Abstract
Purpose.
To report feasibility and reliability of a semi-automated image analysis method for retinal vessel diameter measurements in subjects with papilledema before and after treatment.
Methods.
Scanning laser ophthalmoscopy (SLO) was performed in seven normal, five pseudopapilledema, and seven papilledema subjects. In four papilledema subjects, SLO was performed both before and following treatment. Two observers measured diameters of superior and inferior retinal arteries and veins from SLO images using two methods: manual analysis and semi-automated customized analysis. Vessel measurements were compared between observers and between image analysis methods. Retinal vein and artery diameters for each subject were compared between papilledema, pseudopapilledema, and normal subjects, and before and following treatment for papilledema subjects.
Results.
Interobserver reliability was 0.97 (Pearson's correlation, r) and 0.90 for semi-automated and manual measurements, respectively. Correlation coefficient of manual and semi-automated measurements was 0.85. Retinal vein diameter in papilledema subjects was larger than in pseudopapilledema and normal subjects (P = 0.03, 0.04, Mann-Whitney). Papilledema subjects had a decrease in retinal vein diameter following treatment for and resolution of papilledema (P = 0.04, Wilcoxon signed rank). Retinal artery diameters were not significantly different between papilledema and pseudopapilledema or normal groups, and did not significantly change following papilledema treatment.
Conclusions.
A feasible and reliable semi-automated image analysis method for measurement of retinal artery and vein diameters from SLO images of elevated optic nerves is reported. Further studies are needed to determine the clinical utility of retinal vein diameter measurements as a marker for diagnosis and treatment of papilledema.
Keywords: papilledema, idiopathic intracranial hypertension, retinal blood vessels
Retinal vessel diameters measured from scanning laser ophthalmoscopic images using semi-automated image analysis are feasible and reliable. Retinal vein diameters are larger in papilledema subjects before treatment than in pseudopapilledema, normal, and papilledema subjects after treatment.
Introduction
Permanent vision loss caused by papilledema, the swelling of the optic nerve heads due to elevation in intracranial pressure (ICP), occurs in 50% of people with idiopathic intracranial hypertension (IIH).1,2 This newly affects 1:100,000 people annually, with a 20-fold higher incidence in otherwise healthy, young, obese women.3,4 Direct monitoring of ICP, which is the root cause of papilledema and target of papilledema treatment, is limited to invasive techniques such as spinal taps. Therefore, most treatment decisions are based on downstream outcome markers such as peripheral vision and optic disc appearance, changes of which are delayed following changes in intracranial pressure.5 There is a critical need for identifying markers of IIH disease status that, when combined with current clinical measures, will improve clinical management of IIH and IIH outcomes.
Retinal blood vessel diameter changes are promising indirect markers for intra-cerebral and intra-optic nerve mechanical forces in IIH because they are a consequence of compression of the ophthalmic artery and vein caused by the swollen optic nerve and ICP. A small study demonstrated decrease in retinal vein diameters in eyes with papilledema following treatment with optic nerve sheath fenestration (a surgery designed to release pressure around the optic nerve6). A descriptive study noted increased retinal venous diameters and pressures in response to short-term ICP increases an animal model.7 Retinal blood vessel diameters are methodologically attractive quantities because they can be easily visualized through the pupil and quantitatively measured with noninvasive imaging technologies with high spatial and temporal resolution.8–12
Algorithms developed for measurement of retinal blood vessel diameters in other diseases are not directly applicable to the study of retinal blood vessels in papilledema. Available methods for study of vessel size utilize fundus photographs.10,12,13 There is a theoretical concern for relative magnification of vessels elevated out of the image plane caused by linear perspective. Available analysis methods use optic disc diameter as a reference against which to standardize vessel measurements.12 These cannot be applied to papilledema because optic disc swelling prevents identification of disc diameter. Confocal scanning laser ophthalmoscopy (SLO) is an imaging modality that overcomes these challenges. This digital imaging technology is sensitive, has high resolution, and can be used without dilating the pupils. Because it is a planar imaging technique, it will not magnify out of plane objects. It is intrinsically calibrated during acquisition and therefore, does not require a reference structure in the image to generate a quantitative measurement.
