Abstract
Bradykinin causes vascular relaxations through release of endothelial relaxing factors including prostacyclin, nitric oxide (NO) and epoxyeicosatrienoic acids (EETs). Bradykinin is metabolized by angiotensin converting enzyme (ACE) and ACE inhibition enhances bradykinin relaxations. Our goal was to characterize the role of bradykinin receptors and endothelial factors in ACE inhibitor-enhanced relaxations in bovine coronary arteries. In U46619 preconstricted arteries, bradykinin (10−11−10−8M) caused concentration-dependent relaxations (maximal relaxation ≥100%, log EC50=−9.8±0.1). In the presence of the NO synthase inhibitor, N-nitro-L-arginine (L-NA, 30 µM) and the cyclooxygenase inhibitor, indomethacin (10 µM), relaxations were reduced by an inhibitor of EET synthesis, miconazole (10 µM) (maximal relaxation =55±10%). Bradykinin relaxations were inhibited by the bradykinin 2 (B2) receptor antagonist, D-Arg0-Hyp3-Thi5,8-D-Phe7-bradykinin (1 µM) (log EC50=−8.5±0.1) but not altered by the B1 receptor antagonist, des-Arg9[Leu8]bradykinin (1 µM). Mass spectrometric analysis of bovine coronary artery bradykinin metabolites revealed a time-dependent increase in bradykinin (1–5) and (1–7) suggesting metabolism by ACE. ACE inhibition with captopril (50 µM) enhanced bradykinin relaxations (log EC50=−10.3±0.1). The enhanced relaxations were eliminated by L-NA, the B1 receptor antagonist but not the B2 receptor antagonist. Our results demonstrate that ACE inhibitor-enhanced bradykinin relaxations of bovine coronary arteries occur through endothelial cell B1 receptor activation and NO.
Keywords: bradykinin receptors, captopril, endothelium, epoxyeicosatrienoic acids
Introduction
In bovine coronary arteries, the nonapeptide bradykinin causes potent endothelium-dependent relaxations that are mediated through two distinct pathways; nitric oxide (NO) and an endothelium-derived hyperpolarizing factor (EDHF) (Pratt et al., 1996; Campbell et al., 2001). In this vasculature, the epoxyeicosatrienoic acids (EETs), arachidonic acid cytochrome P450 epoxygenase metabolites, function as transferable EDHFs (Campbell et al., 1996; Gebremehdin et al., 1998; Fisslthaler et al., 1999; Gauthier et al., 2005). They activate smooth muscle large-conductance, calcium-activated potassium channels to cause membrane hyperpolarization and vascular relaxation (Campbell et al., 1996; Pratt et al., 2001).
Kinin biological actions are mediated through the activation of two G protein coupled receptors, B1 and B2 (for reviews see Marceau and Regoll, 2004; McLean et al., 2000). The B2 receptor is constitutively expressed in many tissues types including the vasculature, whereas B1 receptor expression is regulated by cytokines and inflammatory regulators although some cell types have some constitutive expression (Hall, 1992; Marceau et al., 1998; McLean et al., 2000; Figueroa et al., 2001; Passos et al., 2004). Under physiological conditions, bradykinin relaxations of many arteries are mediated through endothelial cell B2 receptor activation (Mombouli et al., 1992; Cockcroft et al., 1994; Koller et al., 1995; Miyamoto et al., 1999; Su et al,. 2000; Ren et al., 2002).
In vivo, bradykinin’s half-life is estimated to be 17 sec (Ferreira and Vane., 1967). Enzymes responsible for bradykinin degradation include angiotensin converting enzyme (ACE, kinase II), carboxypeptidase N (kininase I), neutral endopeptidase and aminopeptidase P (Murphy et al., 2000). The stable plasma bradykinin metabolite is the pentapeptide bradykinin 1–5 (B(1–5)) formed by sequential ACE metabolism (Murphy et al., 2000). The ACE activity responsible for this metabolism is most likely of endothelial cell origin since ACE is highly expressed in this cell type (Baudin et al., 1997).
ACE inhibitors are utilized for the treatment of numerous cardiovascular diseases including hypertension and heart failure (Smith and Ball, 2000). They suppress the conversion of angiotensin I to angiotensin II as well as bradykinin metabolism to inactive peptides B(1–7) and B(1–5) (Skeggs et al., 1956; Yang et al., 1971). Acute ACE inhibitor exposure potentiates bradykinin relaxations in arteries from numerous vascular beds. Possible mechanisms of this potentiation include increased local concentrations of bradykinin or direct interaction of the ACE inhibitor with B1 receptors (Mombouli et al., 1992, 2002; Beril et al., 2002, Erdös et al., 2010). The goal of our study was to characterize the role of B1 and B2 receptors and endothelial relaxing factors in ACE inhibitor-enhanced bradykinin relaxations in bovine coronary arteries. The results from our study indicate that the ACE inhibitor, captopril, enhances bradykinin relaxation of bovine coronary arteries through endothelial B1 receptor-mediated NO release.
