used PR-deficient mice (PR−/−) and compared them with PR+/− and PR+/+ mice and checked their susceptibility to stroke. We also examined the therapeutic potential of PR as a direct drug target by administrating progesterone or the potent fourth-generation PR agonist Nestorone (22) or allopregnanolone after ischemia and reperfusion.
Materials and Methods
Animals
Mice were housed in a temperature-controlled room on a 12-h light, 12-h dark cycle with food and water ad libitum. Experimental protocols were approved by the Direction départementale de la protection des populations du Val-de-Marne, France, authorization number 94-345 to R.G., accredited establishment number 94-043-13). Experiments were performed in accordance with French ethical laws (Act 87--848; Ministère de l'Agriculture et de la Forêt) and the European Communities Council Directives of November 24, 1986 (86/609/EEC) guidelines for the care and use of laboratory animals.
A breeding colony of PRlacz mice was established in our animal facility. PRlacz mice (C57BL6/129SvEv background) were generated by inserting the lacZ reporter and neomycin resistance (neor) genes into exon 1. Their insertion site was chosen to place the lacZ reporter under the control of the endogenous PR gene promoter and to effectively disrupt the transcription of both PR isoforms (23). The PRlacz mice are a phenocopy of the previously described PR-knockout (PRKO) mouse (24). Each mouse was identified for its PR genotype by using a validated genotyping protocol (25). Genomic DNA from mouse tails was extracted using direct PCR lysis reagent (Viagen Biotech, Euromedex, France). One microgram of DNA was subjected to PCR amplification using Taq DNA polymerase (Invitrogen, Inc., Carlsbad, CA). PCR was performed by denaturing the DNA at 94 C for 3 min, followed by 35 cycles of amplification: 94 C for 1 min, 55 C for 1 min, 72 C for 1 min, and a final extension step at 72 C for 10 min. The following PR-specific primers were used: P1 (5′-TAG ACA GTG TCT TAG ACT CGT TGT TG-3′), P3 (5′-GAT GGG CAC ATG GAT GAA ATC-3′), and a lacZ-specific primer, lacZ (5′-CTT CAC CCA CCG GTA CCT TAC GCT TC-3′). The presence of primer-amplified PCR product was detected on agarose gel and visualized by ethidium bromide fluorescence. We observed the presence of a 590-bp DNA band for PR+/+ mice (corresponding to the PR gene, P1/P3 primers), a 148-bp band for PR−/− mice (P1/lacZ primers), or both bands for PR+/− mice.
Anesthesia
Before surgery, adult male PRlacz mice weighting 26–30 g were anesthetized with ketamine (50 mg/kg) and xylazine hydrochloride (6 mg/kg). Although the most commonly used anesthetic drugs may provide a certain degree of neuroprotection (25), ketamine, a noncompetitive antagonist of N-Methyl-D-aspartate (NMDA) receptors, has the advantage over the others not to interact with GABAA receptors (26). The choice of ketamine reduces potential interferences between the anesthetic drug and the treatments we are studying (progesterone and allopregnanolone).
Transient stroke model
Throughout surgery, body temperature was monitored by a rectal probe and maintained at 37 ± 0.5 C with a homeothermic blanket control unit (Harvard Apparatus, Edenbridge, Kent, UK). The middle cerebral artery (MCA) was occluded for 1 h with an intraluminal filament (27). After ligature of the left common carotid artery, a nylon monofilament coated with thermomelting glue (4 mm long, 190 μm diameter) was introduced through an arteriotomy performed on the external carotid artery and advanced into the internal carotid artery. Occlusion of the MCA was controlled by monitoring the cerebral blood flow within the MCA territory by laser Doppler flowmetry (Moor Instruments, France). Mice with less than a 50% drop in blood flow were excluded from the studies. The filament was withdrawn 1 h after occlusion to allow reperfusion, and the common carotid artery ligature was also removed. Sham-operated mice underwent the same surgical procedure except that no filament was inserted. After surgery, the wound was sutured.
In vivo steroid treatments
To test the effect of steroid treatment after reperfusion, mice were randomly and blindly assigned to either progesterone (8 mg/kg; Sigma), allopregnanolone (8 mg/kg; Sigma), Nestorone (0.08 mg/kg; Population Council, Rockefeller University, New York, NY) or vehicle-treated (sesame oil; Sigma) group. All steroids (progesterone, allopregnanolone, and Nestorone) were initially dissolved in a small volume of ethanol and further diluted in sesame oil to obtain the desired final steroid concentrations. The vials containing the steroid solutions were placed in an incubator overnight to allow the evaporation of ethanol. The vehicle solution was prepared according to the same procedure (ethanol plus sesame oil) without steroids. Injections were given ip at 1, 6, and 24 h after MCA occlusion (MCAO) according to established neuroprotective protocols (9, 16). Mice in the vehicle group underwent the same experimental protocol, except that they received the same volume/weight of vehicle only. After treatments, mice were returned to their cage at 29 C with free access to food and water. Treated mice were killed at 48 h after MCAO.
Analysis of infarct-size and edema
Cerebral infarct volumes and areas were determined after triphenyltetrazolium chloride (TTC) staining of brain sections (28, 29). Brains were cut into seven 1-mm-thick coronal sections using a MacIlwain tissue chopper (Mickle Laboratory Engineering, Gomshall, Surrey, UK). Slices were quickly immersed in 0.1 m PBS (pH 7.4) containing 2% TTC for 20 min at room temperature and were then stored in phosphate-buffered 4% paraformaldehyde (Acros, Noisy-le-Grand, France) overnight before analysis. The area of damaged unstained brain tissue was measured on the posterior surface of each slice using a computer image analysis system (NIH Image). To correct for brain swelling, each infarct area was multiplied by the ratio of the surface of the intact (contralateral) hemisphere to the infarcted (ipsilateral) hemisphere at the same level. Total volume of damaged tissue, expressed as cubic millimeters, was calculated by linear integration of the corrected lesion areas (30). Edema volume was calculated by computing the ratio of the volume of the infarcted to the intact hemisphere. The rationale for this method is that the accumulation of water, within the infarction, proportionally enlarges the infarcted hemisphere (30).
Rotarod test
Motor coordination of mice was evaluated by the time they remained on a constant-speed rotarod (10 revolutions/min). A trial ended when the animal fell off or gripped the device without attempting to walk. The day before MCAO, mice were trained in three trials to obtain stable baselines. Just before ischemia, the test was performed again, and the mean duration of three trials on the device was recorded, representing the preischemic value. On the day of killing, the mice were again tested on the rotarod, and the mean duration of three trials represented the postischemic value.
Analysis of steroids by gas chromatography/mass spectrometry (GC/MS)
Six hours after MCAO, brain and plasma levels of progesterone, 5α-DHP, and allopregnanolone were determined by GC/MS according to a validated protocol (31, 32). Corticosterone was measured in mice plasma. Steroids were extracted from tissues and plasma with methanol, and internal standards were added for steroid quantification: 2 ng [2H6]5α-DHP (CDN Isotopes) for 5α-DHP, 2 ng 19-norprogesterone (Steraloids, Newport, Rhode Island) for progesterone and allopregnanolone, and 10 ng [2H8]corticosterone (CDN Isotopes, Sainte Foy La Grande, France) for corticosterone. Unconjugated and conjugated steroids were separated by a previously described solid-phase extraction and a recycling procedure (33). The fraction containing the unconjugated steroids was filtered and further purified and fractionated by HPLC (Thermo Fisher Scientific, San Jose, CA). Three fractions were collected from the HPLC system: the first containing 5α-DHP, silylated with a mixture of N-methyl-N-trimethylsilyltrifluoroacetamide, ammonium iodide (NH4I), and dithioerythritol (1000:2:5, vol/wt/wt), the second containing progesterone and allopregnanolone derivatized with heptafluorobutyric anhydride, and the third with corticosterone also derivatized with heptafluorobutyric anhydride. GC/MS analysis of the steroid derivatives was performed using an AS 3000 autosampler, a Focus GC gas chromatograph, and a DSQII mass spectrometer (Thermo Fisher Scientific San Jose, CA). Identification of each steroid derivative was supported by its retention time during GC and two diagnostic ions resulting from electron impact ionization. Quantification was performed in single ion monitoring mode according to the major diagnostic ion (Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org).