The objectives of this study are to report measurements of retinal vessel diameters in eyes with pseudopapilledema, untreated papilledema, and treated papilledema by a semi-automated image analysis method applied to SLO images and demonstrate, in a pilot manner, vascular diameter differences between untreated papilledema in both psuedopapilledema and normal subjects, and papilledema before and after therapeutic intervention.
Methods
Subjects
Subjects from the Neuro-ophthalmology Service at the University of Illinois with clinically suspected papilledema were identified retrospectively (n = 11 subjects) and prospectively (1 subject). Subjects were included in the study if SLO imaging of the optic disc had been performed prior to lumbar puncture (LP). Subjects were females aged 38 ± 20 years, with normal brain imaging and no neurological or ophthalmic disease other than idiopathic intracranial hypertension (IIH) or pseudopapilledema. Lumbar puncture opening pressure (LPOP), performed after baseline SLO imaging in all cases, was measured using a manometer when the patient was in the lateral decubitus position. Subjects were classified as untreated papilledema due to IIH (n = 7 subjects) or pseudopapilledema (n = 5 subjects) if the LPOP was greater or less than 25 cm water, respectively. All papilledema subjects met diagnostic criteria for IIH.14 All pseudopapilledema subjects did not have an alternative diagnosis for disc elevation other than drusen or anomalous discs based on review of magnetic resonance imaging and clinical testing. Normal subjects (n = 7, 6 male, aged 37 ± 13 years) with no ophthalmic or neurological disease and no history of headaches were identified prospectively.
SLO images after LP (follow-up) were included in the study for papilledema subjects if they were obtained following resolution of papilledema and symptoms attributable to elevated ICP (n = 4 subjects). Follow-up SLO images were included for pseudopapilledema subjects if they were obtained more than 6 months after the baseline image (n = 1 subject). Follow-up images were not available for normal subjects. The research followed the tenets of the Declaration of Helsinki and was approved by an institutional review board of the University of Illinois at Chicago.
SLO Imaging
Infrared SLO Images were obtained using a commercially available instrument (Spectralis; Heidelberg Engineering, Heidelberg, Germany). SLO images consisted of 1536 × 1536 pixels with 3-μm resolution, covering a retinal area of 30 × 30°. The image was captured using the high-resolution retinal nerve fiber layer scanning protocol. The optic disc was centered on the SLO image for optimal visualization of major retinal vessels (Fig. 1a).
Figure 1.
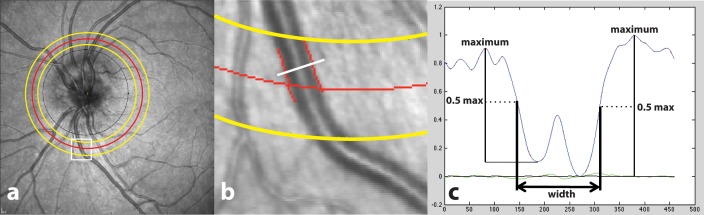
Example of semi-automated analysis of retinal vessel diameter. (a) SLO image obtained in a subject with papilledema prior to treatment. The black circle displays the location of the OCT RNFL measurement, which was not used in our study. The red circle is the center of the ROI, located 2.25 mm from the optic disc center. The yellow circles are the boundaries of the ROI. The white box outlines a region with a selected inferior vein. (b) Magnified region outlined by white box shown in (a) with inferior vein vessel boundary as identified by the semi-automated image segmentation method indicated by red lines. The white line is a representative vessel cross-section. (c) Normalized intensity profile derived across the cross-section of the inferior retinal vein shown in (b). Width of the profile at half maximum intensity is the measure of vessel width (indicated by double-headed arrow).
Image Analysis
Vessel diameter was measured using semi-automated and manual techniques in a ring region of interest (ROI) with inner and outer radii of 2.0 and 2.5 mm, respectively, from the optic disc center. This region was selected for vessel diameter measurement because vessels were not distorted and obscured due to disc swelling. Vessel diameter was measured in two major retinal arteries and two major retinal veins in each eye. Inferior- and superior-temporal veins and inferior- and superior-temporal arteries were chosen, as these were reliably identifiable across most subjects.