Results
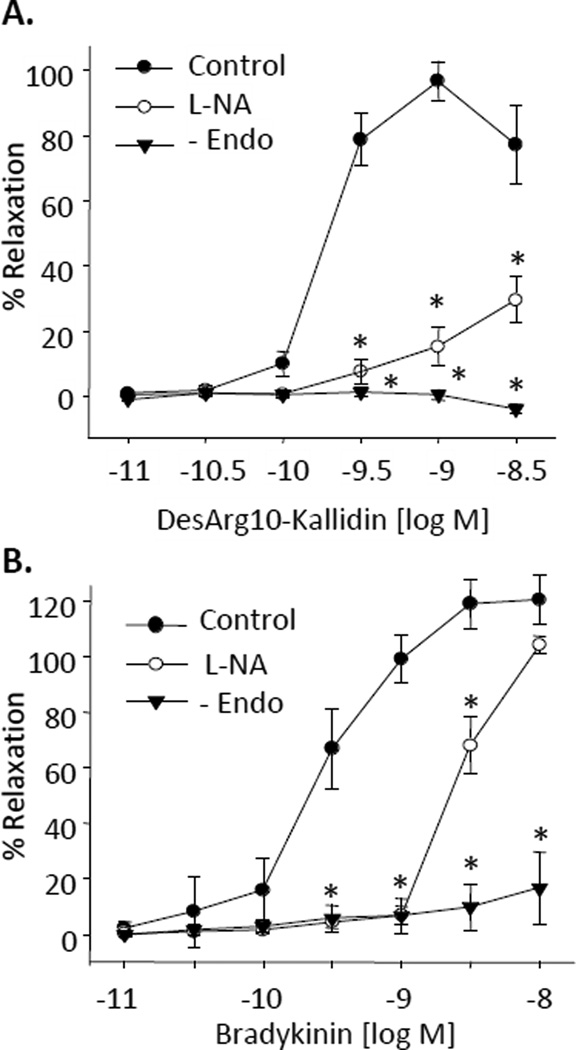
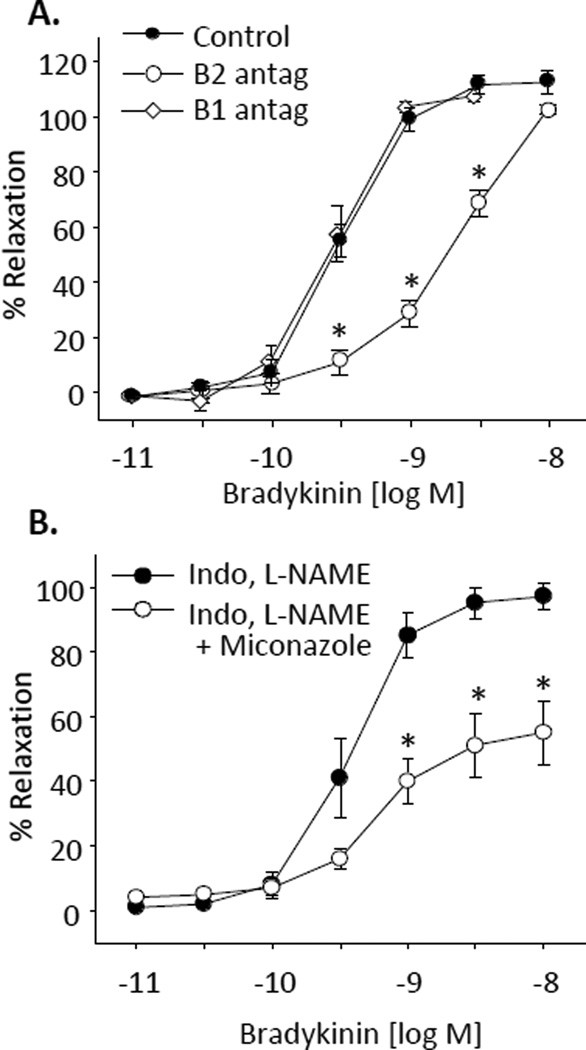
In bovine coronary arterial rings preconstricted with U46619, the B1 receptor agonist, DesArg10-kallidin, caused potent concentration-related relaxations (maximal relaxations = 97 ± 6%, log EC50 = −9.9 ± 0.8) (Figure 1A). The relaxations were eliminated by endothelium removal and greatly reduced by NO synthase inhibition with L-nitro-arginine (L-NA, 30 µM) (maximal relaxations = 30 ± 7%). Similarly, bradykinin, caused concentration-dependent relaxations (maximal relaxations = 122 ± 9%, log EC50 = −9.5 ± 0.1 (Figure 1B) that were eliminated by endothelium removal and inhibited, but not blocked by L-NA (log EC50 = −8.2 ± 0.1). To clarify the role of specific receptors in bradykinin relaxations, the relaxations were repeated with and without the B1 receptor antagonist desArg9-Leu8-bradykinin (1 µM) or the B2 receptor antagonist, D-Arg0-Hyp3-Thi5,8-D-Phe7-bradykinin (1 µM) (Figure 2A). Maximal relaxations to bradykinin were significantly reduced by the B2 receptor antagonist (log EC50=−8.5±0.1). In contrast, the B1 receptor antagonist did not alter the relaxation response to bradykinin. Thus, under control conditions, the endothelium-dependent relaxations to bradykinin are mediated by B2 receptors only.
Figure 1.
Effect of NO inhibition and endothelium removal on DesArg10-Kallidin (A) and bradykinin (B) relaxations of bovine coronary arteries. Relaxations responses were recorded in arterial rings preconstricted with the thromboxane mimetic U46619 (10 – 50 nM). Arteries were pretreated with the NO inhibitor L-NA (30 µM). n = 4–8. Each value represents the mean ± standard error of the mean (SEM). * significantly different from control.
Figure 2.
Effect of bradykinin receptor antagonists (A) and cytochrome p450 inhibition (B) on bradykinin relaxations of bovine coronary arteries. Relaxations responses were recorded in arterial rings preconstricted with the thromboxane mimetic U46619 (10 – 50 nM). (A). Arteries were pretreated with the B1 receptor antagonist (B1 antag), des-Arg9[Leu8]bradykinin (1 µM) or the B2 receptor antagonist (B2 antag), D-Arg-0Hyp3-Thi5,8-D-Phe7] bradykinin (1 µM). (B) Arteries were treated with L-NA (30 µM), indomethacin (10 µM) with or without miconazole (10 µM). n = 4–9. Each value represents the mean ± SEM. * significantly different from control.
