Abstract
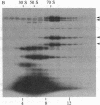
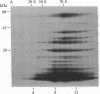
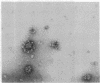
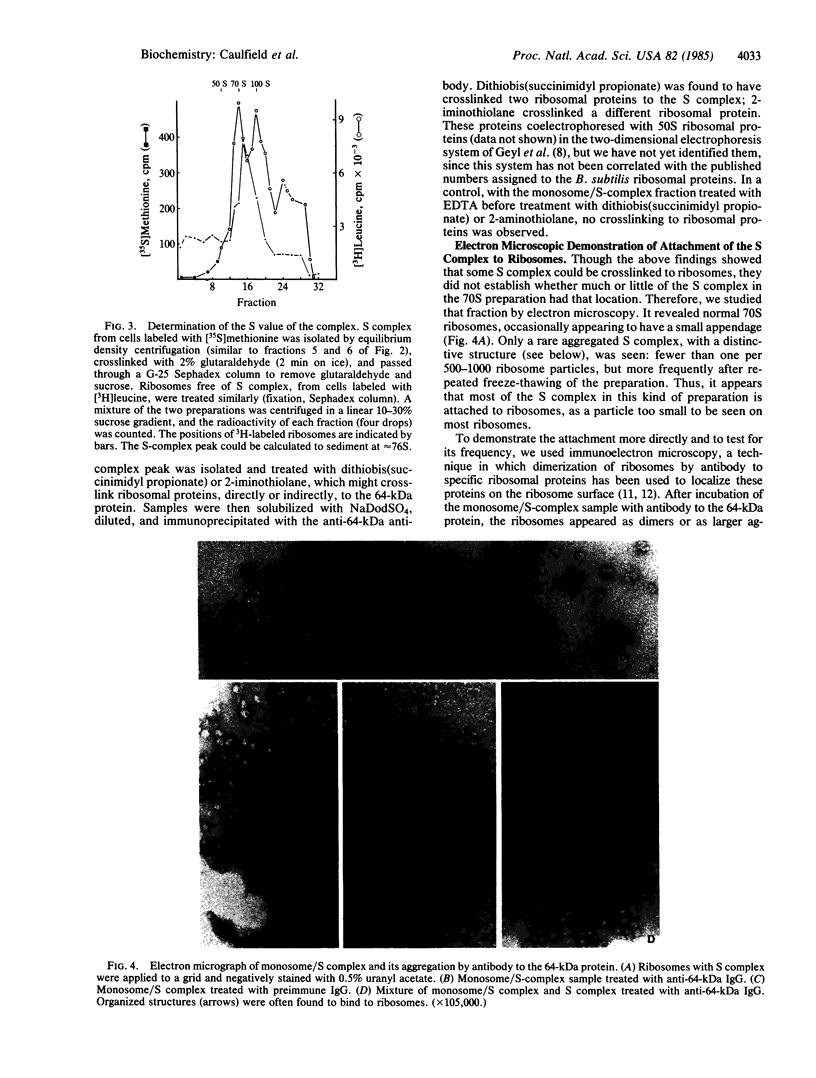
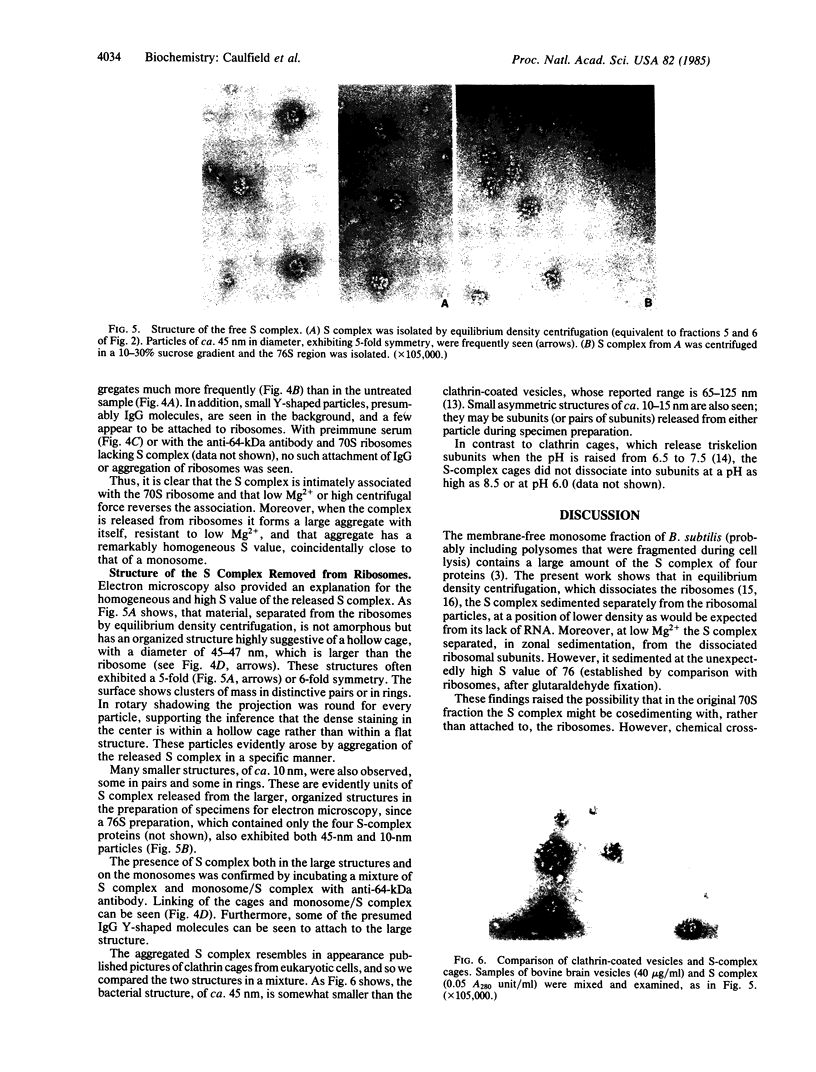
The S complex of Bacillus subtilis, a set of four proteins that appears to be involved in protein secretion, is shown to be attached to 70S ribosomes: antibody to its 64-kDa component can aggregate these ribosomes, and the complex can be chemically crosslinked to ribosomal proteins. Low Mg2+ or prolonged high-speed centrifugation in a sucrose gradient releases the S complex from the ribosomes, and it is recovered as an aggregate with an S value of 76. Electron microscopy shows that these aggregates have a regular structure, somewhat resembling clathrin cages, with a diameter of about 45 nm. If these aggregates are physiological, their function would differ significantly from that of the signal recognition particle of eukaryotes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caulfield M. P., Horiuchi S., Tai P. C., Davis B. D. The 64-kilodalton membrane protein of Bacillus subtilis is also present as a multiprotein complex on membrane-free ribosomes. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7772–7776. doi: 10.1073/pnas.81.24.7772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Rhoads D., Tai P. C. Alkaline phosphatase and OmpA protein can be translocated posttranslationally into membrane vesicles of Escherichia coli. J Bacteriol. 1985 Mar;161(3):973–980. doi: 10.1128/jb.161.3.973-980.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyl D., Böck A., Isono K. An improved method for two-dimensional gel-electrophoresis: analysis of mutationally altered ribosomal proteins of Escherichia coli. Mol Gen Genet. 1981;181(3):309–312. doi: 10.1007/BF00425603. [DOI] [PubMed] [Google Scholar]
- Horiuchi S., Marty-Mazars D., Tai P. C., Davis B. D. Localization and quantitation of proteins characteristic of the complexed membrane of Bacillus subtilis. J Bacteriol. 1983 Jun;154(3):1215–1221. doi: 10.1128/jb.154.3.1215-1221.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi S., Tai P. C., Davis B. D. A 64-kilodalton membrane protein of Bacillus subtilis covered by secreting ribosomes. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3287–3291. doi: 10.1073/pnas.80.11.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infante A. A., Baierlein R. Pressure-induced dissociation of sedimenting ribosomes: effect on sedimentation patterns. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1780–1785. doi: 10.1073/pnas.68.8.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny J. W., Traut R. R. Identification of fifteen neighboring protein pairs in the Escherichia coli 50 S ribosomal subunit crosslinked with 2-iminothiolane. J Mol Biol. 1979 Jan 25;127(3):243–263. doi: 10.1016/0022-2836(79)90328-0. [DOI] [PubMed] [Google Scholar]
- Kirchhausen T., Harrison S. C. Protein organization in clathrin trimers. Cell. 1981 Mar;23(3):755–761. doi: 10.1016/0092-8674(81)90439-6. [DOI] [PubMed] [Google Scholar]
- Lake J. A., Pendergast M., Kahan L., Nomura M. Localization of Escherichia coli ribosomal proteins S4 and S14 by electron microscopy of antibody-labeled subunits. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4688–4692. doi: 10.1073/pnas.71.12.4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake J. A. Practical aspects of immune electron microscopy. Methods Enzymol. 1979;61:250–257. doi: 10.1016/0076-6879(79)61014-5. [DOI] [PubMed] [Google Scholar]
- Marty-Mazars D., Horiuchi S., Tai P. C., Davis B. D. Proteins of ribosome-bearing and free-membrane domains in Bacillus subtilis. J Bacteriol. 1983 Jun;154(3):1381–1388. doi: 10.1128/jb.154.3.1381-1388.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M., Blobel G. In vitro translocation of bacterial proteins across the plasma membrane of Escherichia coli. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7421–7425. doi: 10.1073/pnas.81.23.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse B. M. Coated vesicles from pig brain: purification and biochemical characterization. J Mol Biol. 1975 Sep 5;97(1):93–98. doi: 10.1016/s0022-2836(75)80024-6. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Fine R. E. Coated vesicles transport newly synthesized membrane glycoproteins from endoplasmic reticulum to plasma membrane in two successive stages. Proc Natl Acad Sci U S A. 1980 Feb;77(2):780–784. doi: 10.1073/pnas.77.2.