RNA isolation and quantitative PCR
Total RNA was extracted from cerebral tissues using Trizol reagent (Life Technologies, Invitrogen, Saint Aubin, France). The concentration and purity of total RNA was determined by measuring the OD at 260 and 280 nm. All samples were precipitated with ethanol and then dissolved in distilled water to a concentration of 1 μg/μl, and their quality was verified by gel electrophoresis. Total RNA was subjected to deoxyribonuclease 1 (Invitrogen, Saint Aubin, France) treatment to remove residual genomic DNA. cDNA templates for PCR amplification were synthesized from 2 μg total RNA using a SuperScript II ribonuclease H reverse transcriptase kit (Invitrogen) for 60 min at 42 C in the presence of random hexamer primers. Primers for quantitative PCR were designed using Primer Express version 3.0, Applied Biosystems (Foster City, CA) (Supplemental Table 2). Cyclophilin-B was chosen as the housekeeping gene. The relative gene expression for the mRNA of PR was determined using the ABI PRISM 700 sequence detection system (Applied Biosystems) and the standard curve method (34). Specificity of PCR amplification and the absence of dimers were confirmed by melting curve analysis. In addition, PCR products were verified with high-resolution gel electrophoresis. Linearity and efficiency of PCR amplification were validated before quantification (Supplemental Table 2). Both parameters were assessed using standard curves generated by six increasing amounts of cDNA over a 10-fold range. The relationship between the cycle threshold (CT) and the logarithm of the cDNA concentration was determined from the correlation coefficient (r) and the amplification efficiency. Correlation coefficients confirm the linearity, and amplification efficiency was calculated using the equation Ex = (10−1/slope) − 1 × 100 (35), where Ex is amplification efficiency. The linearity and efficiency of the amplification of PCR assays among different templates permitted accurate quantification. Each reaction mixture contained 2 ng cDNA/μl reaction, 0.2 μm primers, and 1× Sybr Green (Applied Biosystems) in a final volume of 25 μl. PCR were performed in triplicate under optimized conditions: 95 C at 10 min followed by 40 cycles at 95 C for 15 sec and 60 C for 1 min. The concentration of the target genes was calculated by referring CT values in each sample with CT values of the internal standard curve.
Statistics
Statistical analysis was performed by using Statistica version 9.1 (StatSoft, Tulsa, OK). Comparisons between multiple groups were made by one-way or two-way ANOVA and followed by Newman-Keuls tests. Correlation between two continuous variables was determined by Pearson's correlation test. P values <0.05 were considered statistically significant.
Results
PR-dependent signaling of endogenous brain progesterone confers early protection after stroke
PRKO mice exhibited an increased susceptibility to stroke damage
To determine whether nuclear PR might play a role in the resistance of the brain to ischemic injury, we used adult male PRlacz mice, here referred to as wild-type PR+/+, heterozygous PR+/−, and homozygous knockout PR−/− mice, generated by inserting the lacZ reporter and neomycin resistance genes into exon 1 of the PR gene to effectively disrupt its transcription (23). We used males because of their low and stable levels of circulating progesterone and because a protective treatment for stroke targeting the PR should also be applicable in men, who show a high risk and poor outcome when compared with women (36). The uterus and brain of PR+/− mice have been reported to contain about half of the number of PR binding sites when compared with PR+/+ mice (24, 37). Using quantitative PCR, we showed that PR mRNA expression is absent in PR−/− mice and reduced by about 60% in cerebral cortex, subcortical regions, and hypothalamus of PR+/− mice (Supplemental Fig. 1).
Transient focal cerebral ischemia was induced by occluding the left MCA during 1 h with an intraluminal filament followed by reperfusion (27). During MCAO, PR+/+, PR+/−, and PR−/− mice showed similar reductions in cerebral blood flow recorded by laser Doppler flowmetry (respectively, 68.8 ± 3, 68.1 ± 2.8, and 72.7 ± 3.5%, means ± sem, P = 0.47).
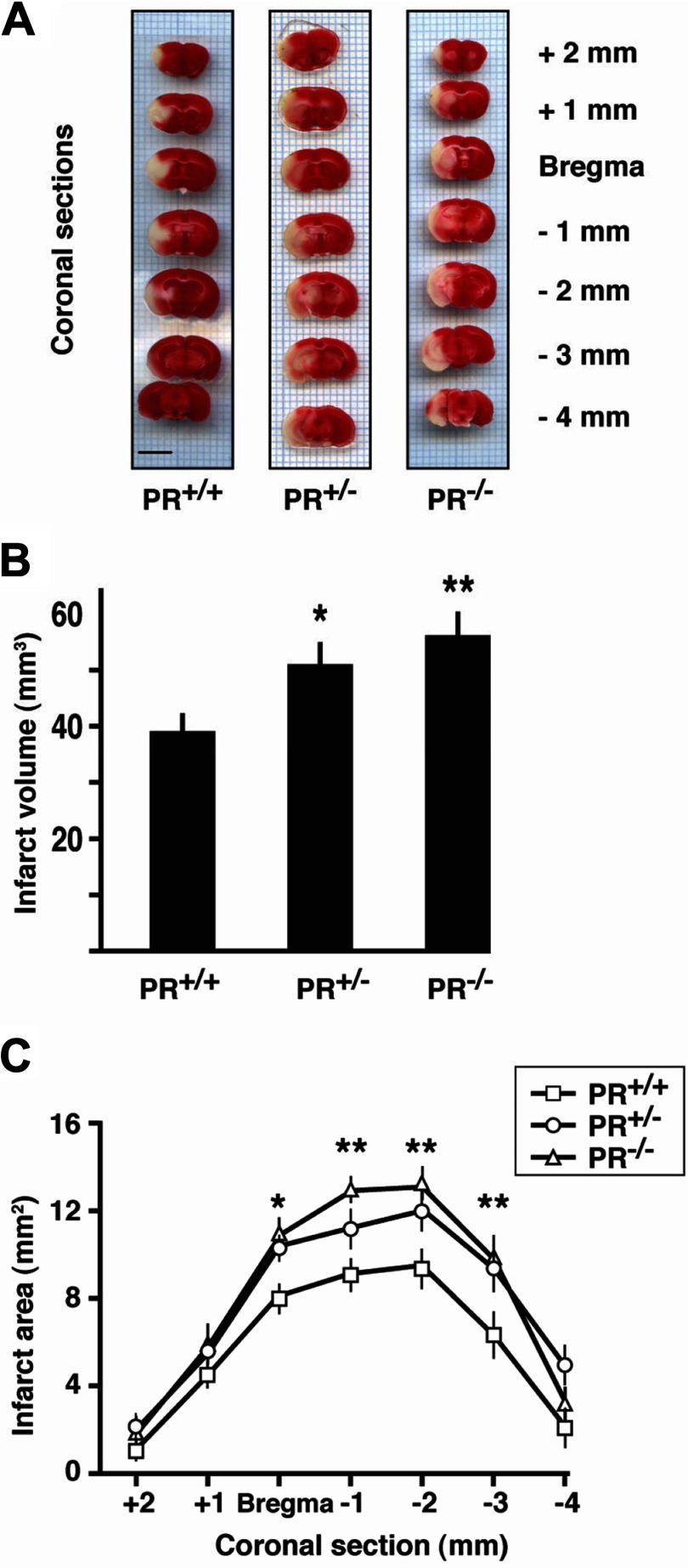
Ischemic brain damage was first assessed at 24 h after MCAO by staining brain sections with TTC (Fig. 1A). The total infarct volume was increased by 31% in PR+/− and by 46% in PR−/− mice when compared with wild-type PR+/+ mice (Fig. 1, A and B). Similarly, infarct areas were significantly larger on successive 1-mm coronal brain sections (Fig. 1C).
