Abstract
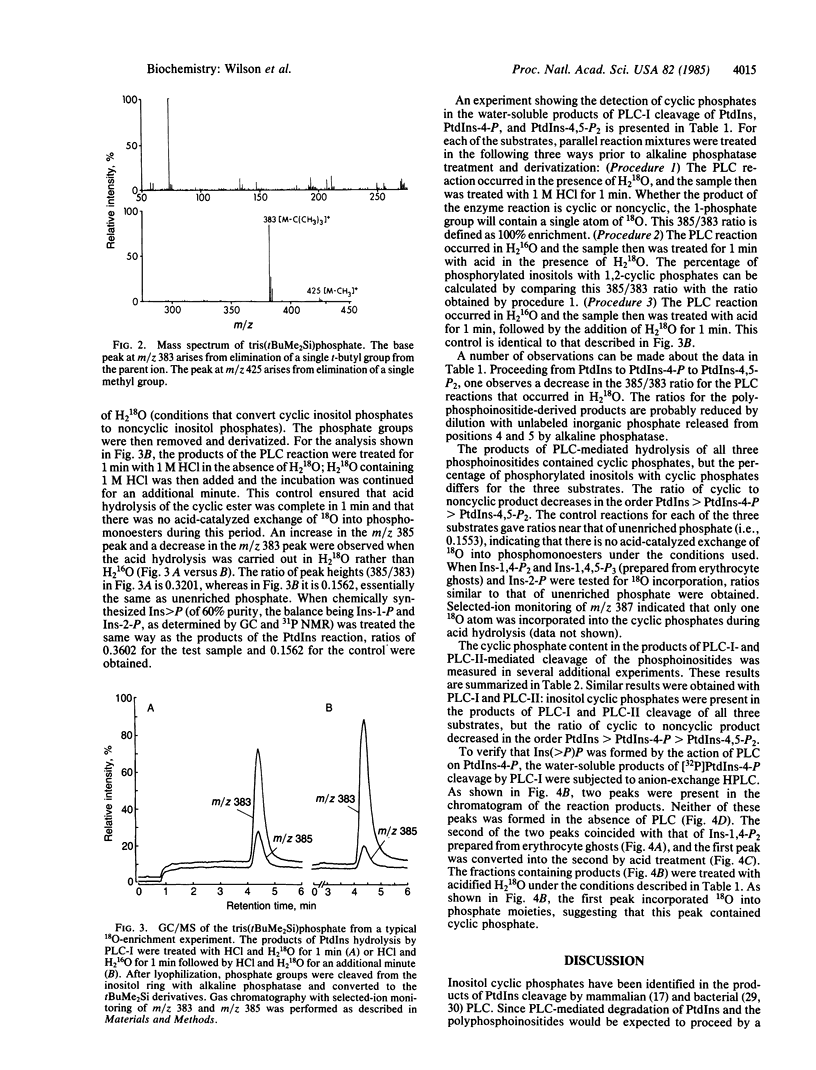
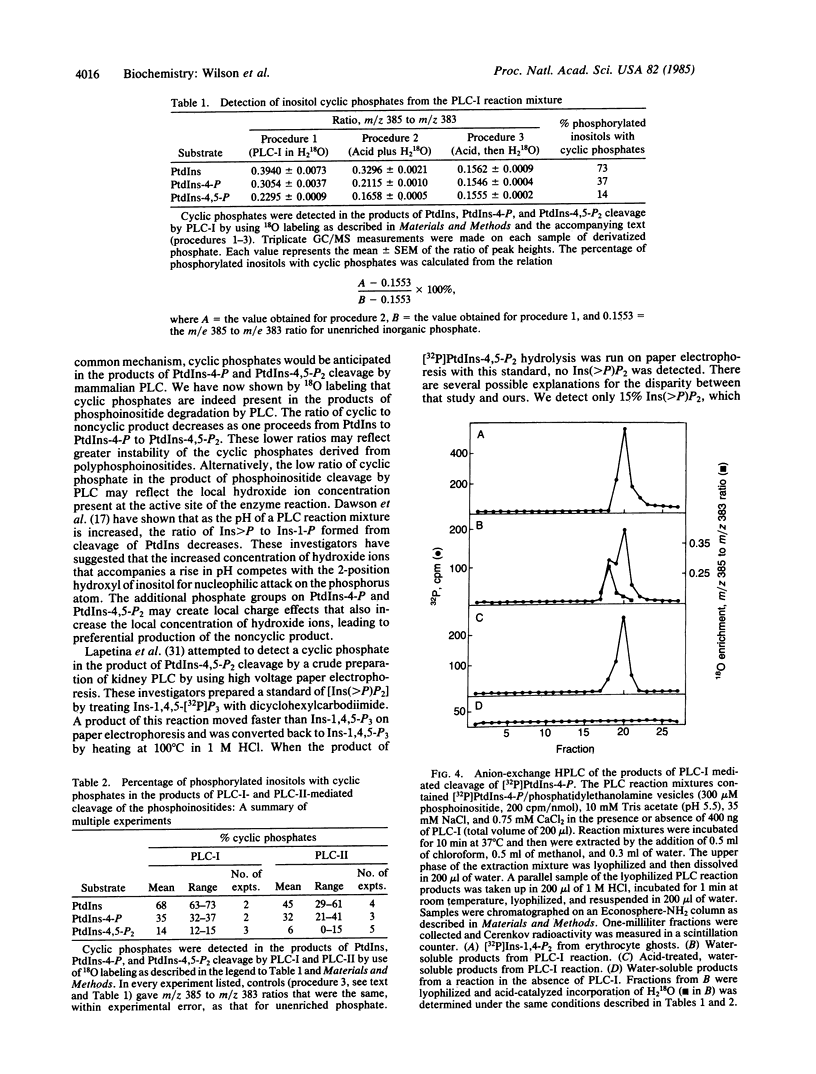
Previous studies have shown that metabolism of phosphatidylinositol by phospholipase C produces a mixture of two water-soluble products: inositol 1-phosphate and inositol 1,2-(cyclic)phosphate. In the present study, we demonstrate that the water-soluble products of phosphatidylphosphoinositol (polyphosphoinositide) cleavage by purified ram seminal vesicle phospholipase C enzymes also contain cyclic phosphates. Inositol cyclic phosphates were detected by 18O labeling. In the presence of acid, cyclic phosphates are rapidly hydrolyzed to phosphomonoesters, and when the hydrolysis is carried out in H2 18O, the resultant phosphomonoesters will contain 18O. The 18O content of the phosphomonoesters was measured following alkaline phosphatase treatment and conversion of the inorganic phosphate to a volatile derivative for gas chromatography/mass spectrometry. Inositol cyclic phosphates were found in the phospholipase C cleavage products of all three phosphoinositides, but the ratio of cyclic to noncyclic product was found to decrease in the order phosphatidylinositol greater than phosphatidylinositol 4-phosphate greater than phosphatidylinositol 4,5-bisphosphate. The formation of myo-inositol 1,2(cyclic)-4-bisphosphate was further substantiated by anion-exchange HPLC of the water-soluble products of [32P]phosphatidylinositol 4-phosphate metabolism by phospholipase C. Two peaks were detected one of which, on acid treatment, incorporated 18O from H2 18O into phosphate groups, consistent with this peak containing the cyclic phosphate product. These results suggest that polyphosphoinositide breakdown in stimulated cells may occur via a cyclic phosphate intermediate, as has been described for phosphatidylinositol. These cyclic phosphates contain a reactive bond that may play a role in phosphoinositide-derived signal transduction.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Agranoff B. W., Murthy P., Seguin E. B. Thrombin-induced phosphodiesteratic cleavage of phosphatidylinositol bisphosphate in human platelets. J Biol Chem. 1983 Feb 25;258(4):2076–2078. [PubMed] [Google Scholar]
