Abstract
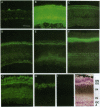
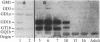
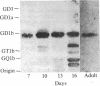
Mouse monoclonal antibody 18B8 detects developmentally regulated antigens in chicken retina and brain. The antigens detected by immunofluorescence appear initially on cell bodies in retinas of 6-13 day embryos. In older embryos during synapse formation and in adults, the antigen is localized in discrete laminae within the inner synaptic layer of retina and also is present in the outer synaptic layer and the outer segments of photoreceptor cells. The antigens from retina and brain were purified partially and were shown to be gangliosides of unknown structure that contain at least two sialic acid residues. Gangliosides that are recognized by antibody 18B8 change both qualitatively and quantitatively during neuronal development. These changes were correlated with the spatial and temporal changes in antigen expression detected histochemically.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnstable C. J. Monoclonal antibodies which recognize different cell types in the rat retina. Nature. 1980 Jul 17;286(5770):231–235. doi: 10.1038/286231a0. [DOI] [PubMed] [Google Scholar]
- Chaudhari N., Hahn W. E. Genetic expression in the developing brain. Science. 1983 May 27;220(4600):924–928. doi: 10.1126/science.6189184. [DOI] [PubMed] [Google Scholar]
- Cheresh D. A., Reisfeld R. A., Varki A. P. O-acetylation of disialoganglioside GD3 by human melanoma cells creates a unique antigenic determinant. Science. 1984 Aug 24;225(4664):844–846. doi: 10.1126/science.6206564. [DOI] [PubMed] [Google Scholar]
- Chikaraishi D. M. Complexity of cytoplasmic polyadenylated and nonpolyadenylated rat brain ribonucleic acids. Biochemistry. 1979 Jul 24;18(15):3249–3256. doi: 10.1021/bi00582a009. [DOI] [PubMed] [Google Scholar]
- Dodd J., Solter D., Jessell T. M. Monoclonal antibodies against carbohydrate differentiation antigens identify subsets of primary sensory neurones. Nature. 1984 Oct 4;311(5985):469–472. doi: 10.1038/311469a0. [DOI] [PubMed] [Google Scholar]
- Dreyfus H., Urban P. F., Edel-Harth S., Mandel P. Developmental patterns of gangliosides and of phospholipids in chick retina and brain. J Neurochem. 1975 Sep;25(3):245–250. doi: 10.1111/j.1471-4159.1975.tb06960.x. [DOI] [PubMed] [Google Scholar]
- Eisenbarth G. S., Walsh F. S., Nirenberg M. Monoclonal antibody to a plasma membrane antigen of neurons. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4913–4917. doi: 10.1073/pnas.76.10.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finne J. Occurrence of unique polysialosyl carbohydrate units in glycoproteins of developing brain. J Biol Chem. 1982 Oct 25;257(20):11966–11970. [PubMed] [Google Scholar]
- Fredman P., Magnani J. L., Nirenberg M., Ginsburg V. Monoclonal antibody A2B5 reacts with many gangliosides in neuronal tissue. Arch Biochem Biophys. 1984 Sep;233(2):661–666. doi: 10.1016/0003-9861(84)90492-2. [DOI] [PubMed] [Google Scholar]
- Fredman P., Nilsson O., Tayot J. L., Svennerholm L. Separation of gangliosides on a new type of anion-exchange resin. Biochim Biophys Acta. 1980 Apr 18;618(1):42–52. doi: 10.1016/0005-2760(80)90052-1. [DOI] [PubMed] [Google Scholar]
- Fredman P., Norén R., Månsson J. E., Svennerholm L. Concentration, composition and structure of gangliosides from the central nervous systems of dogfish, Squalus acanthia, and cod, Gadus callarias. Biochim Biophys Acta. 1982 Nov 12;713(2):410–418. doi: 10.1016/0005-2760(82)90260-0. [DOI] [PubMed] [Google Scholar]
- Grunwald G. B., Pratt R. S., Lilien J. Enzymic dissection of embryonic cell adhesive mechanisms. III. Immunological identification of a component of the calcium-dependent adhesive system of embryonic chick neural retina cells. J Cell Sci. 1982 Jun;55:69–83. doi: 10.1242/jcs.55.1.69. [DOI] [PubMed] [Google Scholar]
- Hakomori S., Kannagi R. Glycosphingolipids as tumor-associated and differentiation markers. J Natl Cancer Inst. 1983 Aug;71(2):231–251. [PubMed] [Google Scholar]
- Johnson G. D., Nogueira Araujo G. M. A simple method of reducing the fading of immunofluorescence during microscopy. J Immunol Methods. 1981;43(3):349–350. doi: 10.1016/0022-1759(81)90183-6. [DOI] [PubMed] [Google Scholar]