Fig. 1.
Influence of PR disruption on infarct volume and areas at 24 h after MCAO. A, Successive 1-mm-thick coronal brain sections of representative PR+/+, PR+/−, and PR−/− mice stained with TTC (viable tissue in red; infarcted tissue appears pale). Scale bar, 5 mm. B, Quantitative analysis of total infarct volumes calculated from brain sections. C, Areas of damaged brain tissue on successive brain sections. Data represent means ± sem; n = 14–18 per group. *, P < 0.05; **, P < 0.01 as compared with PR+/+ mice after one-way (B) or two-way (C) ANOVA (PR genotype × brain section level).
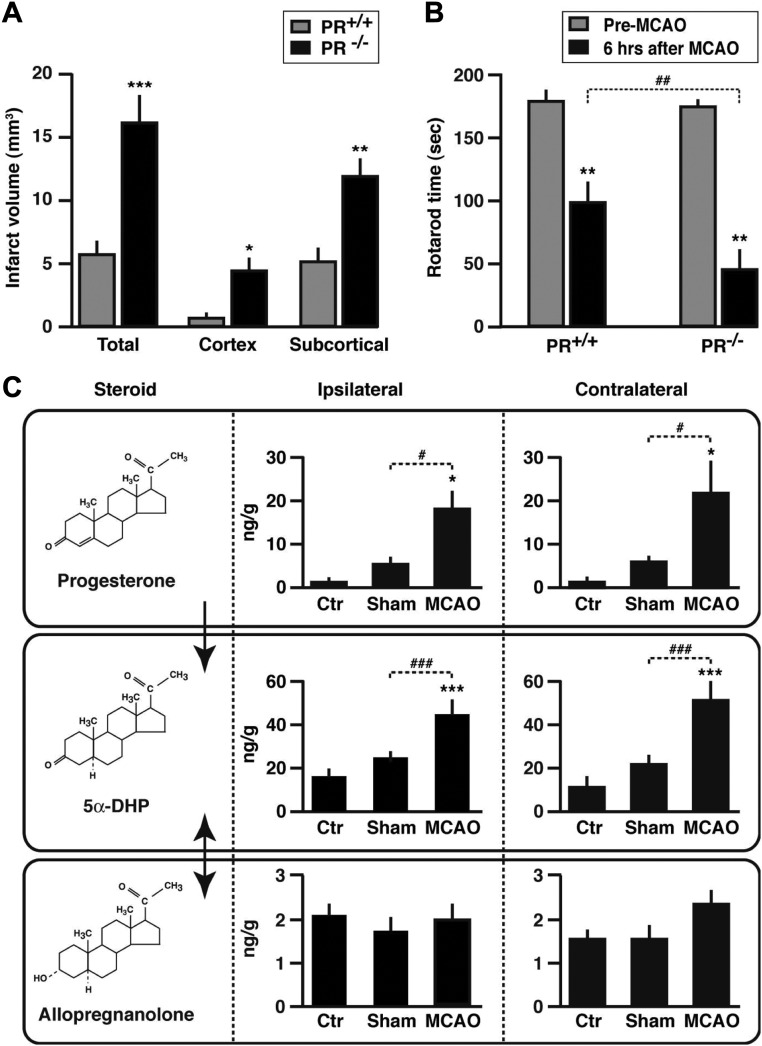
To investigate whether PR-dependent neuroprotection may be an early event, brain damage was examined at 6 h after MCAO in PR+/+, and PR−/− mice. As in the previous experiment, there was no difference in the reduction in blood flow between the two genotypes after MCAO, and there was no correlation between decrease in blood pressure and infarct volume. Although the total infarct volume was still small at this time, it was three times larger in PR−/− mice than in PR+/+ mice. Moreover, infarct volumes were significantly larger in both the cortex and subcortical structures of PR−/− mice (Fig. 2A). Areas of damage on successive brain sections, up to −4 mm from Bregma, were also much greater in PR−/− mice when compared with PR+/+ mice (not shown). Linear regression yielded a high positive correlation between ischemic brain damage and edema (r = 0.65; Pearson's correlation P < 0.01). There was, however, no correlation between infarct volume and weight of the mice (P = 0.6). To assess neurological deficits, mice were tested on a rotating cylinder (rotarod), which functions as a treadmill and is widely used to assess genetic and drug effects on motor coordination. There was no difference between PR+/+ and PR−/− mice in the time spent on the rotarod before ischemic injury. Motor functions were altered after MCAO in both groups, but they were more impaired in PR−/− than in PR+/+ mice (Fig. 2B). At this early stage, no correlation was observed between rotarod performance and infarct volume (Pearson's correlation P = 0.19).
Fig. 2.
Endpoint measures at 6 h after MCAO. A, Infarct volumes in PR+/+ and PR−/− mice. Two-way ANOVA (PR genotype × brain region) revealed a significant effect of PR genotype (P < 0.001). ***, P < 0.001; **, P < 0.01; *, P < 0.05 as compared with PR+/+ mice. B, Time spent on a rotarod before or 6 h after MCAO. **, P < 0.01 as compared with preischemia performance; ##, P < 0.01 as indicated. C, Brain levels of progesterone, 5α-DHP, and allopregnanolone in PR+/+ male mice analyzed by GC/MS (nanograms per gram of tissue). ***, P < 0.001; *, P < 0.05 as compared with control mice (Ctr); ###, P < 0.001; #, P < 0.05 as indicated. Data represent means ± sem; n = 6 per group.
Stroke induced an increase in the levels of the two neurosteroids progesterone and 5α-DHP, both endogenous ligands of PR
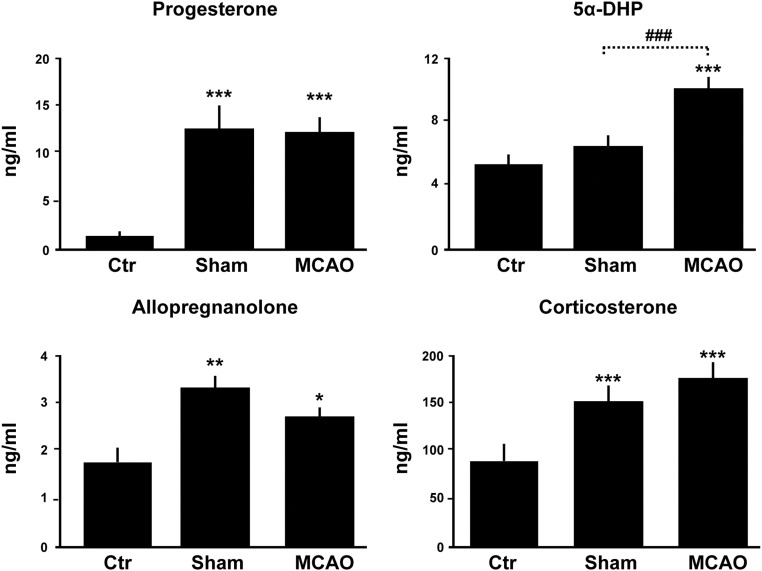
The above results showed that PR inactivation results in increased vulnerability of the brain to ischemic damage. In addition, they pointed to a role of endogenous brain progesterone in PR-dependent neuroprotective signaling. We therefore measured the levels of progesterone and its reduced derivatives by GC/MS in brain and plasma. Levels of progesterone were strongly increased, more than 25-fold, in the brains of male mice as early as 6 h after MCAO, reaching levels observed in females during pregnancy (Fig. 2C). The increase in brain progesterone was observed both in the lesioned ipsilateral and in the contralateral brain sides. We also observed a 2- to 3-fold increase in brain levels of the progesterone metabolite 5α-DHP, which also increases the transcriptional activity of PR (19). In contrast, stroke did not significantly affect brain levels of allopregnanolone, which were about 10 times lower than those of progesterone and 5α-DHP (Fig. 2C). Part of the progesterone present in brain tissue may be of adrenal origin, because plasma levels of the hormone were increased in response to both sham surgery and MCAO (Fig. 3). Consistently, plasma levels of adrenal corticosterone were up-regulated in response to surgery stress (Fig. 3). However, ischemic injury selectively stimulated progesterone synthesis within the brain, because brain but not plasma levels were significantly higher after MCAO than after sham surgery (Figs. 2C and 3). Moreover, the ratios of brain to plasma levels of progesterone and 5α-DHP were significantly increased after MCAO but not in response to sham surgery (Table 1). Up-regulation of the brain's endogenous PR ligands (progesterone and 5α-DHP) may thus confer resistance to ischemic damage.