Semi-automated measurements of vessel diameter were obtained using a customized software written in a computing environment (MATLAB; MathWorks, Inc., Natick, MA, USA), similar to a method previously applied for measurement of conjunctival blood vessel diameters (Fig. 1b).15 Briefly, a two-dimensional Hessian filter was applied to the SLO image to create a “vesselness” image, according to a previously reported method.16 This image was then binarized using a threshold to assign pixels corresponding to vessels to 1, and other pixels to 0. A vessel of interest was manually identified on the binary image within the ROI. The centerline of the vessel was automatically extracted using distance transform after manually selecting starting and end points for the centerline.17 Vessel width was automatically calculated by first generating intensity profiles across the vessel (perpendicular to the vessel centerline), then determining the full width at half maximum of the profile (Fig. 1c). This calculation was performed repeatedly along the vessel segment and the mean value was calculated as the vessel diameter. Two observers independently measured the diameter of each target vessel on each baseline image using this methodology. One observer measured the diameter of the same vessels on each follow-up image.
Manual measurements of vessel diameter were performed using the ophthalmic software provided with the SLO instrument (Eye Explorer; Heidelberg Engineering). Two observers independently measured the diameter of each target vessel on baseline papilledema and pseudopapilledema images (n = 84 vessels). They were instructed to use measurement tools provided with the software to identify the ROI and to select a clear vascular segment within the ROI. They used the same measurement tools to measure the vessel diameter perpendicular to the vessel walls in the center of the segment.
Statistical Analysis
Interobserver reliability of each image analysis method (manual, semi-automated) was evaluated using Pearson correlation and calculation of interobserver variability applied to the diameter measurements for each vessel (n = 84). Interobserver variability was evaluated by calculating the difference between the observer's measurements for each vessel. These differences were compared between image analysis methods using t-test for independent samples. Multivariate linear regression analyses were performed to evaluate the effects of vessel type (artery, vein) on the relationship between vessel measurements by two observers for each analysis method. The full regression model included a term representing vessel type (artery or vein) and an interaction term (vessel type ⊥ vessel diameter). This permitted calculation of the constants (y-intercept) and slopes that were dependent on vessel type. Backward elimination techniques were used to incrementally remove the least significant term to arrive at the final model. P < 0.10 was required for a term to be included in the final model.
Manual and semi-automated measurements of each vessel (n = 84) were compared using Pearson's correlation and multivariate linear regression analysis. Vessel diameters representing each method were calculated as the mean of the two observers' measurements for each vessel.
For each subject at each time point (baseline, follow-up), retinal vessel diameters in both eyes obtained by a single observer using semi-automated analysis were averaged to derive a retinal artery diameter index and a retinal vein diameter index. Due to the small sample size and inability to confirm normal distribution of the population data, nonparametric methods were used to compare between and within subjects. Baseline vessel diameter indices were compared in a pairwise manner between untreated papilledema, pseudopapilledema, and normal subjects by applying Mann-Whitney tests. Vessel diameter indices in papilledema subjects were compared between baseline (untreated) and follow-up (treated) using Wilcoxon signed ranks test. To evaluate segmental effects, index diameters for superior artery, inferior artery, superior vein, and inferior vein were calculated by averaging regional vessel diameters in both eyes for each patient. The superior and inferior artery and vein index diameters were compared between papilledema and pseudopapilledema groups using Mann-Whitney tests. All analyses were performed using statistical software (SPSS 21; IBM Corp., Armonk, NY, USA). Statistical significance was accepted at P < 0.05.