- Aub D. L., Putney J. W., Jr Metabolism of inositol phosphates in parotid cells: implications for the pathway of the phosphoinositide effect and for the possible messenger role of inositol trisphosphate. Life Sci. 1984 Apr 2;34(14):1347–1355. doi: 10.1016/0024-3205(84)90006-7. [DOI] [PubMed] [Google Scholar]
- Bell R. L., Kennerly D. A., Stanford N., Majerus P. W. Diglyceride lipase: a pathway for arachidonate release from human platelets. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3238–3241. doi: 10.1073/pnas.76.7.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Heslop J. P., Irvine R. F., Brown K. D. Inositol trisphosphate formation and calcium mobilization in Swiss 3T3 cells in response to platelet-derived growth factor. Biochem J. 1984 Aug 15;222(1):195–201. doi: 10.1042/bj2220195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Brown J. E., Rubin L. J. A direct demonstration that inositol-trisphosphate induces an increase in intracellular calcium in Limulus photoreceptors. Biochem Biophys Res Commun. 1984 Dec 28;125(3):1137–1142. doi: 10.1016/0006-291x(84)91402-5. [DOI] [PubMed] [Google Scholar]
- Brown J. E., Rubin L. J., Ghalayini A. J., Tarver A. P., Irvine R. F., Berridge M. J., Anderson R. E. myo-Inositol polyphosphate may be a messenger for visual excitation in Limulus photoreceptors. Nature. 1984 Sep 13;311(5982):160–163. doi: 10.1038/311160a0. [DOI] [PubMed] [Google Scholar]
- Burgess G. M., Godfrey P. P., McKinney J. S., Berridge M. J., Irvine R. F., Putney J. W., Jr The second messenger linking receptor activation to internal Ca release in liver. Nature. 1984 May 3;309(5963):63–66. doi: 10.1038/309063a0. [DOI] [PubMed] [Google Scholar]
- Dawson A. P., Irvine R. F. Inositol (1,4,5)trisphosphate-promoted Ca2+ release from microsomal fractions of rat liver. Biochem Biophys Res Commun. 1984 May 16;120(3):858–864. doi: 10.1016/s0006-291x(84)80186-2. [DOI] [PubMed] [Google Scholar]
- Dawson R. M., Clarke N. D-myoinositol 1:2-cyclic phosphate 2-phosphohydrolase. Biochem J. 1972 Mar;127(1):113–118. doi: 10.1042/bj1270113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson R. M., Freinkel N., Jungalwala F. B., Clarke N. The enzymic formation of myoinositol 1:2-cyclic phosphate from phosphatidylinositol. Biochem J. 1971 May;122(4):605–607. doi: 10.1042/bj1220605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Michell R. H. The polyphosphoinositide phosphodiesterase of erythrocyte membranes. Biochem J. 1981 Jul 15;198(1):133–140. doi: 