Fig. 3.
Plasma levels of progesterone, 5α-DHP, allopregnanolone, and corticosterone as analyzed by GC/MS. Plasma was sampled from wild-type control mice (Ctr) and at 6 h after either sham operation or MCAO. ***, P < 0.001; **, P < 0.01; *, P < 0.05 as compared with control; ###, P < 0.001 as indicated by Newman-Keuls tests after one-way ANOVA. Data represent means ± sem; n = 5 per group.
Table 1.
Ratios of brain levels of progesterone, 5α-DHP, and allopregnanolone to plasma levels
| Control | Sham | MCAO | |
|---|---|---|---|
| Progesterone | 1.13 ± 0.3 | 0.50 ± 0.2 | 1.96 ± 0.3a |
| 5α-DHP | 3.30 ± 0.2 | 3.80 ± 0.3 | 4.40 ± 0.2a |
| Allopregnanolone | 1.40 ± 0.2 | 0.57 ± 0.3 | 0.80 ± 0.3 |
Results are shown means ± sem; n = 5 per group. Brain levels are nanograms per gram, and plasma levels are nanograms per milliliter.
P < 0.05 as compared with the corresponding control group by Newman-Keuls tests after one-way ANOVA.
Longer-term neuroprotection after stroke requires additional treatment with progesterone and is also PR dependent
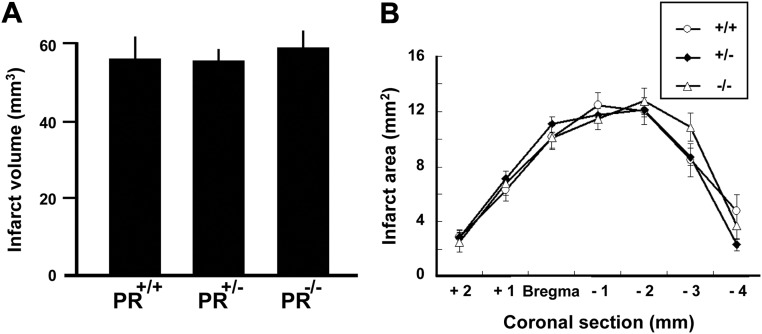
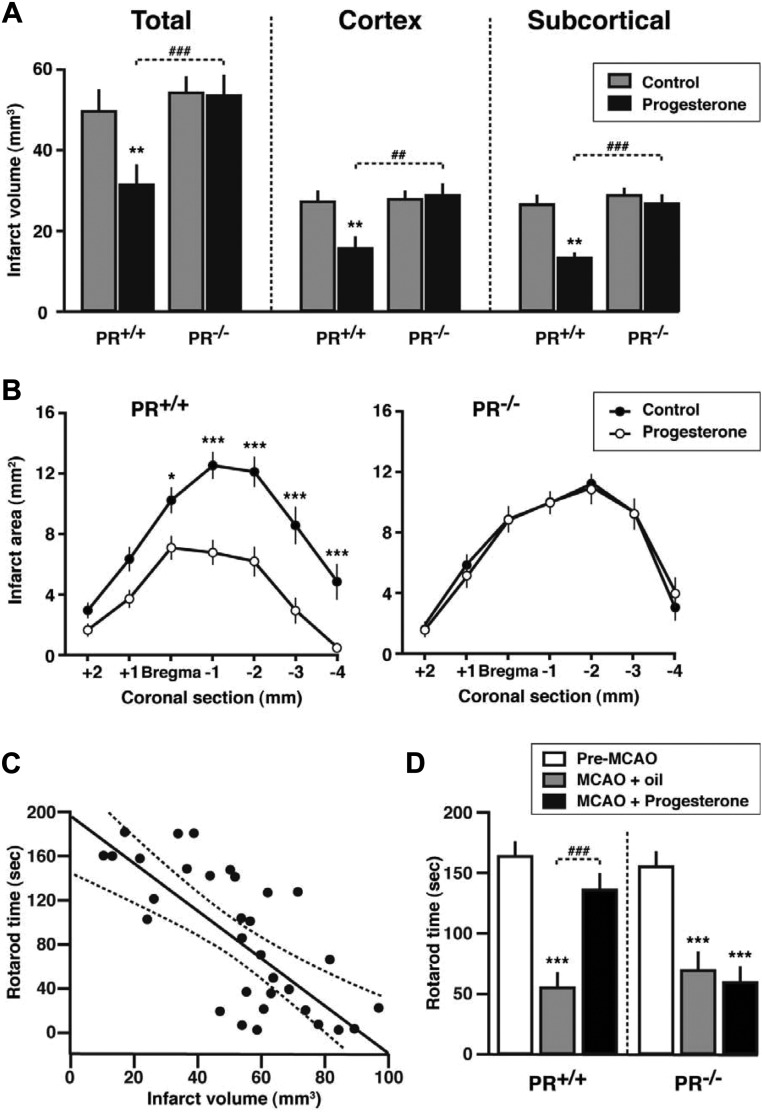
The presence of PR confers resistance to ischemic damage during the first 24 h. We then investigated whether a beneficial influence of PR can still be observed in PR+/+, PR+/−, and PR−/− mice at 48 h after MCAO. Although endogenous progesterone via PR did appear to protect the brain during the first 24 h of recovery, the damage continued to evolve during the next 24 h, rendering PR+/+, PR+/−, and PR−/− groups equally damaged at 48 h. Indeed, in contrast to the 6- and 24-h time points, no influence of the PR genotype on infarct volume and areas was observed (Fig. 4). These results suggest an acute and transient protective effect of endogenous PR activation, albeit insufficient to overcome the deleterious effects of ischemia on delayed cell death. Previous work has shown that treatment with progesterone after MCAO results in longer-term improvement of neurological outcomes (9). To confirm that the administration of exogenous progesterone can indeed provide longer-lasting protection against stroke injury, and to investigate whether its beneficial effects are PR dependent, PR+/+ and PR−/− mice received three injections of progesterone (8 mg/kg) at 1, 6, and 24 h after MCAO, according to a previously established neuroprotective treatment (9). Morphological and functional outcomes were examined at 48 h after MCAO. The total infarct volume and ischemic lesions in both cerebral cortex and subcortical structures were reduced by progesterone treatment in PR+/+ but not in PR−/− mice (Fig. 5A). Likewise, the administration of progesterone reduced areas of damage in the different brain areas only in PR+/+ mice (Fig. 5B). Motor coordination, when assessed on the rotarod, revealed a highly significant negative correlation with the infarct volume (Fig. 5C). Both PR+/+ and PR−/− mice displayed functional motor deficits at 48 h after MCAO. Whereas progesterone treatment significantly improved the ability of PR+/+ mice to remain on the rotarod as compared with vehicle treatment, it was inefficient in PR−/− mice (Fig. 5D). We also observed a strong positive correlation between infarct volume and brain edema, and progesterone treatment reduced brain edema in PR+/+ mice, but not in PR−/− mice (Fig. 6, A and B). Thus, at 48 h after MCAO, the improvement of histological and functional outcomes by progesterone treatment is PR dependent.