Results
Reliability and Comparison of Image Analysis Methods
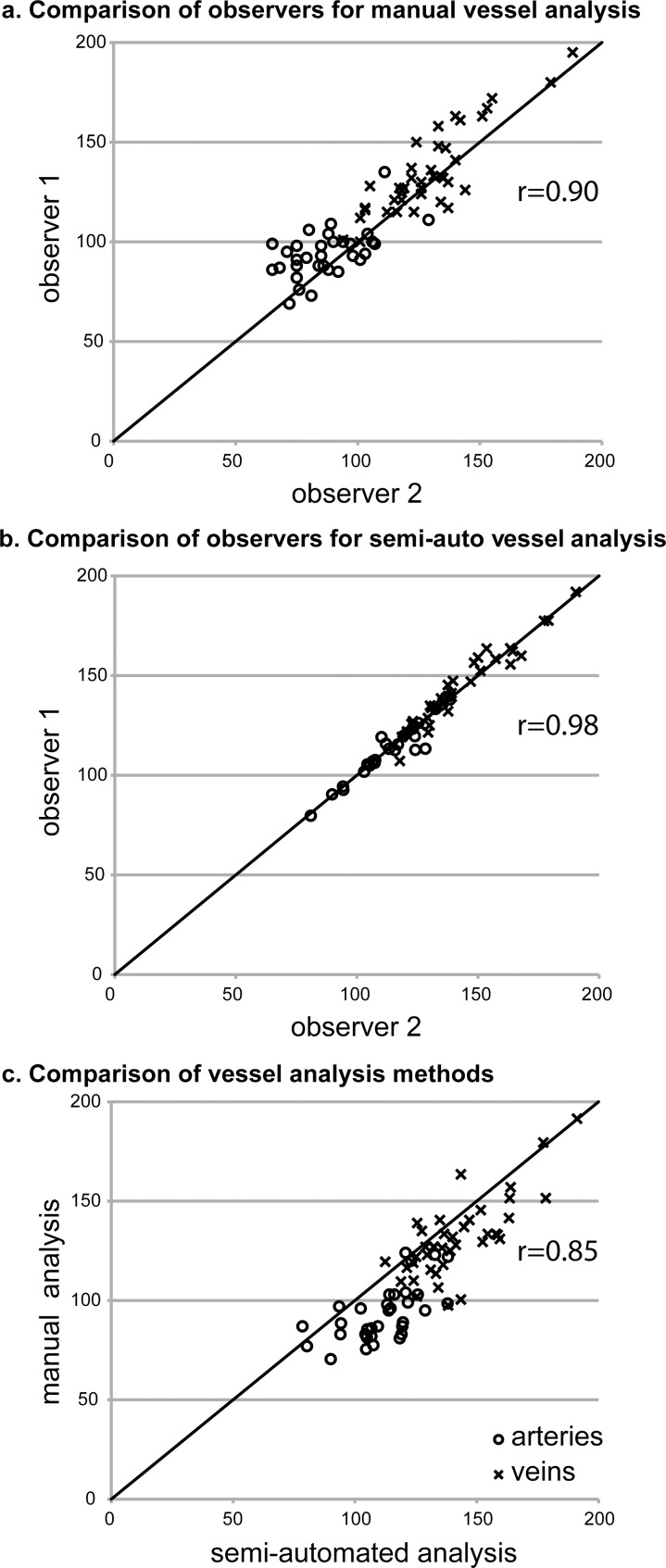
Manual diameter measurements were obtained for 83% to 90% (observer 1: 38/42; observer 2: 35/42) of retinal arteries and 92% to 98% (observer 1: 41/42; observer 2: 39/42) of retinal veins. Semi-automated diameter measurements were obtained for 64% to 67% (observer 1: 27/42; observer 2: 28/42) of retinal arteries and 82% to 88% of retinal veins (observer 1: 37/42; observer 2: 35/42). The relationship between observers for each method and between methods is shown graphically in Figure 2 and quantitatively in the Table. The interobserver variability was significantly less for semi-automated measurements than for manual measurements (P < 0.001, t-test). The vessel type dependent constant and slope in the multivariate linear regression models were not significantly different from zero for either image analysis method. The y-intercepts for the interobserver comparisons were also not significantly different from zero. Therefore, these terms were not included in the final regression models.
Figure 2.
Comparison of retinal blood vessel diameter measurements obtained by different observers using manual and semi-automated image analysis methods. (a) Interobserver reliability for manual measurements. (b) Interobserver reliability for semi-automated measurements. (c) Comparison of manual and semi-automated measurements. The line in each panel is the identity line. All axes are vessel diameter in micrometers.
Table.