- Kahn A. J. An autoradiographic analysis of the time of appearance of neurons in the developing chick neural retina. Dev Biol. 1974 May;38(1):30–40. doi: 10.1016/0012-1606(74)90256-5. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Lemmon V., Gottlieb D. I. Monoclonal antibodies selective for the inner portion of the chick retina. J Neurosci. 1982 May;2(5):531–535. doi: 10.1523/JNEUROSCI.02-05-00531.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J. M., Beasley L., Stallcup W. B. The D1.1 antigen: a cell surface marker for germinal cells of the central nervous system. J Neurosci. 1984 Mar;4(3):820–831. doi: 10.1523/JNEUROSCI.04-03-00820.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linser P., Moscona A. A. Hormonal induction of glutamine synthetase in cultures of embryonic retina cells: requirement for neuron-glia contact interactions. Dev Biol. 1983 Apr;96(2):529–534. doi: 10.1016/0012-1606(83)90190-2. [DOI] [PubMed] [Google Scholar]
- Magnani J. L., Nilsson B., Brockhaus M., Zopf D., Steplewski Z., Koprowski H., Ginsburg V. A monoclonal antibody-defined antigen associated with gastrointestinal cancer is a ganglioside containing sialylated lacto-N-fucopentaose II. J Biol Chem. 1982 Dec 10;257(23):14365–14369. [PubMed] [Google Scholar]
- Mintz G., Gottlieb D. I., Reitman M. L., Derby M., Glaser L. Developmental changes in glycoproteins of the chick nervous system. Brain Res. 1981 Feb 9;206(1):51–70. doi: 10.1016/0006-8993(81)90100-1. [DOI] [PubMed] [Google Scholar]
- Nilsson O., Fredman P., Klinghardt G. W., Dreyfus H., Svennerholm L. Chloroquine-induced accumulation of gangliosides and phospholipids in skeletal muscles. Quantitative determination and characterization of stored lipids. Eur J Biochem. 1981 Jun 1;116(3):565–571. doi: 10.1111/j.1432-1033.1981.tb05373.x. [DOI] [PubMed] [Google Scholar]
- Panzetta P., Maccioni H. J., Caputto R. Synthesis of retinal gangliosides during chick embryonic development. J Neurochem. 1980 Jul;35(1):100–108. doi: 10.1111/j.1471-4159.1980.tb12494.x. [DOI] [PubMed] [Google Scholar]
- Rothbard J. B., Brackenbury R., Cunningham B. A., Edelman G. M. Differences in the carbohydrate structures of neural cell-adhesion molecules from adult and embryonic chicken brains. J Biol Chem. 1982 Sep 25;257(18):11064–11069. [PubMed] [Google Scholar]
- Rougon G., Deagostini-Bazin H., Hirn M., Goridis C. Tissue- and developmental stage-specific forms of a neural cell surface antigen linked to differences in glycosylation of a common polypeptide. EMBO J. 1982;1(10):1239–1244. doi: 10.1002/j.1460-2075.1982.tb00019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rösner H. Ganglioside changes in the chicken optic lobes as biochemical indicators of brain development and maturation. Brain Res. 1982 Mar 18;236(1):49–61. doi: 10.1016/0006-8993(82)90033-6. [DOI] [PubMed] [Google Scholar]
- Sheffield J. B., Fischman D. A. Intercellular junctions in the developing neural retina of the chick embryo. Z Zellforsch Mikrosk Anat. 1970;104(3):405–418. doi: 10.1007/BF00335691. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G., Milner R. J., Gottesfeld J. M., Lerner R. A. Identifier sequences are transcribed specifically in brain. Nature. 1984 Mar 15;308(5956):237–241. doi: 10.1038/308237a0. [DOI] [PubMed] [Google Scholar]
- Svennerholm L., Fredman P. A procedure for the quantitative isolation of brain gangliosides. Biochim Biophys Acta. 1980 Jan 18;617(1):97–109. doi: 10.1016/0005-2760(80)90227-1. [DOI] [PubMed] [Google Scholar]
- Thiery J. P., Brackenbury R., Rutishauser U., Edelman G. M. Adhesion among neural cells of the chick embryo. II. Purification and characterization of a cell adhesion molecule from neural retina. J Biol Chem. 1977 Oct 10;252(19):6841–6845. [PubMed] [Google Scholar]
- Trisler G. D., Schneider M. D., Nirenberg M. A topographic gradient of molecules in retina can be used to identify neuron position. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2145–2149. doi: 10.1073/pnas.78.4.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel Z., Daniels M. P., Nirenberg M. Synapse and acetylcholine receptor synthesis by neurons dissociated from retina. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2370–2374. doi: 10.1073/pnas.73.7.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WITKOVSKY P. AN ONTOGENETIC STUDY OF RETINAL FUNCTION IN THE CHICK. Vision Res. 1963 Nov;61:341–355. doi: 10.1016/0042-6989(63)90008-7. [DOI] [PubMed] [Google Scholar]