Fig. 4.
Endpoint measures at 48 h after MCAO. A, Total brain infarct volume in PR+/+, PR+/−, and PR−/− mice. There were no significant differences between the three groups. There was also no effect of the PR genotype on lesion volume in cerebral cortex and subcortical structures (not shown). B, The areas of damaged brain tissue measured on seven successive 1-mm-thick brain sections (section 3 = bregma level) were similar in PR+/+, PR+/−, and PR−/− mice. Data represent means ± sem; n = 15 per group.
Fig. 5.
Improvement of neurological outcomes at 48 h after MCAO requires treatment by with progesterone and is PR dependent. A, Reduction in infarct volume by progesterone. **, P < 0.01 as compared with control mice; ###, P < 0.001; ##, P < 0.01 as indicated. B, Infarct areas on successive brain sections of PR+/+ and PR−/− mice. ***, P < 0.001; *, P < 0.05 as compared with the corresponding sections of progesterone-treated mice. C, Scatter plot and Pearson's correlation analysis of infarct volume vs. rotarod performance (r = −0.70; P < 0.001; dotted lines delimit the 0.95 confidence interval). D, Effects of MCAO and progesterone treatment on the time mice remained on a rotarod. ***, P < 0.001 compared with preischemia performance; ###, P < 0.001 as indicated. Data represent means ± sem; n = 16 per group.
Fig. 6.
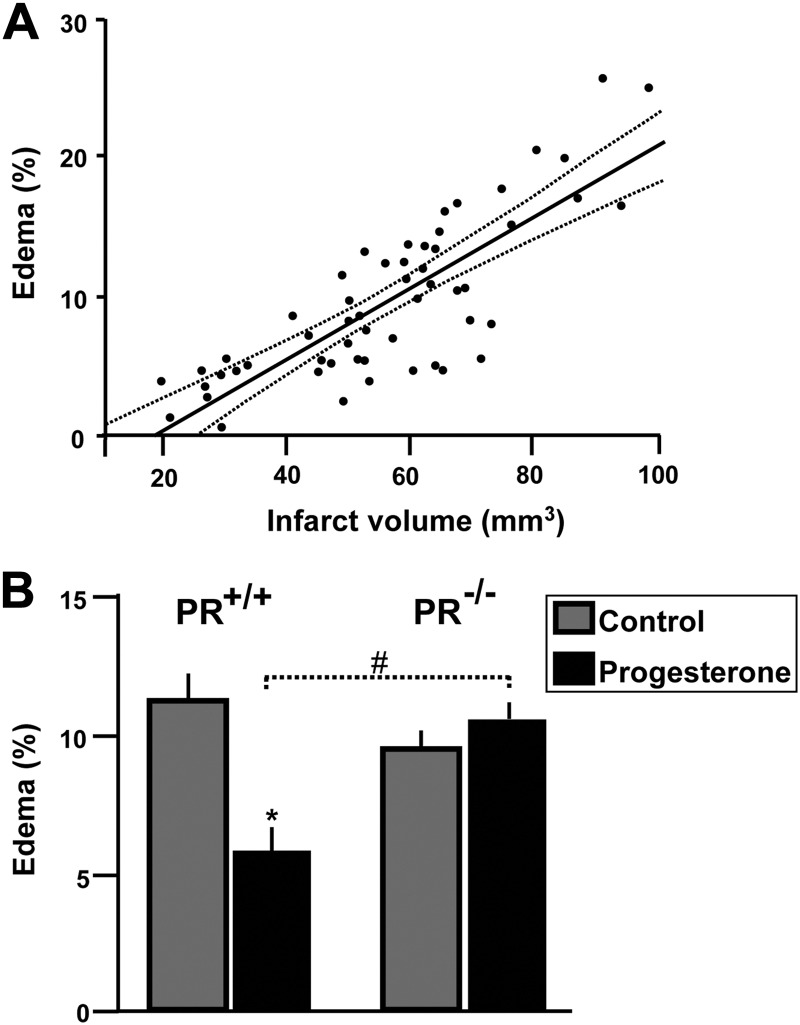
Effects of progesterone treatment on brain edema at 48 h after MCAO. A, Pearson's correlation analysis revealed a high positive correlation between ischemic brain damage and edema (r = 0.79; P < 0.001; dotted lines delimit the 0.95 confidence interval). B, Effect of progesterone on brain edema in PR+/+ and PR−/− mice. #, P < 0.05 as indicated by Newman-Keuls tests after two-way ANOVA (PR genotype × treatment). Data represent means ± sem; n = 17–19.
The potent and selective PR agonist and contraceptive agent Nestorone is also neuroprotective
The identification of PR as a neuroprotective drug target opens new therapeutic indications for selective synthetic progestins, already validated for contraception or hormone therapy. Nestorone (16-methylene-17α-acetoxy-19-norpregn-4-ene-3, 20-dione) is a 19- norprogesterone derivative that shows high specific binding to PR and is about 100 times more potent than progesterone (22, 38). PR+/+ mice received three injections of Nestorone (only 0.08 mg/kg at 1, 6, and 24 h after MCAO), and neurological outcomes were examined at 48 h. When compared with oil treatment, Nestorone reduced the total infarct volume by 32% (P < 0.01), the ischemic lesions in cerebral cortex and in subcortical structures, respectively, by 22% (P < 0.01) and 52% (P < 0.01) and increased the time PR+/+ mice remained on the rotarod by 43% (P < 0.05).