Comparison of 84 Retinal Vessel Diameter Measurements Made From SLO Fundus Images of Elevated Optic Discs Using Manual and Semi-Automated Analysis Techniques
|
Interobserver Comparison |
Analysis Method Comparison |
||
|
Manual Analysis |
Semi-Automated Analysis |
||
| Variability, mean, ±SD (mean, ±) | 11 ± 8 μm (10% ± 8%) | 3.2 ± 3.5 μm (2% ± 3%) | |
| Pearson's correlation, r | 0.90 | 0.98 | 0.85 |
| Regression full model | |||
| Y-intercept (95% CI) | 22.9 μm (−0.3 to 46.0) | 7.3 μm (−4.8 to 19.4) | 56.3 μm (30.2–82.4) |
| Slope (95% CI) | 0.79 (0.62–0.96) | 0.94 (0.86–1.03) | 0.66 (0.46–0.86) |
| Vessel-dependent constant (95% CI) | −3.9 μm (−40.7 to 33.0) | −2.2 μm (−21.5 to 17.1) | −5.4 μm (−44.4 to 33.6) |
| Vessel-dependent slope (95% CI) | −0.06 (−0.40 to 0.28) | 0.02 (−0.14 to 0.18) | 0.0 (−0.36 to 0.37) |
| Regression final model | |||
| Y-intercept (95% CI) | 42.3 μm (28.6–56.0) | ||
| Slope (95% CI) | 0.91 (0.81–1.01) | 0.95 (0.90–0.99) | 0.75 (0.64–0.86) |
Retinal Vessel Diameters in Papilledema, Pseudopapilledema, and Normal Subjects
Papilledema, pseudopapilledema, and normal subjects did not differ in age (P = 0.64–0.80, Mann-Whitney). Opening pressure was higher in the papilledema group (mean: 36; range, 34–37 cm H2O) than in the pseudopapilledema group (14–21 cm H2O; P = 0.008 Mann-Whitney). Pseudopapilledema was due to ultrasound- or OCT-confirmed drusen and anomalous discs in three and two subjects, respectively. No pseudopapilledema subject had empty sella on magnetic resonance imaging. Frisen grading of papilledema18 was grade 1, 2, 3, and 4 in one, one, three, and two papilledema subjects, respectively. No subject had disc pallor. LPs were performed an average of 12.5 days (range, 0–53 days) after initial SLO imaging in papilledema subjects.
Papilledema subjects with follow-up imaging were treated medically with acetazolamide monotherapy (n = 2); acetazolamide and furosemide (n = 1); and acetazolamide, furosemide, topiramate, and transverse venous sinus stenting (n = 1). Follow-up SLO imaging was completed after an average of 5.75 months of medical therapy in the medically treated subjects and 1 week after venous sinus stenting in the surgically treated subject. Improvement in disc swelling was seen in all papilledema subjects to Frisen grade 1. Flat temporal disc margins were confirmed on OCT B-scan of the optic discs performed at the time of follow-up SLO imaging. The pseudopapilledema subject with follow-up imaging showed no change in disc appearance or contour on OCT B-scan.
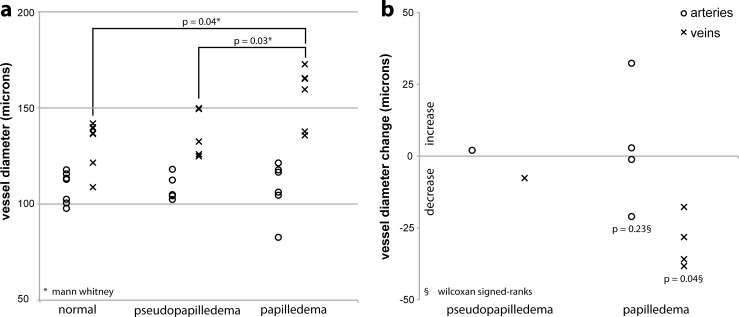
Artery diameters were not different between untreated papilledema (median: 112 μm; interquartile range, 13 μm) and pseudopapilledema (median: 105 μm; interquartile range, 12 μm; P = 0.57, Mann-Whitney) or normal (median: 112 μm; interquartile range, 15 μm, P = 0.66, Mann-Whitney) subjects. Vein diameters were larger in untreated papilledema subjects (median 160 μm, interquartile range 28, μm) compared with pseudopapilledema (median: 133 μm, interquartile range: 24 μm; P = 0.03 Mann-Whitney) and normal (median: 137 μm; interquartile range, 18 μm; P = 0.04 Mann-Whitney) subjects (Fig. 3a). Superior artery diameters and inferior artery diameters were not different between papilledema and pseudopapilledema groups. Superior vein and inferior vein diameters were larger in the papilledema group compared with the pseudopapilledema group. Artery and vein diameters were not different between the normal and pseudopapilledema groups.