To verify the role of cytochrome P450 metabolites in mediating the bradykinin NO-resistant relaxations, relaxations to bradykinin were repeated in the presence of the cyclooxygenase inhibitor, indomethacin (10 µM) plus the NOS inhibitor, L-NAME (30 µM) with and without the cytochrome P450 inhibitor, miconazole (10 µM) (Figure 2B). Miconazole reduced the maximal relaxations over 40% to 55 ± 10%. Thus, in bovine coronary arteries, bradykinin relaxations occur through endothelial B2 receptor activation to increase NO release and the release of cytochrome P450 metabolites, an EET.
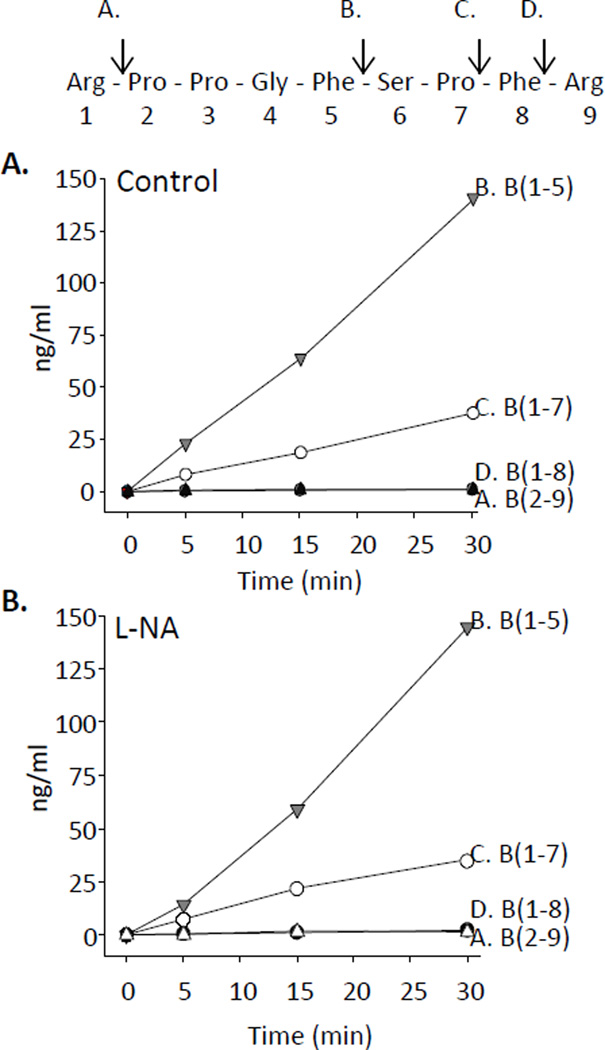
We next examined bradykinin metabolism by bovine coronary arteries. Bovine coronary arteries were incubated with bradykinin (1 µM) for 0, 5, 15 and 30 minutes with and without L-NA (30 µM). Metabolites were extracted and analyzed by liquid chromatography-mass spectroscopy (LC-MS). Elution times of bradykinin standards, the respective mass to charge ratios (m/z) used for their detection and lower limit sensitivities are shown in Table 1. Bradykinin metabolite concentrations increased with time (Figure 3A). The major metabolites were B(1–5) and B(1–7) whereas B(1–8) and B(2–9), the kininase I and aminopeptidase P metabolites, respectively, were minor metabolites (≤ 2.3 ng/ml). B(1–5) was produced in the highest concentration (141 ng/ml at 30 min). B(1–5) represents two sequential C-terminal cleavages by ACE and B(1–7) represents one C-terminal cleavage by ACE. Bradykinin metabolism was not altered by pretreatment with L-NA (Figure 3B). Thus, ACE activity represents the primary metabolic pathway for bradykinin degradation in bovine coronary arteries.
Table 1.
Liquid chromatography-mass spectrometry (LC-MS) assay parameters. Elution times of bradykinin and the various bradykinin metabolite standards, mass to charge ratios (m/z) used for their respective detection and lower limit detection sensitivities.
| Elution times (min) | m/z | Sensitivity | |
|---|---|---|---|
| Internal Std | 20.06 | 517 | --- |
| B(1–5) | 11.72 | 573 | 25 pg/µl |
| B(1–7) | 13.98 | 379 | 5 pg/µl |
| Bradykinin | 21.49 | 531 | 5 pg/µl |
| B(2–9) | 25.04 | 453 | 5 pg/µl |
| B(1–8) | 28.92 | 453 | 5 pg/µl |
Figure 3.
Bradykinin metabolism in bovine coronary arteries. Bovine coronary arteries were incubated with bradykinin (1 µM) for 0, 5, 15 and 30 min under control conditions (A) without L-NA and (B) with L-NA (30 µM). Metabolites were extracted and analyzed by LC-MS. Bradykinin peptide sequence and metabolic cleavage sites are noted above panel A. Site A represents cleavage by aminopeptidase P and formation of B(2–9). Site B represents 2 successive cleavages by ACE and B(1–5) formation. Site C represents 1 cleavage by ACE and B(1–7) formation. Site D represents cleavage by carboxypeptidase and B(1–8) formation.
Since B(1–7) is a primary metabolite of bradykinin, we next examined its activity in preconstricted coronary arterial rings. B(1–7) was without activity. It did not cause relaxation or further constriction (data not shown). Thus, ACE metabolism represents an inactivation pathway for the vascular relaxation effects of bradykinin in bovine coronary arteries.