Relative role of PR in the neuroprotective effect of the neurosteroid allopregnanolone
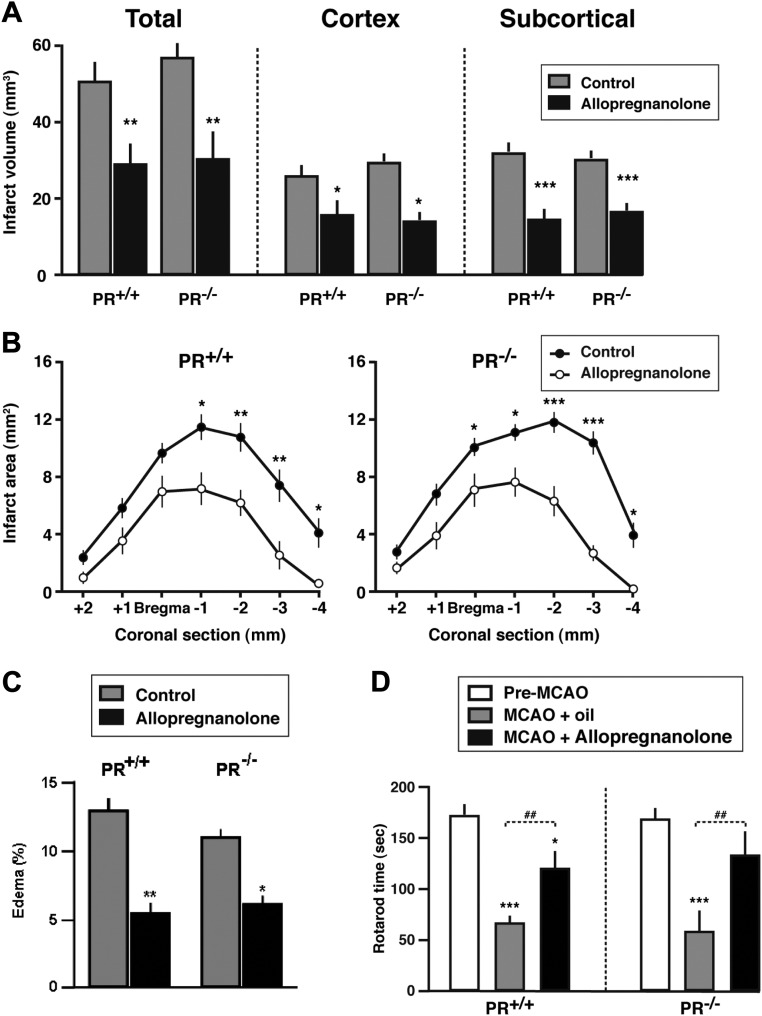
The fact that progesterone does not reduce ischemic brain damage in PR−/− mice and that Nestorone is also neuroprotective strongly suggested that the effects of progesterone may be mainly mediated by PR and not after conversion to its derivative allopregnanolone, which does not bind to PR. Because 1) previous work has shown that allopregnanolone has neuroprotective effects after stroke and 2) allopregnanolone can be converted back to 5α-DHP, which binds PR, we checked whether there may be a contributory role of PR in mediating the effects of allopregnanolone. If the protective effects of allopregnanolone are mediated via its conversion to PR-active 5α-DHP (19), they should no longer be observed in PR−/− mice. To test this possibility, PR+/+and PR−/− mice received three ip injections of a neuroprotective dose of allopregnanolone (8 mg/kg) at 1, 6, and 24 h after MCAO (16), and neurological outcomes were examined at 48 h. Treatment with allopregnanolone markedly reduced total infarct volume, ischemic damage in cerebral cortex and subcortical structures, and the extension of lesions at different brain levels in both PR+/+ and PR−/− mice (Fig. 7, A and B). Allopregnanolone also reduced brain edema independently of the genotype (Fig. 7C). The brain-protective effects of allopregnanolone were accompanied by improved functional outcomes evaluated on the rotarod, which were again independent of PR (Fig. 7D).
Fig. 7.
Improvement of neurological outcomes by allopregnanolone at 48 h after MCAO does not involve PR. A, Reduction in infarct volume by allopregnanolone. Two-way ANOVA revealed a significant effect of allopregnanolone treatment (P < 0.01) but no influence of PR genotype for total, cortical, and subcortical infarct volumes. ***, P < 0.001; **, P < 0.01; *, P < 0.05 as compared with the corresponding control group. B, Infarct areas on successive brain sections of PR+/+ and PR−/− mice. ***, P < 0.001; **, P < 0.01; *, P < 0.05 as compared with the corresponding sections of allopregnanolone-treated mice. C, Reduction of edema by allopregnanolone (means ± sem) in both PR+/+ and PR−/− mice. Two-way ANOVA (PR genotype × treatment) showed a significant effect of treatment (P < 0.001) but no effect of PR genotype. **, P < 0.01; *, P < 0.05 as compared with corresponding control group by Newman-Keuls tests. D, The time mice remained on a rotarod. ***, P < 0.001, *, P < 0.05 compared with preischemia performance; ##, P < 0.01 as indicated. Data represent means ± sem; n = 6–8 for vehicle- and allopregnanolone-treated mice.
Discussion
Here, we identify PR as an important component of neuroprotective signaling after ischemic stroke. Both short-term neuroprotective effects of endogenous brain progesterone and longer-term neuroprotective effects of progesterone treatment indeed require the presence of PR.
In our experiments, deficiency of PR results in increased susceptibility of the brain to ischemic damage. Intriguingly, at 24 h after MCAO, brain damage was nearly as severe in heterozygous PR+/− mice, which lack only one allele of the PR gene, as in PR−/− mice. PR haploinsufficiency increases the vulnerability of brain cortical and subcortical regions to ischemic insult. This is a first example of PR haploinsufficiency, because decreased expression of the receptor in hypothalamus or uterus of PR+/− mice does not result in a particular reproductive phenotype (37). Moreover, only part of hypothalamic PR needs to be activated for high levels of sexual receptivity in females (39). Our results suggest that brain PR may be a limiting factor for reactive neuroprotective processes after ischemia.
Six hours after MCAO, we observed a specific increase (not observed in sham-operated mice) of cerebral levels of progesterone and 5α-DHP, contrasting with stable levels of allopregnanolone. These data suggest an activation of the cerebral biosynthesis of progesterone and its reduced metabolite after stroke. The up-regulation of the endogenous PR ligands, progesterone and 5α-DHP, may confer resistance to ischemic damage and is consistent with the observed neuroprotective role of PR early after stroke. Increased synthesis of progesterone and 5α-DHP within the central nervous system has previously been reported after traumatic injury and spinal cord injury (31, 40), and both steroids can be synthesized de novo from cholesterol by neurons and glial cells, thus qualifying as neurosteroids (41). The rapid activation of cerebral biosynthesis of PR ligands may be a part of endogenous neuroprotective mechanisms in response to lesions of the central nervous system, and it may contribute to the extended window of opportunity for progesterone treatment to prevent neuron loss after ischemic or TBI injury (3, 42).
Although endogenous progesterone protected the brain during the first 24 h of recovery, no differences between PR+/+ and PR−/− mice were observed in infarct size and motor coordination at 48 h after MCAO. These findings suggest that the reactive increase in brain steroidogenesis and endogenous PR activation in response to stroke are not sufficient for providing protection against ischemic brain damage for as long as 48 h and to overcome the deleterious effect of ischemia on delayed cell death. Our results show that longer-term neuroprotective effects require additional treatment with exogenous progesterone and the presence of PR.
We showed that progesterone specifically acts through PR. Indeed, treatment with progesterone is effective in PR+/+ mice but failed to confer neuroprotection in PR-deficient mice, and the potent and selective PR agonist Nestorone was also effective in wild-type PR+/+ mice. It is important to note that Nestorone improved neurological outcomes at a dose 100 times lower than progesterone, confirming the potency of this progestin and a key role of the PR in neuroprotection. Nestorone is a highly selective progestin derived from 19 nor-progesterone and exerts its activity at much lower doses than progesterone (22). Moreover, this progestin does not convert into 5α-DHP or allopregnanolone, and its action has been shown via the PR with a higher transactivation effect of the PR than progesterone itself. In addition, it was found that this progestin shows interesting properties in neurogenesis, similar to progesterone and superior to other progestins (43) as well as in myelin repair (44) justifying its testing in this stroke model. In addition, although progesterone is used in relatively high doses (8 mg/kg), the possibility to use a much lower dose (100-fold less) with Nestorone to induce the same effect on the stroke model represents a promising avenue for future therapeutic use.
Until now, there was a widely accepted assumption that neuroprotective effects of progesterone may be mainly mediated by its metabolite allopregnanolone, which does not bind to PR but activates membrane GABAA receptors (17, 18). Our results suggest that the biosynthesis of allopregnanolone in the brain is not sufficient for efficient neuroprotection because 1) stroke did not significantly affect brain levels of allopregnanolone and 2) neither endogenous allopregnanolone nor the treatment with an elevated dose of exogenous progesterone protected against ischemic brain injury in PR−/− mice.