10.1042/bj1980133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Mussat M. C., Michell R. H. The inositol trisphosphate phosphomonoesterase of the human erythrocyte membrane. Biochem J. 1982 Apr 1;203(1):169–177. doi: 10.1042/bj2030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Wusteman M. M. Breakdown of polyphosphoinositides and not phosphatidylinositol accounts for muscarinic agonist-stimulated inositol phospholipid metabolism in rat parotid glands. Biochem J. 1983 Dec 15;216(3):633–640. doi: 10.1042/bj2160633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein A., Payne R., Corson D. W., Berridge M. J., Irvine R. F. Photoreceptor excitation and adaptation by inositol 1,4,5-trisphosphate. Nature. 1984 Sep 13;311(5982):157–160. doi: 10.1038/311157a0. [DOI] [PubMed] [Google Scholar]
- Hofmann S. L., Majerus P. W. Identification and properties of two distinct phosphatidylinositol-specific phospholipase C enzymes from sheep seminal vesicular glands. J Biol Chem. 1982 Jun 10;257(11):6461–6469. [PubMed] [Google Scholar]
- Hofmann S. L., Majerus P. W. Modulation of phosphatidylinositol-specific phospholipase C activity by phospholipid interactions, diglycerides, and calcium ions. J Biol Chem. 1982 Dec 10;257(23):14359–14364. [PubMed] [Google Scholar]
- Ikezawa H., Yamanegi M., Taguchi R., Miyashita T., Ohyabu T. Studies on phosphatidylinositol phosphodiesterase (phospholipase C type) of Bacillus cereus. I. purification, properties and phosphatase-releasing activity. Biochim Biophys Acta. 1976 Nov 19;450(2):154–164. [PubMed] [Google Scholar]
- Irvine R. F., Brown K. D., Berridge M. J. Specificity of inositol trisphosphate-induced calcium release from permeabilized Swiss-mouse 3T3 cells. Biochem J. 1984 Aug 15;222(1):269–272. doi: 10.1042/bj2220269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph S. K., Thomas A. P., Williams R. J., Irvine R. F., Williamson J. R. myo-Inositol 1,4,5-trisphosphate. A second messenger for the hormonal mobilization of intracellular Ca2+ in liver. J Biol Chem. 1984 Mar 10;259(5):3077–3081. [PubMed] [Google Scholar]
- Lapetina E. G., Seguin E. B., Agranoff B. W. Preparation of 32P-labeled inositides and their degradation by soluble kidney enzymes. Biochim Biophys Acta. 1975 Jul 22;398(1):118–124. doi: 10.1016/0005-2760(75)90175-7. [DOI] [PubMed] [Google Scholar]