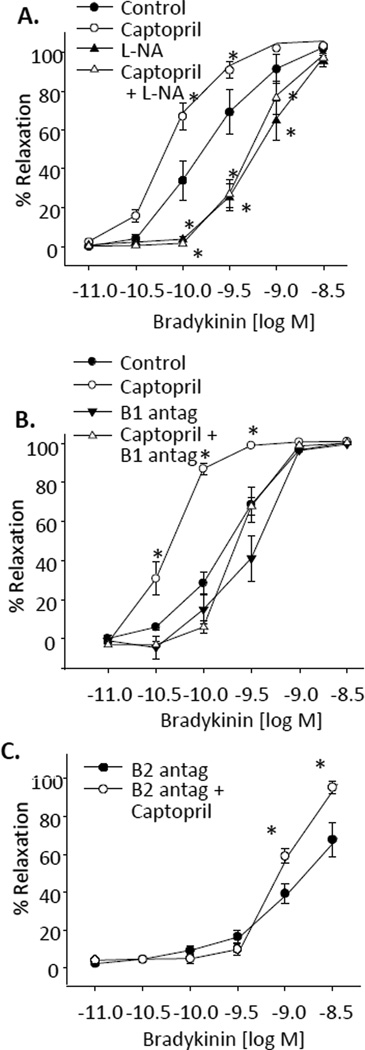
To determine the effect of ACE metabolism on bradykinin relaxations, we repeated the relaxations in the presence of the ACE inhibitor, captopril (50 µM) (Figure 4A). Captopril enhanced the relaxations (log EC50 = −10.3±0.1) compared to control bradykinin relaxations (log EC50 = −9.8±0.1). Control relaxations without captopril were inhibited by L-NA (log EC50 =−9.0±0.1). Captopril-enhanced relaxations were eliminated by L-NA (log EC50=−9.1±0.1), (Figure 4A) and B1 receptor antagonism (log EC50 = −9.4±0.1) (Figure 4B). Alternatively, captopril-enhanced relaxations to bradykinin remained when relaxations were performed with the B2 receptor antagonist (B2 antagonist alone, max relaxations = 67.9±9.1%, B2 antagonist plus captopril, max relaxations = 95.6±3.2%) (Figure 4C). Thus, captopril-enhanced relaxations to bradykinin occur through B1-receptor-mediated, NO-dependent mechanisms.
Figure 4.
Effect of ACE inhibition on bradykinin relaxations of bovine coronary arteries. Relaxations responses were recorded in arterial rings preconstricted with the thromboxane mimetic U46619 (10 – 50 nM). (A) Effect of NOS inhibition with L-NA (30 µM). (B) Effect of B1 receptor inhibition (B1 antag) with des-Arg9[Leu8]bradykinin (1 µM). (C) Effect of the B2 receptor antagonist (B2 antag), D-Arg0-Hyp3-Thi5,8-D-Phe7-bradykinin (1 µM). n = 7–18. Each value represents the mean ± SEM. * = significantly different from control or B2 antagonist.
Discussion
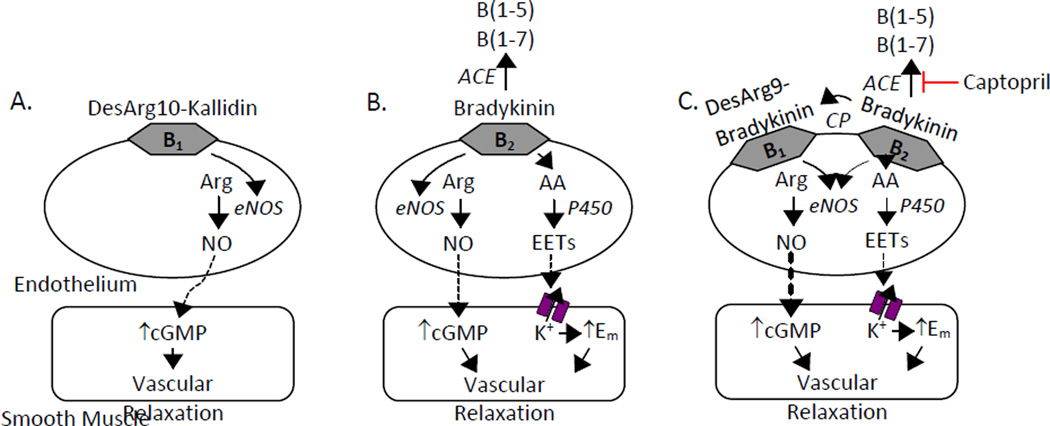
This study clarifies the roles of B1 and B2 receptors in bradykinin relaxations of bovine coronary arteries. Under control conditions, bradykinin relaxation occurs through activation of endothelial cell B2 receptors and are coupled to the release of NO and a cytochrome P450 metabolite. Previous studies have characterized the cytochrome P450 metabolite as an EET (Campbell et al., 1996; Fisslthaler et al., 1998; Gebremehdin et al., 1998; Campbell et al., 2001; Gauthier et al., 2005). In contrast, ACE inhibitor enhanced relaxations to bradykinin are mediated by B1 receptors and NO release. These pathways are illustrated in Figure 5.
Figure 5.
Mechanisms of kinin relaxations in bovine coronary arteries. Kinins interact with endothelial cell B1 and B2 receptors to stimulate relaxing factor release. (A) The B1 receptor agonist DesArg10-kallidin stimulates endothelial NOS (eNOS) NO production. NO diffuses to the smooth muscle and activates cGMP to cause vascular relaxation. (B) B2 receptor activation with bradykinin stimulates the release of 2 relaxing factors: NO plus the cytochrome P450 arachidonic acid metabolite, the EETs. EETs activate smooth muscle potassium channels to cause membrane hyperpolarization and vascular relaxation. (C) The presence of captopril enhances bradykinin relaxations through B1 receptor-dependent NO production. This occurs through increased bradykinin availability for carboxypeptidase metabolism resulting in increased DesArg9-bradykinin concentrations and the subsequent stimulation of B1 receptors. CP, carboxypeptidase.