We showed that treatment with allopregnanolone reduced brain edema and infarct volume independently of the genotype. The brain-protective effects of allopregnanolone were accompanied by improved functional outcomes evaluated on the rotarod, which were again independent of PR. These results demonstrate that allopregnanolone treatment can protect the brain against ischemic damage by signaling mechanisms not involving PR. This neuroprotection may implicate binding of allopregnanolone to membrane GABAA receptors (17) or to the nuclear pregnane X receptor (45) or by its direct actions on mitochondria (46).
Although we demonstrate a key role of PR in the neuroprotective effects of progesterone, our results do not rule out an involvement of additional signaling pathways of progesterone. In addition to PR and GABAA receptors, progesterone-regulated neural responses may be mediated by putative membrane receptors such as the mPR (α-, β-, and γ-isoforms) and PGRMC1. However, it is very unlikely that a down-regulation of the alternative receptors contributes to the absence of progesterone responses in PRKO mice: 1) actions of allopregnanolone that are dependent on GABAA receptors (47) are preserved in PRKO mice; 2) the expression of PGRMC1, previously known as 25-Dx, is up-regulated in PRKO mice (48); and 3) we have recently reported that the expression of mPRα, mPRβ, and mPRγ in the central nervous system is similar in wild-type and PRKO mice (49).
In conclusion, this study demonstrates that 1) PR is an essential key for early endogenous neuroprotection, and it might serve as pharmacological drug target for stroke therapy. With the success, in terms of safety and improved outcome, of the first two clinical trials of progesterone after TBI (4, 5), trials targeting PR may be realistic strategies to promote recovery after stroke. We also demonstrate that 2) allopregnanolone may not be an endogenous neuroprotective agent. However allopregnanolone treatment can protect the brain against ischemic damage by signaling mechanisms not involving PR.
Supplementary Material
Acknowledgments
We thank Françoise Robert for the art work and Krzysztof Rajkowski for critical reading of the manuscript. We also acknowledge the excellent technical assistance for steroid analysis of Antoine Pianos, Bernard Eychenne, and Annie Cambourg.
This work was supported by Institut National de la Santé et de la Recherche Médicale (INSERM), University Paris-Sud, a cooperative program between the Governments of France and Argentina (INSERM/CONICET), and by the Barrus Foundation. A.L. was beneficiary of a postdoctoral fellowship from University Paris-Sud. M.S. is the beneficiary of an Interface Program of INSERM and the Assistance Publique-Hôpitaux de Paris.
Disclosure Summary: The authors have no competing financial interests. R.S.W., M.S., and R.G. declare being co-inventors of a patent concerning the neuroprotective effects of Nestorone: International Application No. PCT/US2010/053201. The other authors have nothing to declare.
Footnotes
- CT
- Cycle threshold
- 5α-DHP
- 5α-dihydroprogesterone
- GABAA
- γ-aminobutyric acid type A
- GC/MS
- gas chromatography/mass spectrometry
- MCA
- middle cerebral artery
- MCAO
- MCA occlusion
- mPR
- membrane PR
- NMDA
- N-Methyl-D-aspartate
- PGRMC1
- progesterone membrane receptor component 1
- PR
- progesterone receptors
- PRKO
- PR-knockout
- TBI
- traumatic brain injury
- TTC
- triphenyltetrazolium chloride.
References
- 1. Stein DG. 2008. Progesterone exerts neuroprotective effects after brain injury. Brain Res Rev 57:386–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schumacher M, Guennoun R, Ghoumari A, Massaad C, Robert F, El-Etr M, Akwa Y, Rajkowski K, Baulieu EE. 2007. Novel perspectives for progesterone in hormone replacement therapy, with special reference to the nervous system. Endocr Rev 28:387–439 [DOI] [PubMed] [Google Scholar]
- 3. Gibson CL, Gray LJ, Bath PM, Murphy SP. 2008. Progesterone for the treatment of experimental brain injury; a systematic review. Brain 131:318–328 [DOI] [PubMed] [Google Scholar]
- 4. Xiao G, Wei J, Yan W, Wang W, Lu Z. 2008. Improved outcomes from the administration of progesterone for patients with acute severe traumatic brain injury: a randomized controlled trial. Crit Care 12:R61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wright DW, Kellermann AL, Hertzberg VS, Clark PL, Frankel M, Goldstein FC, Salomone JP, Dent LL, Harris OA, Ander DS, Lowery DW, Patel MM, Denson DD, Gordon AB, Wald MM, Gupta S, Hoffman SW, Stein DG. 2007. ProTECT: a randomized clinical trial of progesterone for acute traumatic brain injury. Ann Emerg Med 49:391–402, 402e1–e2 [DOI] [PubMed] [Google Scholar]
- 6. Dobkin BH. 2008. Training and exercise to drive poststroke recovery. Nat Clin Pract Neurol 4:76–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van der Worp HB, Sena ES, Donnan GA, Howells DW, Macleod MR. 2007. Hypothermia in animal models of acute ischaemic stroke: a systematic review and meta-analysis. Brain 130:3063–30674 [DOI] [PubMed] [Google Scholar]
- 8. Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJL, eds. 2006. Measuring the global burden of disease and risk factors. Washington, DC: World Bank; [PubMed] [Google Scholar]
- 9. Gibson CL, Murphy SP. 2004. Progesterone enhances functional recovery after middle cerebral artery occlusion in male mice. J Cereb Blood Flow Metab 24:805–813 [DOI] [PubMed] [Google Scholar]
- 10. Sayeed I, Wali B, Stein DG. 2007. Progesterone inhibits ischemic brain injury in a rat model of permanent middle cerebral artery occlusion. Restor Neurol Neurosci 25:151–159 [PubMed] [Google Scholar]
- 11. Gibson CL, Coomber B, Murphy SP. 2011. Progesterone is neuroprotective following cerebral ischaemia in reproductively ageing female mice. Brain 134:2125–2133 [DOI] [PubMed] [Google Scholar]
- 12. Conneely OM, Mulac-Jericevic B, DeMayo F, Lydon JP, O'Malley BW. 2002. Reproductive functions of progesterone receptors. Recent Prog Horm Res 57:339–355 [DOI] [PubMed] [Google Scholar]
- 13. Parsons B, Rainbow TC, MacLusky NJ, McEwen BS. 1982. Progestin receptor levels in rat hypothalamic and limbic nuclei. J Neurosci 2:1446–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gofflot F, Chartoire N, Vasseur L, Heikkinen S, Dembele D, Le Merrer J, Auwerx J. 2007. Systematic gene expression mapping clusters nuclear receptors according to their function in the brain. Cell 131:405–418 [DOI] [PubMed] [Google Scholar]
- 15. Blaustein JD. 2008. Progesterone and progestin receptors in the brain: the neglected ones. Endocrinology 149:2737–2738 [DOI] [PubMed] [Google Scholar]
- 16. Sayeed I, Guo Q, Hoffman SW, Stein DG. 2006. Allopregnanolone, a progesterone metabolite, is more effective than progesterone in reducing cortical infarct volume after transient middle cerebral artery occlusion. Ann Emerg Med 47:381–389 [DOI] [PubMed] [Google Scholar]
- 17. Hosie AM, Wilkins ME, da Silva HM, Smart TG. 2006. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature 444:486–489 [DOI] [PubMed] [Google Scholar]
- 18. Belelli D, Lambert JJ. 2005. Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat Rev Neurosci 6:565–575 [DOI] [PubMed] [Google Scholar]