Figure 3.
Cross sectional and longitudinal retinal vessel diameter comparisons. (a) Retinal blood vessel diameters in normal, pseudopapilledema, and untreated papilledema subjects. Each marker represents the index artery or vein diameter for a single subject. (b) Change in retinal blood vessel diameters following treatment in papilledema subjects and following 6 months in an untreated pseudopapilledema subject. Each marker represents the increase (>0) or decrease (<0) in index artery or vein diameter for a single subject.
Artery diameter did not change in papilledema subjects following treatment (median: 5.2-μm increase; P = 0.23, Wilcoxon signed ranks). Vein diameter decreased in papilledema subjects following treatment (median 32.9-μm decrease, P = 0.04; Wilcoxon signed ranks). The absolute change in vein diameter (7.2 μm) in the pseudopapilledema subject with follow-up was less than that of all papilledema subjects (Fig. 3b).
Discussion
We have demonstrated feasibility of retinal vessel diameter measurements in eyes with elevated optic discs using a semi-automated image analysis method applied to SLO images. The interobserver vessel diameter measurements for the semi-automated analysis method were highly correlated and measurements obtained by observers were near identical. The mean interobserver difference in retinal vessel diameter measurements of the semi-automated method (3 μm) is at the level of image resolution. This was lower than that of our manual method (11 μm) and similar to previously reported semi-automated methods.10,19 This illustrates the advantage of automated image analysis to reduce interobserver variability, which is particularly important in larger studies that necessitate multiple observers.20,21
Manual and semi-automated vessel diameter measurements were correlated, but had nonzero regression intercepts indicating consistent bias between analysis methods. This finding is likely due to differences in identification of vessel boundaries by humans and computer. Due to unavailability of a gold standard for vessel diameter measurement, the accuracy of the methods was not determined.
In the current study, larger vein diameters were observed in subjects with papilledema compared with subjects with pseudopapilledema and normal subjects. To our knowledge, this is the first report comparing retinal vessel diameters between subjects with different kinds of optic nerve elevation. Retinal vein diameters decreased, while no consistent change in retinal artery diameters was detected, following treatment and resolution of papilledema, in agreement with reported observations of Lee et al. following surgical treatment of papilledema.6 A prospective longitudinal study will be important for study of the influence of covariates, study of intereye correlations, and assessment of the utility of retinal vein diameter as a clinical marker of papilledema.
A theoretical framework to account for the association between large retinal vein diameters and untreated papilledema is based on the Hagen-Poiseuille equation that describes the relationship between pressure gradient (Δ P), volumetric fluid flow (Q) rate and tube radius (Δ P α Q/r4). Elevated ICP in the subarachnoid space directly compresses the vessels draining the central retinal vein. In addition, swollen axons in the optic nerve with papilledema directly compress the central retinal vascular bundle as it passes through the optic nerve.22,23 Compressed vessels have increased resistance to blood flow with upstream effects of increased retinal venous pressure, which is expected to manifest as increased retinal vein diameter, increased blood vessel tortuosity, and loss of spontaneous venous pulsations.8,24 Observations of retinal vein diameter and pressure increases in a short-term model of ICP elevation without papilledema suggest that ICP changes in the absence of optic nerve changes are sufficient to alter retinal hemodynamics.7 Characterization of retinal blood flow is the next step for evaluating papilledema within this framework. While retinal and optic nerve blood flow have been studied in ischemic optic neuropathy, optic neuritis and optic nerve elevation, they have not been specifically studied in papilledema.25–27
Strengths of our study include images obtained prior to lumbar puncture to ensure appropriately categorization of papilledema and pseudopapilledema subjects' ICP at the time of imaging and use of semi-automated methodology for vessel diameter measurements. A limitation is use of an index variable different than the central retinal artery and vein equivalents, used in other population studies of retinal vessel diameters.12,28 This reflects a limitation in the ability to distinguish small diameter vessel type (vein or artery) on SLO images. A limitation of the semi-automated analysis methodology was the proportion of vessels for which a diameter could not be calculated. Vessel anatomy was an important factor as the semi-automated method failed to distinguish and segment individual vessels that were in close proximity. SLO image quality was another factor contributing to unsuccessful semi-automated measurement. The rate of successful measurement might be improved through modifications to image acquisition procedures that ensure focus on the plane of interest and by improving the image segmentation algorithm for application to lower quality images. The small sample size and retrospective nature of this study are limitations, as the patient selections and timing of testing were not standardized.