Numerous studies have demonstrated a role of B2 receptors in bradykinin relaxations (Mombouli et al., 1992; Cockcroft et al., 1994; Koller et al., 1995; Miyamoto et al., 1999; Su et al., 2000; Ren et al., 2002). Since B2 receptors are constitutively expressed, their contribution to bradykinin relaxations is expected. Similar to our results, B2 receptor-dependent dilations of canine and porcine coronary arteries are partially mediated by NO plus an additional relaxing factor, EDHF (Momboulli et al., 1992; Fisslthaler et al., 1998; Su et al., 2000). Alternatively, the B1 receptor specific agonist, DesArg10-kallidin, stimulated potent endothelium-dependent relaxations in bovine coronary arteries. B1-mediated relaxations were unexpected since this receptor subtype is not normally expressed, but induced by inflammatory mediators. Similar to our observation, B1 receptor-specific relaxations were observed in canine, porcine and human coronary arteries (Drummond and Cocks, 1995; Pruneau et al., 1996; Su et al., 2000). B1 receptor expression is observed in endothelial cells from human coronary, pulmonary, renal and carotid arteries plus others (McLean et al., 2000). This suggests that arterial B1 receptor expression may be constitutive. The role of NO in B1 receptor-dependent relaxations is in agreement with previous studies establishing NO release from endothelial cells during B1 receptor activation (Ignjatovic et al., 2004; Stanisavljevic et al., 2006; Skidgel et al., 2006; Zhang et al., 2011).
The LC-MS analysis of bradykinin metabolism by bovine coronary arteries identified the major metabolites as B(1–5) and B(1–7). This indicates that ACE is predominately involved in bradykinin degradation in these arteries. Studies of isolated rat aorta, canine coronary arteries or in vivo analysis of human plasma demonstrated a similar metabolite profile of bradykinin degradation (Murphey et al., 2000; Momboulli et al., 2002; Bujak-Gizycka et al., 2011). A logical expectation is that ACE inhibition enhances bradykinin relaxations by blocking metabolism and increasing the local concentrations of bradykinin at the level of the vascular endothelium. This expectation is supported by our observation that ACE metabolism of bradykinin is an inactivation pathway since B(1–7) was without vascular activity in bovine coronary arteries. In porcine coronary arteries, Beril and colleagues have suggested that endothelial cell co-localization of ACE and B2 receptors accounts for ACE inhibitor potentiation of bradykinin relaxations (Beril et al., 2002). In isolated canine coronary arteries, investigators similarly concluded that ACE inhibitor potentiation of bradykinin relaxation involved the protection of bradykinin from ACE metabolism (Momboulli et al., 2002). Following this reasoning, enhanced relaxations secondary to increasing bradykinin concentrations would be sensitive to B2 receptor blockade and not B1 receptor blockade. However, the captopril-enhanced relaxations of bovine coronary arteries remained intact in the presence of the B2 receptor antagonist. It is possible that the overall bath concentrations of bradykinin overwhelm and masks small increases in bradykinin concentrations during ACE inhibition and therefore a role of B2 receptors was not observed. In contrast, the captopril-enhanced relaxations were eliminated by the B1 receptor antagonist. Taken together, this suggests that enhanced relaxations that occur with captopril are complex and not simply due to increases in bradykinin since bradykinin is not a B1 receptor agonist.
Des-Arg9-bradykinin(B(1–8)), the bradykinin carboxypeptidase metabolite lacking the C-terminal arginine, is an endogenous B1 receptor agonist (Marceau and Regoll, 2004; Skidgel et al., 2006). This metabolite was produced in low concentrations when bradykinin was incubated with bovine coronary arteries. Previous studies indicate that carboxypeptidase M is co-localized with B1 receptors in the plasma membrane of endothelial cells. This provides for local B(1–8) delivery to the B1 receptor (Skidgel et al., 2006; Zhang et al, 2011). ACE metabolism of bradykinin removes the C-terminal Phe8-Arg9 decreasing bradykinin availability for carboxypeptidase metabolism to B(1–8) and limits local B(1–8) concentrations. ACE inhibition would therefore increase bradykinin availability for carboxypeptidase metabolism, increasing B(1–8) concentrations and the subsequent stimulation of B1 receptor, NO-dependent relaxations. It should be noted that our metabolism studies measured metabolites released into the incubation media. Local bradykinin metabolite concentrations at the endothelial cell may be underestimated by measurements in the incubation media. However, under control conditions, it does not appear that bradykinin metabolism to B(1–8) is of significance in the bovine coronary artery since the B1 receptor antagonist had little effect on the relaxations to bradykinin.
A direct interaction of ACE inhibitors and B1 receptors has been previously demonstrated in pulmonary endothelial cells with ACE inhibitors stimulating NO release through direct B1 receptor activation (Ignjatovic et al., 2002; Stanisavljevic et al., 2006; Zhang et al, 2011). Most importantly, Erdös and colleagues indicated that ACE inhibitors are allosteric enhancers of B1 receptor function and bind a Zn-binding consensus sequence, a site independent of the bradykinin orthostatic ligand binding site (Ignjatovic et al., 2002; Erdos et al., 2010). This interaction could occur in bovine coronary arteries and result in increased B1 receptor sensitivity to bradykinin. However, a direct effect of captopril was not apparent. Captopril alone did not altered vascular function of the bovine coronary arteries as similar concentrations of U46619 were used to constrict arteries treated with captopril as compared to the control arteries (10.3 ± 1.4 nM and 10.8 ± 1.4 nM, respectively).
In summary, our results indicate that captopril enhances relaxations to bradykinin by increased B1 receptor function. The enhanced relaxations to bradykinin with ACE inhibition are mediated by endothelial B1 receptor-mediated NO release.