- Majerus P. W., Neufeld E. J., Wilson D. B. Production of phosphoinositide-derived messengers. Cell. 1984 Jul;37(3):701–703. doi: 10.1016/0092-8674(84)90405-7. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Palmer F. B. Chromatography of acidic phospholipids on immobilized neomycin. J Lipid Res. 1981 Nov;22(8):1296–1300. [PubMed] [Google Scholar]
- Prentki M., Biden T. J., Janjic D., Irvine R. F., Berridge M. J., Wollheim C. B. Rapid mobilization of Ca2+ from rat insulinoma microsomes by inositol-1,4,5-trisphosphate. Nature. 1984 Jun 7;309(5968):562–564. doi: 10.1038/309562a0. [DOI] [PubMed] [Google Scholar]
- Prescott S. M., Majerus P. W. Characterization of 1,2-diacylglycerol hydrolysis in human platelets. Demonstration of an arachidonoyl-monoacylglycerol intermediate. J Biol Chem. 1983 Jan 25;258(2):764–769. [PubMed] [Google Scholar]
- Schacht J. Purification of polyphosphoinositides by chromatography on immobilized neomycin. J Lipid Res. 1978 Nov;19(8):1063–1067. [PubMed] [Google Scholar]
- Sherman W. R., Munsell L. Y., Gish B. G., Honchar M. P. Effects of systemically administered lithium on phosphoinositide metabolism in rat brain, kidney, and testis. J Neurochem. 1985 Mar;44(3):798–807. doi: 10.1111/j.1471-4159.1985.tb12886.x. [DOI] [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Suematsu E., Hirata M., Hashimoto T., Kuriyama H. Inositol 1,4,5-trisphosphate releases Ca2+ from intracellular store sites in skinned single cells of porcine coronary artery. Biochem Biophys Res Commun. 1984 Apr 30;120(2):481–485. doi: 10.1016/0006-291x(84)91279-8. [DOI] [PubMed] [Google Scholar]
- Sundler R., Alberts A. W., Vagelos P. R. Enzymatic properties of phosphatidylinositol inositolphosphohydrolase from Bacillus cereus. Substrate dilution in detergent-phospholipid micelles and bilayer vesicles. J Biol Chem. 1978 Jun 25;253(12):4175–4179. [PubMed] [Google Scholar]
- Watson S. P., McConnell R. T., Lapetina E. G. The rapid formation of inositol phosphates in human platelets by thrombin is inhibited by prostacyclin. J Biol Chem. 1984 Nov 10;259(21):13199–13203. [PubMed] [Google Scholar]
- Wilson D. B., Bross T. E., Hofmann S. L., Majerus P. W. Hydrolysis of polyphosphoinositides by purified sheep seminal vesicle phospholipase C enzymes. J Biol Chem. 1984 Oct 10;259(19):11718–11724. [PubMed] [Google Scholar]