We have demonstrated the feasibility of using semi-automated image analysis techniques to quantify retinal vessel diameters from SLO images of subjects with papilledema and pseudopapilledema. In a pilot manner, we demonstrated that retinal vein diameters are larger in untreated papilledema subjects when compared with psuedopapilledema subjects, normal subjects and when compared with treated papilledema subjects. These findings assert the role of retinal vein diameter measurements for capturing the dynamics of pathophysiological changes associated with evolving and resolving papilledema. Future studies are needed to evaluate the utility of retinal vein measurement as a diagnostic and therapeutic monitoring tool for papilledema as well as to understand the determinants of altered cerebral and retinal hemodynamics that underlie vein diameter alterations in papilledema.
Acknowledgments
Supported by National Institutes of Health Grants K12 EY021475, R01 EY017918, and P30 EY001792, and an unrestricted departmental grant from Research to Prevent Blindness.
Disclosure: H.E. Moss, None; G. Treadwell, None; J. Wanek, None; S. DeLeon, None; M. Shahidi, None
References
- 1. Corbett JJ, Savino PJ, Thompson HS, et al. Visual loss in pseudotumor cerebri. Follow-up of 57 patients from five to 41 years and a profile of 14 patients with permanent severe visual loss. Arch Neurol. 1982; 39: 461–474 [DOI] [PubMed] [Google Scholar]
- 2. Rowe FJ, Sarkies NJ. Assessment of visual function in idiopathic intracranial hypertension: a prospective study. Eye. 1998; 12: 111–118 [DOI] [PubMed] [Google Scholar]
- 3. Radhakrishnan K, Thacker AK, Bohlaga NH, Maloo JC, Gerryo SE. Epidemiology of idiopathic intracranial hypertension: a prospective and case-control study. J Neurol Sci. 1993; 116: 18–28 [DOI] [PubMed] [Google Scholar]
- 4. Radhakrishnan K, Ahlskog JE, Cross SA, Kurland LT, O'Fallon WM. Idiopathic intracranial hypertension (pseudotumor cerebri) descriptive epidemiology in Rochester, Minn, 1976 to 1990. Arch Neurol. 1993; 50: 78–80 [DOI] [PubMed] [Google Scholar]
- 5. Hayreh MS, Hayreh SS. Optic disc edema in raised intracranial pressure. I. Evolution and resolution. Arch Ophthalmol. 1977; 95: 1237–1244 [DOI] [PubMed] [Google Scholar]
- 6. Lee SY, Shin DH, Spoor TC, Chaesik K, McCarty B, Kim D. Bilateral retinal venous caliber decrease following unilateral optic nerve sheath decompression. Ophthalmic Surg. 1995; 26: 25–28 [PubMed] [Google Scholar]
- 7. Rios-Montenegro EN, Anderson DR, David NJ. Intracranial pressure and ocular hemodynamics. Arch Ophthalmol. 1973; 89: 52–58 [DOI] [PubMed] [Google Scholar]
- 8. Moret F, Poloschek CM, Lagrèze WA, Bach M. Visualization of fundus vessel pulsation using principal component analysis. Invest Ophthalmol Vis Sci. 2011; 52: 5457–5464 [DOI] [PubMed] [Google Scholar]
- 9. Patton N, Aslam T, MacGillivray T, Pattie A, Deary IJ, Dhillon B. Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: a rationale based on homology between cerebral and retinal microvasculatures. J Anat. 2005; 206: 319–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. De Jong F, Schrijvers E, Ikram M, et al. Retinal vascular caliber and risk of dementia The Rotterdam Study. Neurology. 2011; 76: 816–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haan M, Espeland M, Klein B, et al. Cognitive function and retinal and ischemic brain changes The Women's Health Initiative. Neurology. 2012; 78: 942–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hubbard LD, Brothers RJ, King WN, et al. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology. 1999; 106: 2269–2280 [DOI] [PubMed] [Google Scholar]