Materials and Methods
Reagents
L-NA, L-NAME, miconazole, indomethacin, desArg10-kallidin, B(1–8) and buffer reagents were purchased from Sigma Aldrich, St. Louis, MO. Bradykinin was purchased from Sigma Aldrich (functional vascular studies) and American Peptide, Sunnyvale, CA (mass spectroscopy standard). Des-Arg9,Leu8-bradykinin and D-Arg0-Hyp3-Thi5,8-D-Phe7-bradykinin were purchased from Bachem, Torrance, CA. U-46619 was purchased from Cayman Chemical, Ann Arbor, MI. DesArg9-bradykinin and Phe8Ψ(CH-NH)-Arg9-bradykinin, the mass spectrometry internal standard, were purchased from Tocris Bioscience, Bristol, United Kingdom. B(1–5), B(1–7), B(2–9), and Lys-des-Arg9-bradykinin were purchased from American Peptide. Captopril was purchased from Spectrum Chemicals & Laboratory Products, New Brunswick, NJ. All solvents were high-performance liquid chromatography grade and were purchased from Sigma Aldrich.
Vascular reactivity studies
Measurements of isometric tone in bovine coronary arterial rings were conducted as described previously (Campbell et al., 1996; Gauthier et al., 2002). Fresh bovine hearts were obtained from a local slaughterhouse. Sections of the left anterior descending coronary artery were dissected, cleaned and cut into 1.5 – 2.0 mm diameter rings (3 mm length). Arterial rings were suspended on two stainless hooks. Tension was measured using either a model FT-03C force transducer (Grass Instruments), ETH-400 bridge amplifier, and MacLab 8e A/D converter with MacLab software and Macintosh computer or a four-chamber myograph (model 610M, Danish Myo Technology) containing a Kreb’s-bicarbonate buffer equilibrated with 95% O2-5% CO2 and maintained at 37°C. Data was recorded using MacLab software and a Macintosh computer. The arterial rings were slowly stretched to a basal tension of 2.5–3.5 grams and equilibrated for 0.5 hours. KCl (60 mM) was repeatedly added and rinsed until reproducible stable contractions were observed. The thromboxane mimetic, U46619 (5–20 nM), was added to increase basal tension to approximately 50 – 75% of maximal KCl contraction. Vessels were pretreated for 10 min with vehicle, L-NA (30 µM), L-NAME (30 µM), indomethacin (10 µM), D-Arg0-Hyp3-Thi5,8-D-Phe7-bradykinin (1 µM), des-Arg9[Leu8]bradykinin (1 µM) or captopril (50 µM) 10 min before preconstruction. Relaxation responses to cumulative additions of bradykinin, B(1–7) and DesArg10-kallidin were recorded. Basal tension represents tension before the addition of U46619. Results are expressed as % relaxation of the U46619-treated rings with 100% relaxation representing basal tension.
Bradykinin metabolism
Bovine coronary arterial rings (35 mg/vial) were incubated in 2 ml HEPES buffer (130 mM NaCl, 5 mM KCl, 20 mM HEPES, 1 mM CaCl2, 2 mM MgCl2, and 5.5 mM glucose, pH 7.4) with bradykinin (1 µM) for 0, 5, 15 and 30 min at 37°C in a shaker bath. In addition, a control sample of bradykinin in buffer alone without incubation was also processed. After incubation, the supernatant was immediately removed and extracted the same day.
Solid-phase extraction of bradykinin and bradykinin metabolites
The internal standard (Phe8Ψ(CH-NH)-Arg9-bradykinin) was added to each sample with ethanol containing 1% trifluoroacetic acid (TFA) to a final volume of 15% followed by 1 ml of water containing 1% TFA. The samples were applied to a preconditioned Sep Pak C18 SPE cartridge (Waters Corp., Milford, MA) and washed with 20 ml of water containing 1% TFA. Peptides were eluted from the column using 6 ml of methanol containing 1% TFA and dried under a stream of nitrogen gas. The sample was dissolved in 500 µl of 75% acetonitrile / 25% water containing 1% TFA, centrifuged, and the supernatant was removed and dried under a stream of nitrogen gas. For LC-MS, the samples were dissolved in 30 µl of 50% methanol / 50% water containing 3% formic acid and 0.01% TFA, centrifuged, and the supernatant analyzed.
Liquid chromatography, mass spectrometry (LC-MS) quantification of bradykinin peptide metabolites
LC-MS was performed using a modification of a previously described method (Cui et al., 2007; Gauthier et al., 2008). Analyses were performed using a Waters-Micromass Quattro micro API electrospray triple quadrupole mass spectrometric system coupled with a Waters 2695 high-performance liquid chromatograph. The mass spectrometer is equipped with a Z-spray dual orthogonal ionization source and is controlled by MassLynx 4.1 software. Samples were separated on a reverse phase C18 column (Jupiter 2.0×250 mm, Phenomenex) using water-methanol with 0.3% formic acid as a mobile phase at a flow rate of 0.2 ml/min. The mobile phase of 20% methanol in water linearly increased to 50% methanol over 30 min, followed by a linear increase to 100% methanol over 5 min. Positive ion electrospray ionization mass spectrometric conditions were as follows: capillary voltage: 3.2 kV, cone voltage: 34 V, desolvation temperature: 400°C, and source temperature: 100°C. Analysis was performed in positive electrospray, single-ion recording mode. Analyte concentrations were calculated using compound peak area / internal standard peak area ratios with comparison to standard curves. Elution times of the standards, mass to charge ratios (m/z) used for detection of the various bradykinin metabolites and the lower limit sensitivity of each metabolite are shown in Table 1.
Statistical Analysis
Data are expressed as means ± SEM. Statistical analysis was performed by a one-way analysis of variance followed by the Student-Newman-Keuls multiple comparison test when significant differences were present. P < 0.05 was considered statistically significant.
Acknowledgements
The authors thank Ms. Gretchen Barg for secretarial assistance and Dr. Kasem Nithipatikom and Ms. Marilyn Isbell for mass spectrometric analyses. This study was supported by a grant from the National Institutes of Health (HL-83297 and HL-51055).