- 19. Rupprecht R, Reul JM, Trapp T, van Steensel B, Wetzel C, Damm K, Zieglgänsberger W, Holsboer F. 1993. Progesterone receptor-mediated effects of neuroactive steroids. Neuron 11:523–530 [DOI] [PubMed] [Google Scholar]
- 20. Rupprecht R, Berning B, Hauser CA, Holsboer F, Reul JM. 1996. Steroid receptor-mediated effects of neuroactive steroids: characterization of structure-activity relationship. Eur J Pharmacol 303:227–234 [DOI] [PubMed] [Google Scholar]
- 21. Thomas P. 2008. Characteristics of membrane progestin receptor alpha (mPRα) and progesterone membrane receptor component 1 (PGMRC1) and their roles in mediating rapid progestin actions. Front Neuroendocrinol 29:292–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kumar N, Koide SS, Tsong Y, Sundaram K. 2000. Nestorone: a progestin with a unique pharmacological profile. Steroids 65:629–636 [DOI] [PubMed] [Google Scholar]
- 23. Ismail PM, Li J, DeMayo FJ, O'Malley BW, Lydon JP. 2002. A novel LacZ reporter mouse reveals complex regulation of the progesterone receptor promoter during mammary gland development. Mol Endocrinol 16:2475–2489 [DOI] [PubMed] [Google Scholar]
- 24. Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr, Shyamala G, Conneely OM, O'Malley BW. 1995. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev 9:2266–2278 [DOI] [PubMed] [Google Scholar]
- 25. Schifilliti D, Grasso G, Conti A, Fodale V. 2010. Anaesthetic-related neuroprotection: intravenous or inhalational agents? CNS Drugs 24:893–907 [DOI] [PubMed] [Google Scholar]
- 26. Franks NP, Lieb WR. 1994. Molecular and cellular mechanisms of general anaesthesia. Nature 367:607–614 [DOI] [PubMed] [Google Scholar]
- 27. Connolly ES, Jr, Winfree CJ, Stern DM, Solomon RA, Pinsky DJ. 1996. Procedural and strain-related variables significantly affect outcome in a murine model of focal cerebral ischemia. Neurosurgery 38:523–531; discussion 532 [DOI] [PubMed] [Google Scholar]
- 28. Türeyen K, Vemuganti R, Sailor KA, Dempsey RJ. 2004. Infarct volume quantification in mouse focal cerebral ischemia: a comparison of triphenyltetrazolium chloride and cresyl violet staining techniques. J Neurosci Methods 139:203–207 [DOI] [PubMed] [Google Scholar]
- 29. Bederson JB, Pitts LH, Germano SM, Nishimura MC, Davis RL, Bartkowski HM. 1986. Evaluation of 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke 17:1304–1308 [DOI] [PubMed] [Google Scholar]
- 30. Golanov EV, Reis DJ. 1995. Contribution of cerebral edema to the neuronal salvage elicited by stimulation of cerebellar fastigial nucleus after occlusion of the middle cerebral artery in rat. J Cereb Blood Flow Metab 15:172–174 [DOI] [PubMed] [Google Scholar]
- 31. Meffre D, Pianos A, Liere P, Eychenne B, Cambourg A, Schumacher M, Stein DG, Guennoun R. 2007. Steroid profiling in brain and plasma of male and pseudopregnant female rats after traumatic brain injury: analysis by gas chromatography/mass spectrometry. Endocrinology 148:2505–2517 [DOI] [PubMed] [Google Scholar]
- 32. Liere P, Pianos A, Eychenne B, Cambourg A, Bodin K, Griffiths W, Schumacher M, Baulieu EE, Sjövall J. 2009. Analysis of pregnenolone and dehydroepiandrosterone in rodent brain: cholesterol autoxidation is the key. J Lipid Res 50:2430–2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liere P, Pianos A, Eychenne B, Cambourg A, Liu S, Griffiths W, Schumacher M, Sjövall J, Baulieu EE. 2004. Novel lipoidal derivatives of pregnenolone and dehydroepiandrosterone and absence of their sulfated counterparts in rodent brain. J Lipid Res 45:2287–2302 [DOI] [PubMed] [Google Scholar]
- 34. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 35. Peinnequin A, Mouret C, Birot O, Alonso A, Mathieu J, Clarençon D, Agay D, Chancerelle Y, Multon E. 2004. Rat pro-inflammatory cytokine and cytokine related mRNA quantification by real-time polymerase chain reaction using SYBR green. BMC Immunol 5:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu M, Dziennis S, Hurn PD, Alkayed NJ. 2009. Mechanisms of gender-linked ischemic brain injury. Restor Neurol Neurosci 27:163–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mani SK, Blaustein JD, O'Malley BW. 1997. Progesterone receptor function from a behavioral perspective. Horm Behav 31:244–255 [DOI] [PubMed] [Google Scholar]
- 38. Attardi BJ, Koduri S, Hild SA. 2010. Relative progestational and androgenic activity of four progestins used for male hormonal contraception assessed in vitro in relation to their ability to suppress LH secretion in the castrate male rat. Mol Cell Endocrinol 328:16–21 [DOI] [PubMed] [Google Scholar]
- 39. Pfaff DW, McEwen BS. 1983. Actions of estrogens and progestins on nerve cells. Science 219:808–814 [DOI] [PubMed] [Google Scholar]
- 40. Labombarda F, Pianos A, Liere P, Eychenne B, Gonzalez S, Cambourg A, De Nicola AF, Schumacher M, Guennoun R. 2006. Injury elicited increase in spinal cord neurosteroid content analyzed by gas chromatography mass spectrometry. Endocrinology 147:1847–1859 [DOI] [PubMed] [Google Scholar]
- 41. Baulieu EE, Robel P, Schumacher M. 2001. Neurosteroids: beginning of the story. Int Rev Neurobiol 46:1–32 [DOI] [PubMed] [Google Scholar]
- 42. Stein DG, Wright DW, Kellermann AL. 2008. Does progesterone have neuroprotective properties? Ann Emerg Med 51:164–172 [DOI] [PubMed] [Google Scholar]
- 43. Liu L, Zhao L, She H, et al. Clinically relevant progestins regulate neurogenic and neuroprotective responses in vitro and in vivo. Endocrinology 151:5782–5794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hussain R, El-Etr M, Gaci O, et al. Progesterone and Nestorone facilitate axon remyelination: a role for progesterone receptors. Endocrinology 152:3820–3831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Langmade SJ, Gale SE, Frolov A, Mohri I, Suzuki K, Mellon SH, Walkley SU, Covey DF, Schaffer JE, Ory DS. 2006. Pregnane X receptor (PXR) activation: a mechanism for neuroprotection in a mouse model of Niemann-Pick C disease. Proc Natl Acad Sci USA 103:13807–13812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sayeed I, Parvez S, Wali B, Siemen D, Stein DG. 2009. Direct inhibition of the mitochondrial permeability transition pore: a possible mechanism for better neuroprotective effects of allopregnanolone over progesterone. Brain Res 1263:165–173 [DOI] [PubMed] [Google Scholar]
- 47. Reddy DS, O'Malley BW, Rogawski MA. 2005. Anxiolytic activity of progesterone in progesterone receptor knockout mice. Neuropharmacology 48:14–24 [DOI] [PubMed] [Google Scholar]
- 48. Krebs CJ, Jarvis ED, Chan J, Lydon JP, Ogawa S, Pfaff DW. 2000. A membrane-associated progesterone-binding protein, 25-Dx, is regulated by progesterone in brain regions involved in female reproductive behaviors. Proc Natl Acad Sci USA 97:12816–12821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Labombarda F, Meffre D, Delespierre B, Krivokapic-Blondiaux S, Chastre A, Thomas P, Pang Y, Lydon JP, Gonzalez SL, De Nicola AF, Schumacher M, Guennoun R. 2010. Membrane progesterone receptors localization in the mouse spinal cord. Neuroscience 166:94–106 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.