- 13. Leung H, Wang JJ, Rochtchina E, et al. Relationships between age, blood pressure, and retinal vessel diameters in an older population. Invest Ophthalmol Vis Sci. 2003; 44: 2900–2904 [DOI] [PubMed] [Google Scholar]
- 14. Friedman DI, Jacobson DM. Diagnostic criteria for idiopathic intracranial hypertension. Neurology. 2002; 59: 1492–1495 [DOI] [PubMed] [Google Scholar]
- 15. Shahidi M, Wanek J, Gaynes B, Wu T. Quantitative assessment of conjunctival microvascular circulation of the human eye. Microvasc Res. 2010; 79: 109–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Frangi AF, Niessen WJ, Vincken KL, Viergever MA. Multiscale vessel enhancement filtering. Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Interventation. Lect Notes Comp Sci. Vol. 1496 Springer; 1998: 130–137 [Google Scholar]
- 17. Teng P-Y, Bagci AM, Alperin N. Automated prescription of an optimal imaging plane for measurement of cerebral blood flow by phase contrast magnetic resonance imaging. IEEE Trans Biomed Eng. 2011; 58: 2566–2573 [DOI] [PubMed] [Google Scholar]
- 18. Frisen L. Swelling of the optic nerve head: a staging scheme. J Neurol Neurosurg Psychiatry. 1982; 45: 13–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sherry LM, Wang JJ, Rochtchina E, et al. Reliability of computer-assisted retinal vessel measurement in a population. Clin Exp Ophthalmol. 2002; 30: 179–182 [DOI] [PubMed] [Google Scholar]
- 20. Newsom RSB, Sullivan PM, Rassam SMB, Jagoe R, Kohner EM. Retinal vessel measurement: comparison between observer and computer driven methods. Graefes Arch Clin Exp Ophthalmol. 1992; 230: 221–225 [DOI] [PubMed] [Google Scholar]
- 21. Delori FC, Fitch KA, Feke GT, Deupree DM, Weiter JJ. Evaluation of micrometric and microdensitometric methods for measuring the width of retinal vessel images on fundus photographs. Graefes Arch Clin Exp Ophthalmol. 1988; 226: 393–399 [DOI] [PubMed] [Google Scholar]
- 22. McCulley TJ, Lam BL, Bose S, Feuer WJ. The effect of optic disk edema on spontaneous venous pulsations. Am J Ophthalmol. 2003; 135: 706–708 [DOI] [PubMed] [Google Scholar]
- 23. Hayreh SS. Pathogenesis of oedema of the optic disc (papilloedema): a preliminary report. Br J Ophthalmol. 1964; 48: 522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kylstra J, Wierzbicki T, Wolbarsht M, Landers M, Stefansson E. The relationship between retinal vessel tortuosity, diameter, and transmural pressure. Graefes Arch Clin Exp Ophthalmol. 1986; 224: 477–480 [DOI] [PubMed] [Google Scholar]
- 25. Collignon-Robe NJ, Feke GT, Rizzo JF. Optic nerve head circulation in nonarteritic anterior ischemic optic neuropathy and optic neuritis. Ophthalmology. 2004; 111: 1663–1672 [DOI] [PubMed] [Google Scholar]
- 26. Arnold AC, Badr MA, Hepler RS. Fluorescein angiography in nonischemic optic disc edema. Arch Ophthalmol. 1996; 114: 293–298 [DOI] [PubMed] [Google Scholar]
- 27. Wang Y, Fawzi AA, Varma R, et al. Pilot study of optical coherence tomography measurement of retinal blood flow in retinal and optic nerve diseases. Invest Ophthalmol Vis Sci. 2011; 52: 840–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Parr JC, Spears GF. General caliber of the retinal arteries expressed as the equivalent width of the central retinal artery. Am J Ophthalmol. 1974; 77: 472–477 [DOI] [PubMed] [Google Scholar]