References
- 1.Baudin B, Berard M, Carrier JL, Legrand Y, Drouet L. Vascular origin determines angiotensin I-converting enzyme expression in endothelial cells. Endothelium. 1997;5:73–84. doi: 10.3109/10623329709044160. [DOI] [PubMed] [Google Scholar]
- 2.Beril T, Dendorfer A, de Vries R, Saxena PR, Danser AHJ. Bradykinin potentiation by ACE inhibitors: a matter of metabolism. Br. J. Pharmacol. 2002;137:276–284. doi: 10.1038/sj.bjp.0704862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bujak-Giżycka B, Olszanecki R, Madej J, Suski M, Gębska A, Korbut R. Metabolism of bradykinin in aorta of hypertensive rats. Acta. Biochim. Pol. 2011;58:199–202. [PubMed] [Google Scholar]
- 4.Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ. Res. 1996;78:415–423. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- 5.Campbell WB, Falck JR, Gauthier KM. Role of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factor in bovine coronary arteries. Med. Sci. Monit. 2001;7:578–584. [PubMed] [Google Scholar]
- 6.Cockcroft JR, Chowienczyk PJ, Brett SE, Bender N, Ritter JM. Inhibition of bradykinin-induced vasodilation in human forearm vasculature by icatibant, a potent B2-receptor antagonist. Br. J. Clin. Pharmacol. 1994;38:317–321. doi: 10.1111/j.1365-2125.1994.tb04360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui L, Nithipatikom K, Campbell WB. Simultaneous analysis of angiotensin peptides by LC-MS and LC-MS/MS: metabolism by bovine adrenal endothelial cells. Anal. Biochem. 2007;369:27–33. doi: 10.1016/j.ab.2007.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drummond GR, Cocks TM. Endothelium-dependent relaxation to the B1 kinin receptor agonist des-Arg9-bradykinin in human coronary arteries. Br. J. Pharmacol. 1995;116:3083–3085. doi: 10.1111/j.1476-5381.1995.tb15108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erdös EG, Tan F, Skidgel RA. Angiotensin I-converting enzyme inhibitors are allosteric enhancers of kinin B1 and B2 receptor function. Hypertension. 2010;55:214–220. doi: 10.1161/HYPERTENSIONAHA.109.144600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferreira SH, Vane JR. The disappearance of bradykinin and eledoisin in the circulation and vascular beds of the cat. Br. J. Pharmacol. Chemother. 1967;30:417–424. doi: 10.1111/j.1476-5381.1967.tb02148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Figueroa CD, Marchant A, Novoa U, Förstermann U, Jarnagin K, Schölkens B, Müller-Esterl W. Differential distribution of bradykinin B(2) receptors in the rat and human cardiovascular system. Hypertension. 2001;37:110–120. doi: 10.1161/01.hyp.37.1.110. [DOI] [PubMed] [Google Scholar]
- 12.Fisslthaler B, Popp R, Kiss L, Potente M, Harder DR, Fleming I, Busse R. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature. 1999;401:493–497. doi: 10.1038/46816. [DOI] [PubMed] [Google Scholar]
- 13.Gauthier KM, Deeter C, Krishna UM, Reddy YK, Bondlela M, Falck JR, Campbell WB. 14,15-Epoxyeicosa-5(Z)-enoic acid: A selective epoxyeicosatrienoic acid antagonist that inhibits endothelium-dependent hyperpolarization and relaxation in coronary arteries. Circ. Res. 2002;90:1028–1036. doi: 10.1161/01.res.0000018162.87285.f8. [DOI] [PubMed] [Google Scholar]
- 14.Gauthier KM, Edwards EM, Falck JR, Reddy DS, Campbell WB. 14,15-Epoxyeicosatrienoic acid represents a transferable endothelium-dependent relaxing factor in bovine coronary arteries. Hypertension. 2005;45:666–671. doi: 10.1161/01.HYP.0000153462.06604.5d. [DOI] [PubMed] [Google Scholar]
- 15.Gauthier KM, Zhang DX, Cui L, Nithipatikom K, Campbell WB. Angiotensin II relaxations of bovine adrenal cortical arteries: role of angiotensin II metabolites and endothelial nitric oxide. Hypertension. 2008;52:150–155. doi: 10.1161/HYPERTENSIONAHA.107.104158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gebremedhin D, Harder DR, Pratt PF, Campbell WB. Bioassay of an endothelium-derived hyperpolarizing factor from bovine coronary arteries: role of a cytochrome P450 metabolite. J. Vasc. Res. 1998;35:274–284. doi: 10.1159/000025594. [DOI] [PubMed] [Google Scholar]
- 17.Hall JM. Bradykinin receptors: Pharmacological properties and biological roles. Pharmacol. Ther. 1992;56:131–190. doi: 10.1016/0163-7258(92)90016-s. [DOI] [PubMed] [Google Scholar]
- 18.Ignjatovic T, Stanisavljevic S, Brovkovych V, Skidgel RA, Erdös EG. Kinin B1 receptors stimulate nitric oxide production in endothelial cells: signaling pathways activated by angiotensin I-converting enzyme inhibitors and peptide ligands. Mol. Pharmacol. 2004;66:1310–1316. doi: 10.1124/mol.104.001990. [DOI] [PubMed] [Google Scholar]
- 19.Koller A, Rodenburg JM, Kaley G. Effects of Hoe-140 and ramiprilat on arteriolar tone and dilation to bradykinin in skeletal muscle of rats. Am. J. Physiol. Heart Circ. Physiol. 1995;268:H1628–H1633. doi: 10.1152/ajpheart.1995.268.4.H1628. [DOI] [PubMed] [Google Scholar]