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid S. L., Matsumoto A. K., Rothman J. E. A domain of clathrin that forms coats. Proc Natl Acad Sci U S A. 1982 Jan;79(1):91–95. doi: 10.1073/pnas.79.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spirin A. S. On the equilibrium of the association-dissociation reaction of ribosomal subparticles and on the existance of the so-called '60 S intermediate' ('swollen 70 S') during centrifugation of the equilibrium mixture. FEBS Lett. 1971 May 20;14(5):349–353. doi: 10.1016/0014-5793(71)80298-3. [DOI] [PubMed] [Google Scholar]
- Valentine R. C., Green N. M. Electron microscopy of an antibody-hapten complex. J Mol Biol. 1967 Aug 14;27(3):615–617. doi: 10.1016/0022-2836(67)90063-0. [DOI] [PubMed] [Google Scholar]
- Van Jaarsveld P. P., Nandi P. K., Lippoldt R. E., Saroff H., Edelhoch H. Polymerization of clathrin protomers into basket structures. Biochemistry. 1981 Jul 7;20(14):4129–4135. doi: 10.1021/bi00517a028. [DOI] [PubMed] [Google Scholar]
- Wabl M. R. Electron microscopic localization of two proteins on the surface of the 50 S ribosomal subunit of Escherichia coli using specific antibody markers. J Mol Biol. 1974 Apr 5;84(2):241–247. doi: 10.1016/0022-2836(74)90582-8. [DOI] [PubMed] [Google Scholar]
- Walter P., Blobel G. Signal recognition particle contains a 7S RNA essential for protein translocation across the endoplasmic reticulum. Nature. 1982 Oct 21;299(5885):691–698. doi: 10.1038/299691a0. [DOI] [PubMed] [Google Scholar]
- Walter P., Blobel G. Translocation of proteins across the endoplasmic reticulum. II. Signal recognition protein (SRP) mediates the selective binding to microsomal membranes of in-vitro-assembled polysomes synthesizing secretory protein. J Cell Biol. 1981 Nov;91(2 Pt 1):551–556. doi: 10.1083/jcb.91.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward M. P., Roth T. F. Coated vesicles: characterization, selective dissociation, and reassembly. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4394–4398. doi: 10.1073/pnas.75.9.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]