- 20.Marceau F, Regoli D. Bradykinin receptor ligands: therapeutic perspectives. Nat. Rev. Drug Discov. 2004;3:845–852. doi: 10.1038/nrd1522. [DOI] [PubMed] [Google Scholar]
- 21.Marceau F, Hess F, Bachvarov DR. The B1 Receptors for Kinins. Pharmacol. Rev. 1998;50:357–386. [PubMed] [Google Scholar]
- 22.McLean PG, Perretti M, Ahluwalia A. Kinin B1 receptors and the cardiovascular system: regulation of expression and function. Cardiovasc. Res. 2000;48:194–210. doi: 10.1016/s0008-6363(00)00184-x. [DOI] [PubMed] [Google Scholar]
- 23.Miyamoto A, Ishiguro S, Nishio A. Stimulation of bradykinin B2-receptors on endothelial cells induces relaxation and contraction in porcine basilar artery in vitro. Br. J. Pharmacol. 1999;128:241–247. doi: 10.1038/sj.bjp.0702783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mombouli JV, Ballard KD, Vanhoutte PM. Kininase-independent potentiation of endothelium-dependent relaxations to kinins by converting enzyme inhibitor perindoprilat. Acta. Pharmacol. Sin. 2002;23:203–207. [PubMed] [Google Scholar]
- 25.Mombouli JV, Illiano S, Nagao T, Scott-Burden T, Vanhoutte PM. Potentiation of endothelium-dependent relaxations to bradykinin by angiotensin I converting enzyme inhibitors in canine coronary artery involves both endothelium-derived relaxing and hyperpolarizing factors. Circ. Res. 1992;71:137–144. doi: 10.1161/01.res.71.1.137. [DOI] [PubMed] [Google Scholar]
- 26.Murphey LJ, Hachey DL, Oates JA, Morrow JD, Brown NJ. Metabolism of bradykinin in vivo in humans: Identification of BK1-5 as a stable plasma peptide metabolite. J. Pharmacol. Exp. Ther. 2000;294:263–269. [PubMed] [Google Scholar]
- 27.Passos GF, Fernandes ES, Campos MM, Araújo JG, Pesquero JL, Souza GE, Avellar MC, Teixeira MM, Calixto JB. Kinin B1 receptor up-regulation after lipopolysaccharide administration: role of proinflammatory cytokines and neutrophil influx. J. Immunol. 2004;172:1839–1847. doi: 10.4049/jimmunol.172.3.1839. [DOI] [PubMed] [Google Scholar]
- 28.Pratt PF, Li P, Hillard CJ, Kurian J, Campbell WB. Endothelium-independent, ouabain-sensitive relaxation of bovine coronary arteries by EETs. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H1113–H1121. doi: 10.1152/ajpheart.2001.280.3.H1113. [DOI] [PubMed] [Google Scholar]
- 29.Pratt PF, Rosolowsky M, Campbell WB. Mediators of arachidonic acid-induced relaxation of bovine coronary artery. Hypertension. 1996;28:76–82. doi: 10.1161/01.hyp.28.1.76. [DOI] [PubMed] [Google Scholar]
- 30.Pruneau D, Luccarini J-M, Defrêne E, Paquet J-L, Bélichard P. Characterisation of bradykinin receptors from juvenile pig coronary artery. Eur. J. Pharmacol. 1996;297:53–60. doi: 10.1016/0014-2999(95)00720-2. [DOI] [PubMed] [Google Scholar]
- 31.Ren Y, Garvin J, Carretero OA. Mechanism involved in bradykinin-induced efferent arteriole dilation. Kidney Int. 2002;62:544–549. doi: 10.1046/j.1523-1755.2002.00482.x. [DOI] [PubMed] [Google Scholar]
- 32.Skeggs LT, Jr, Kahn JR, Shumway NP. The preparation and function of the hypertensin-converting enzyme. J. Exper. Med. 1956;103:295–299. doi: 10.1084/jem.103.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skidgel RA, Stanisavljevic S, Erdös EG. Kinin- and angiotensin-converting enzyme (ACE) inhibitor-mediated nitric oxide production in endothelial cells. Biol. Chem. 2006;387:159–165. doi: 10.1515/BC.2006.021. [DOI] [PubMed] [Google Scholar]
- 34.Smith WH, Ball SG. ACE inhibitors in heart failure: an update. Basic Res. Cardiol. 2000;95:8–14. doi: 10.1007/s003950070003. [DOI] [PubMed] [Google Scholar]
- 35.Stanisavljevic S, Ignjatovic T, Deddish PA, Brovkovych V, Zhang K, Erdös EG, Skidgel RA. Angiotensin I-converting enzyme inhibitors block protein kinase C∈ by activating bradykinin B1 receptors in human endothelial cells. J. Pharmacol. Exp. Ther. 2006;316:1153–1158. doi: 10.1124/jpet.105.093849. [DOI] [PubMed] [Google Scholar]
- 36.Su JB, Hoüel R, Héloire F, Barbe F, Beverelli F, Sambin L, Castaigne A, Berdeaux A, Crozatier B, Hittinger L. Stimulation of bradykinin B1 receptors induces vasodilation in conductance and resistance coronary vessels in conscious dogs: Comparison with B2 receptor stimulation. Circulation. 2000;101:1848–1853. doi: 10.1161/01.cir.101.15.1848. [DOI] [PubMed] [Google Scholar]
- 37.Yang HY, Erdös EG, Levin Y. Characterization of a dipeptide hydrolase (kininase II: angiotensin I converting enzyme) J. Pharmacol. Exp. Ther. 1971;177:291–300. [PubMed] [Google Scholar]
- 38.Zhang X, Tan F, Brovkovych V, Zhang Y, Skidgel RA. Cross-talk between carboxypeptidase M and the kinin B1 receptor mediates a new mode of G protein-coupled receptor signaling. J. Biol. Chem. 2011;286:18547–18561. doi: 10.1074/jbc.M110.214940. [DOI] [PMC free article] [PubMed] [Google Scholar]